Abstract
Lipopolysaccharide-binding protein (LBP), an acute-phase protein recognizing lipopolysaccharide (LPS), catalyzes in low concentrations its transfer to the cellular LPS receptor consisting of CD14 and Toll-like receptor-4. It has recently been shown that high concentrations of recombinant LBP can protect mice in a peritonitis model from the lethal effects of LPS. To determine whether in humans the acute-phase rise of LBP concentrations can inhibit LPS binding to monocytes and induction of proinflammatory cytokines, LBP concentrations were analyzed in 63 patients meeting the American College of Chest Physicians/Society of Critical Care Medicine criteria of severe sepsis or septic shock and the ability of these sera to modulate LPS effects in vitro was assessed employing different assays. Transfer of fluorescein isothiocyanate–labeled LPS to human monocytes was assessed by a fluorescence-activated cell sorter–based method, and activation of monocytes was investigated by measuring LPS-induced tumor necrosis factor-α secretion in the presence of the sera. Anti-LBP antibodies and recombinant human LBP were instrumental for depletion and reconstitution of acute-phase sera and subsequent assessment of their modulating effects on LPS activity. Sera of patients with severe sepsis/septic shock exhibited a diminished LPS transfer activity and LPS-induced tumor necrosis factor-α secretion as compared with sera from healthy controls. LBP depletion of sepsis sera and addition of rhLBP resulting in concentrations found in severe sepsis confirmed that LBP was the major serum component responsible for the observed effects. In summary, the inhibition of LPS effects by high concentrations of LBP in acute-phase serum, as described here, may represent a novel defense mechanism of the host in severe sepsis and during bacterial infections.
Introduction
Recognition of bacterial components such as lipopolysaccharide (LPS) by the innate immune system is an early and key event for triggering the inflammatory host response necessary for clearance of invading microorganisms.1,2 However, uncontrolled, the inflammatory response can be a cause of organ dysfunction remote from the primary site of infection, hypotension, or shock—a syndrome termed severe sepsis or septic shock.3,4Despite highly effective antimicrobial chemotherapy and powerful supportive treatment strategies, no significant reduction of mortality attributable to severe sepsis has been achieved in the last 2 decades. The incidence of severe sepsis and septic shock is 1% to 2% of hospital and 9% to 22% of intensive care unit admissions, with a crude mortality rate of 35% to 60%.5,6 Although novel approaches employing anti-inflammatory agents have been successfully applied for the control of severe sepsis and septic shock in experimental settings, only limited efficacy has been demonstrated in clinical trials.7 8 Thus, a better understanding of the molecular mechanisms of the host response and its regulation is needed to be able to develop successful innovative treatment strategies for patients with severe sepsis or septic shock.
For the initiation of the innate immune response, monocytes and macrophages play an essential role. These cells have the ability to recognize bacterial compounds and to activate the innate immune system by the release of a large number of different mediators, such as tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), and IL-6.9 Furthermore, as professional antigen-processing cells they play a crucial role in the activation of the adaptive immune system.10 In infections caused by Gram-negative microorganisms, the principal bacterial constituent recognized by the innate immune system is LPS, a glycolipid in the outer bacterial membrane.11 The acute-phase protein initiating recognition and monomerization of LPS aggregates and its transfer to mononuclear cells is lipopolysaccharide-binding protein (LBP).12 LBP binds to the amphipathic lipid A moiety of LPS and in low concentrations catalyzes its transfer to membrane-bound CD14, a glycosylphosphatidylinositol (GPI)–linked protein that is part of the cellular receptor for LPS.13 Recently, the Toll-like receptor-4 has been identified as the signal-transducing element of the LPS receptor.10 14
LBP is constitutively synthesized in hepatocytes and is present in serum at concentrations of 5 to 15 μg/mL. During the acute-phase response, IL-1 and IL-6 synergize in inducing LBP synthesis, leading to an increase of LBP serum concentrations.15-18 It has recently been discovered that epithelial cells of the intestines and the lungs may represent additional sources of LBP.19,20Besides its ability to amplify the immune response by recognizing small concentrations of LPS, LBP has also been shown in vitro to catalyze the transfer of LPS into high-density lipoprotein particles, resulting in LPS neutralization.21,22 This function of LBP may be protective during severe sepsis, as suggested by animal studies by both others and ourselves.23,24 Recently, 2 clinical studies were published, one of which showed a positive correlation between high initial serum LBP concentrations and improved patient outcome in severe sepsis.16 The other report demonstrated a correlation between high serum LBP concentrations and a decreased incidence of cardiovascular morbidity in patients with end-stage renal disease and hemodialysis.25
No experimental data, however, up to now have been available regarding the biological activity of high concentrations of LBP in the presence of acute-phase sera from patients. In recent years several studies applying whole blood or isolated peripheral blood mononuclear cells (PBMCs) from patients with severe sepsis have shown a significant reduction of LPS-induced cytokine release and impaired monocytic antigen presentation as compared with whole blood or PBMCs of healthy controls.26-29 The aim of the present study was to find out whether elevated LBP is a key factor for inhibition of LPS activity in acute-phase serum. We analyzed serum concentrations of LBP in patients with severe sepsis or septic shock and investigated in vitro whether acute-phase concentrations of LBP can modulate the LPS-induced monocytic response. We found that serum containing high concentrations of LBP clearly reduce LPS activity, an effect that was reversed by LBP depletion. Addition of recombinant human (rh)LBP to normal or LBP-depleted sepsis serum led to a decrease of LPS effects. These findings support the hypothesis that high concentrations of LBP are a key factor for inhibition of LPS activity by acute-phase sera.
Patients, materials, and methods
Patients
Sixty-three patients meeting the criteria of severe sepsis or septic shock were prospectively enrolled over a 14-month period in a 22-bed noncoronary intensive care unit of a tertiary care university hospital. Severe sepsis and septic shock were defined according to the criteria of the American College of Chest Physicians/Society of Critical Care Medicine consensus conference.3 Briefly, severe sepsis was defined by evidence of infection accompanied by at least 2 of the following criteria: fever or hypothermia (temperature > 38.0°C or < 36.0°C), tachycardia (> 90 ventricular beats/min), tachypnea (respiratory rate > 20 breaths/min or Paco2 < 4.3 kPa), or changes in the white blood cell count (leukocyte count > 12 × 109/L or < 4 × 109/L or > 10% immature band forms) and, in addition, at least one acute organ dysfunction remote from the site of infection as indicated by mental disorientation, oliguria, arterial hypoxemia, thrombocytopenia, unexplained metabolic acidosis, or hypotension. Septic shock was defined as a systolic blood pressure below 90 mm Hg for at least 1 hour despite adequate intravascular volume expansion or the necessity for vasopressor therapy. A total of 60.3% of the study cohort fulfilled the criteria of severe sepsis, and 39.7% were in septic shock. Another 52.4% developed septic shock after the onset of severe sepsis. The median duration of severe sepsis/septic shock was 4.8 days (range 2.3-31 days) in survivors and 5.5 days (range 1.0-35 days) in nonsurvivors. The severity of acute illness was scored daily by means of the Acute Physiology, Age, and Chronic Health Evaluation III (APACHE III) classification system and the Sequential Organ Failure Assessment (SOFA) score.30,31 The site of infection was identified using criteria of the Centers for Disease Control and Prevention except for sepsis.32 The infections included diffuse peritonitis (n = 39), pneumonia (n = 8), severe soft tissue infection (n = 6), catheter-associated bloodstream infection (n = 1), urogenital infection (n = 2), and others (n = 7). Documented bacteremia with Gram-negative microorganisms occurred in 4 cases, Gram-positive microorganisms in 6 cases, and in 5 cases bacteremia was polymicrobial. Twenty-one infections were prospectively classified as moderate (pneumonia, catheter-associated bloodstream infection, and isolated intra-abdominal abscess) and 42 as severe (diffuse peritonitis, severe soft tissue infection, deep organ abscess). One patient in the study cohort had a relevant liver disease as defined by the criteria of the APACHE III score. This patient with a biopsy-proven liver cirrhosis, however, did not exhibit any impaired LBP synthesis with peak serum LBP concentrations of 86.8 mg/L during severe sepsis. The patients' characteristics are given in Table1.
Demographic and clinical characteristics of the study cohort
| . | Severe sepsis/ septic shock . |
|---|---|
| Demographic characteristics | |
| Age, mean ± SD, y | 58.4 ± 16.1 |
| Sex, % female/male | 43/57 |
| Severity of the underlying disease, No. (McCabe/Jackson classification) | |
| Nonfatal | 48 |
| Fatal | 12 |
| Rapidly fatal | 3 |
| APACHE III score, mean ± SD, points | |
| Intensive care unit admission | 61 ± 20 |
| Severe sepsis/septic shock onset | 67 ± 21 |
| SOFA score, mean ± SD, points | |
| Severe sepsis onset/septic shock | 12 ± 4 |
| Length of treatment, median (range), d | |
| Intensive care unit | 14 (1-71) |
| Hospital | 28 (3-148) |
| Mortality, % | |
| Severe sepsis-related | 63.5 |
| Severe sepsis-related less than 3 d | 19.0 |
| All cause 28 d | 63.5 |
| All cause intensive care unit | 65.0 |
| All cause hospital | 69.8 |
| Positive microbial culture, local/blood, % | 55.6/23.8 |
| Gram-positive, No. | 12/6 |
| Gram-negative, No. | 7/4 |
| Polymicrobial, No. | 16/5 |
| Sites of infection, No. | |
| Diffuse peritonitis | 39 |
| Pneumonia | 8 |
| Soft tissue infection | 6 |
| Catheter-associated bloodstream infection | 1 |
| Urogenital infection | 2 |
| Others | 7 |
| . | Severe sepsis/ septic shock . |
|---|---|
| Demographic characteristics | |
| Age, mean ± SD, y | 58.4 ± 16.1 |
| Sex, % female/male | 43/57 |
| Severity of the underlying disease, No. (McCabe/Jackson classification) | |
| Nonfatal | 48 |
| Fatal | 12 |
| Rapidly fatal | 3 |
| APACHE III score, mean ± SD, points | |
| Intensive care unit admission | 61 ± 20 |
| Severe sepsis/septic shock onset | 67 ± 21 |
| SOFA score, mean ± SD, points | |
| Severe sepsis onset/septic shock | 12 ± 4 |
| Length of treatment, median (range), d | |
| Intensive care unit | 14 (1-71) |
| Hospital | 28 (3-148) |
| Mortality, % | |
| Severe sepsis-related | 63.5 |
| Severe sepsis-related less than 3 d | 19.0 |
| All cause 28 d | 63.5 |
| All cause intensive care unit | 65.0 |
| All cause hospital | 69.8 |
| Positive microbial culture, local/blood, % | 55.6/23.8 |
| Gram-positive, No. | 12/6 |
| Gram-negative, No. | 7/4 |
| Polymicrobial, No. | 16/5 |
| Sites of infection, No. | |
| Diffuse peritonitis | 39 |
| Pneumonia | 8 |
| Soft tissue infection | 6 |
| Catheter-associated bloodstream infection | 1 |
| Urogenital infection | 2 |
| Others | 7 |
For determination of reference values for LBP and soluble CD14 (sCD14), blood samples were drawn from 40 healthy hospital employees (median age 31 years; range 20-57 years). These sera also served as controls for the LPS binding and stimulation assays.
The investigation was carried out in agreement with the ethical standards of the declaration of Helsinki/Tokyo. The study protocol was approved by the Committee on Medical Ethics of the University Hospital Benjamin Franklin, and informed consent was obtained from volunteers and the patients or their nearest relatives before enrollment.
Sample collection
The first blood sample was drawn within 24 hours after onset of severe sepsis and subsequently once daily until recovery or the patient died. Depending on the duration of the severe sepsis, a median of 7 samples (range 1-38) per patient was collected. Blood samples were obtained either from an arterial line or by peripheral venous puncture and collected in sterile 8-mL tubes with SST Gel and Clot Activator and in 4-mL tubes with ethylenediaminetetraacetic acid (K2) 7.2 mg (Vacutainer) (Becton Dickinson, Meylan, France). After centrifugation at 3000g for 15 minutes and separation, serum and plasma samples were split into 6 aliquots and stored at −70°C until assayed.
LBP and sCD14 assays
Serum LBP concentrations were assessed by an enzyme-linked immunosorbent assay (ELISA) employing the monoclonal antibodies 1E8 and 2B5 (kindly provided by Dr A. Moriarty, Johnson & Johnson, La Jolla, CA). The lower limit of detection of this assay is 1.0 ng/mL. The reference range of serum LBP in healthy humans was found to be 1.85 to 17.4 mg/L (median 7.94 mg/L). Soluble CD14 was measured using a commercially available ELISA according to the manufacturer's instructions (sCD14 ELISA, IBL, Hamburg, Germany). All assays were performed in duplicate.
LBP-dependent binding of LPS to monocytes
Blood was obtained from 6 healthy volunteers, collected in endotoxin-free 8 mL tubes containing heparine (Vacutainer CPT, Becton Dickinson, Brussels, Belgium), and peripheral mononuclear cells subsequently were isolated by density gradient centrifugation according to the manufacturer's instructions. Cells in the interphase were collected, washed twice, and brought to a concentration of 5 × 105 PMBCs per milliliter, corresponding to 1.5 × 105 monocytes per milliliter. The cells were incubated with 30 μL acute-phase serum of patients with severe sepsis/septic shock, or with serum from healthy controls, in a total volume of 200 μL. One microgram per milliliter fluorescein isothiocyanate (FITC)–conjugated LPS from Escherichia coliO111:B4 (Sigma, Deisenhofen, Germany) was added and incubated with the cells for 1 hour at 37 °C. After centrifugation and washing of the adherent cells with cold phosphate-buffered saline, cells were subjected to flow cytometry analysis. In certain experiments rhLBP (kindly provided by Dr S. F. Carroll, Xoma, Berkeley, CA) was added to the sera as indicated. Partial LBP depletion from severe sepsis sera, as well as depletion below the lower detection limit of the LBP ELISA, was performed by immunoprecipitation with a rabbit monoclonal anti–human LBP antibody (kindly provided by Dr S. F. Carroll) and protein A/G–Sepharose (Santa Cruz Biotechnology, Santa Cruz, CA) according to the manufacturer's instructions.
FITC-LPS–labeled monocytes were analyzed by flow cytometry with the FACScan analyser using the Cellquest software (Becton Dickinson, San Jose, CA). Monocytes were gated according to their forward and side scatter characteristics. The fluorescence signal was expressed in fluorescence units and recorded on a logarithmic scale.
LPS-induced TNF-α secretion by monocytes
PBMCs were adjusted to 5 × 105/mL and incubated for 2 hours at 37°C in 96-well plastic plates containing RPMI 1640 cell culture medium (PAA Laboratories, Linz, Austria). After removal of nonadherent cells by washing with RPMI 1640, sera of patients with severe sepsis/septic shock or sera of healthy controls preincubated for 15 hours with 10 ng/mL nonfluorescenceinated LPS from E coli0111:B4 (Sigma) were added. Supernatants were taken after 4 hours of stimulation at 37°C and stored at −70°C until assayed. TNF-α was assessed employing a commercially available ELISA with 2 monoclonal mouse anti–human TNF-α antibodies (Pharmingen, Heidelberg, Germany).
Limulus-amebocyte-lysate assay
The chromogenic limulus-amebocyte-lysate (LAL) assay was performed in a modified form of a published protocol.33Serum samples were not heated in order to preserve binding of LPS to LBP or lipoproteins and to measure unbound endotoxin. Samples were not sterile-filtered for elimination of bacteria. A total of 50 μL LAL reagent (Endo KTA-LAL, Charles River Endosafe, Charleston, SC) was added to 50 μL serum in a 96-well flat bottom microtiter plate (Becton Dickinson, Brussels). After incubation at room temperature for 25 minutes, samples were supplemented with 100 μL substrate (Perfachrome LAL, Pentapharm, Basel, Switzerland). For quantification of the endotoxin concentration, an E coli O111:B4 endotoxin was used as standard according to the manufacturer's instructions (Charles River Endosafe). After 5 minutes the reaction was stopped with acetic acid 40% and the reaction was quantified by an ELISA reader (Spectra Fluor plus, Tecan, Crailsheim, Germany).
Statistical analysis
Data are presented as absolute or relative frequencies for categorical variables, mean ± SD or SEM, or 25th, 50th, and 75th percentiles and, also, range for continuous parameters. All assays were performed in duplicate, and the mean value was calculated. Differences between groups were evaluated using the Mann-Whitney U test or the Wilcoxon test, where appropriate. The association between mean fluorescence units and LBP serum concentrations was analyzed using the Pearson correlation coefficient and the corresponding test based on the bivariate normal distribution, after double logarithmic transformation. Furthermore, the corresponding ordinary least squares regression line was fitted to the data. The prognostic values of onset LBP and sCD14 serum concentrations were assessed by logistic regression modeling in the severe sepsis/septic shock cohort. All tests were 2-sided, and P < .05 was considered statistically significant.
Results
Serum LBP concentrations in severe sepsis or septic shock
For the entire study cohort the median serum LBP concentration at onset of severe sepsis/septic shock was 46.2 mg/L (range 3.74-155), and the median sCD14 serum concentration 9.05 mg/L (range 3.64-37.1). These values were significantly different to the serum LBP and sCD14 concentrations of the healthy volunteers (7.94 mg/L [range 1.85-17.4] and 3.16 mg/L [range 2.48-4.36], respectively;P < .001). At onset of severe sepsis the serum LBP and sCD14 concentrations were not significantly different in the patients with severe sepsis as compared with patients with septic shock. No significant difference in serum LBP and sCD14 concentrations could be observed in survivors as compared with nonsurvivors at onset of severe sepsis or septic shock (44.2 mg/L [range 3.74-112] vs 55.5 mg/L [range 7.36-155] and 8.04 mg/L [range 3.64-15.4] vs 9.79 mg/L [range 4.98-37.1], respectively). Multivariate analyses including severity of infection as a potential confounder revealed that neither LBP nor sCD14 concentrations were independent significant as prognostic indicators for severe sepsis-related mortality.
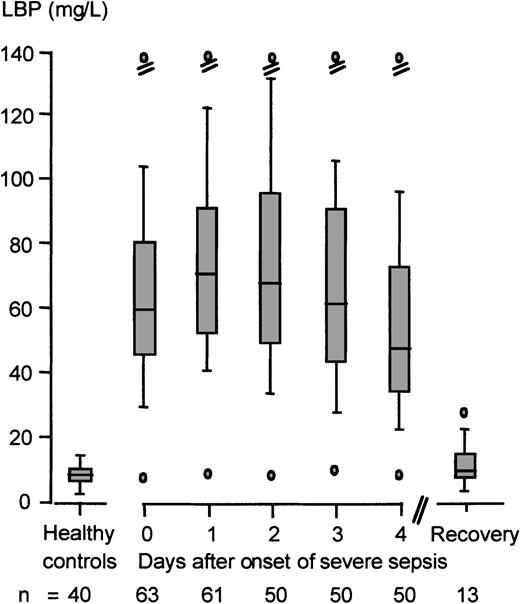
During the inflammatory host response in severe sepsis or septic shock, peak serum LBP and sCD14 concentrations increased 10.5-fold and 4.7-fold (83.1 mg/L [range 11.8-275] and 14.7 mg/L [range 5.18-39.4], respectively) as compared with the reference values of the healthy controls. Peak LBP concentrations were reached after a median time of 40 hours (range 1-120) from the onset of severe sepsis (Figure1). No significant differences in peak serum LBP concentrations were observed in Gram-negative versus Gram-positive bacteremia in the present study cohort (72.4 mg/L [range 28.6-143] and 88.6 mg/L [range 33.3-133], respectively). Furthermore, we failed to detect a significant correlation between age and serum LBP concentrations (Pearson correlation coefficientr = −0.218, P = .088).
Kinetics of LBP serum concentrations in severe sepsis or septic shock.
Shown is the reference range of serum LBP concentrations of healthy controls (n = 40) and the serum LBP kinetics of patients with severe sepsis or septic shock (n = 63) beginning with the onset of severe sepsis (day 0) and ending with the day of hospital discharge. Data are presented as minimum (lower o); 10th (lower dash), 25th (lower end of box), 50th (median, line in box), 75th (upper end of box), and 90th (upper dash) percentiles; and maximum (upper o).
Kinetics of LBP serum concentrations in severe sepsis or septic shock.
Shown is the reference range of serum LBP concentrations of healthy controls (n = 40) and the serum LBP kinetics of patients with severe sepsis or septic shock (n = 63) beginning with the onset of severe sepsis (day 0) and ending with the day of hospital discharge. Data are presented as minimum (lower o); 10th (lower dash), 25th (lower end of box), 50th (median, line in box), 75th (upper end of box), and 90th (upper dash) percentiles; and maximum (upper o).
FITC-LPS binding to monocytes in the presence of different concentrations of patient sera and sera of healthy controls
To investigate the ability of acute-phase sera to modulate LPS binding and response of monocytes, 2 in vitro experiments employing those severe sepsis sera containing peak LBP concentrations and control sera from healthy volunteers were performed: A fluorescence-activated cell sorter (FACS)–based assay was used to assess the binding of FITC-labeled LPS to PBMCs from healthy donors in the absence or presence of serum. In a second assay the serum-dependent LPS-induced TNF-α secretion by these monocytes was measured by ELISA. Peak LBP serum concentrations of patients with severe sepsis were identified by serial measurements as described above.
The correlation between LBP serum concentrations and mean fluorescence units was statistically significant (Pearson correlation coefficient:r = −0.64, P < .001). The inverse relation between log LBP concentrations and log mean fluorescence units is demonstrated in Figure 2. Both subpopulations seem to follow a common trend.
Correlation between FITC-LPS binding to monocytes and LBP serum concentrations.
The scattergram shows the association between log serum LBP concentrations of the severe sepsis and control cohort and the log mean fluorescence units as a measure of FITC-LPS binding to monocytes. The Pearson correlation coefficient and ordinary least squares regression line are supplemented (r = −0.64,P < .001).
Correlation between FITC-LPS binding to monocytes and LBP serum concentrations.
The scattergram shows the association between log serum LBP concentrations of the severe sepsis and control cohort and the log mean fluorescence units as a measure of FITC-LPS binding to monocytes. The Pearson correlation coefficient and ordinary least squares regression line are supplemented (r = −0.64,P < .001).
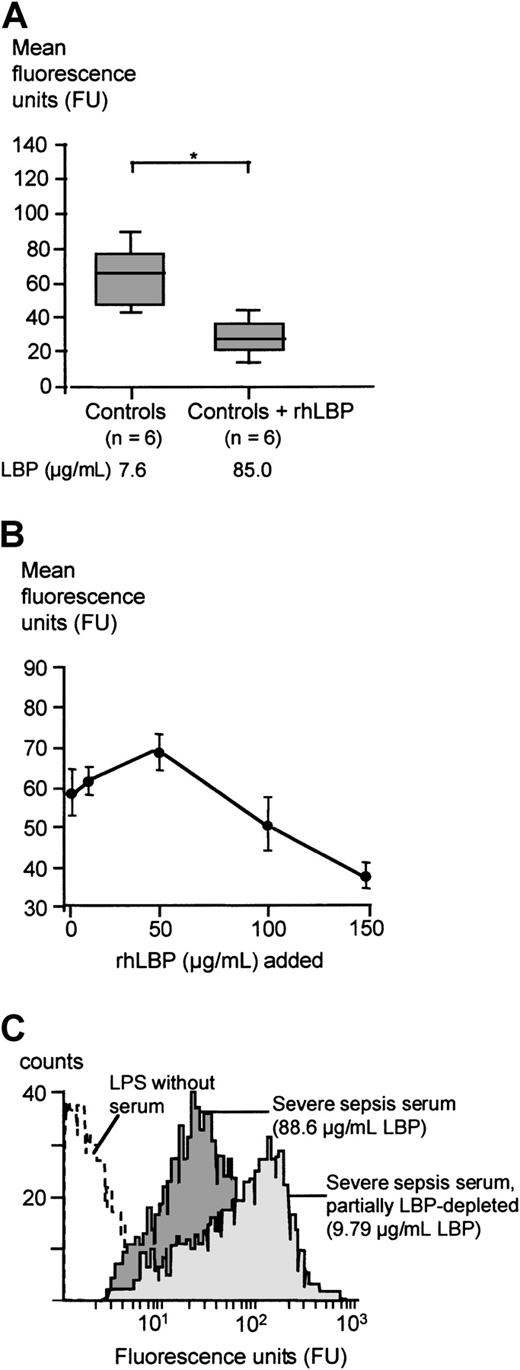
The mean fluorescence units representing the LPS transfer to monocytes in the presence of sera of patients with severe sepsis or septic shock were significantly lower as compared with the mean fluorescence units obtained with the control group sera (Mann-WhitneyU test, P < .05; Figure3A). An example of the FITC-LPS FACS assay employing serum from a randomly chosen severe sepsis patient containing 93.7 μg/mL LBP and of a healthy control serum with an LBP concentration of 9.88 μg/mL is shown in Figure 3B.
FITC-LPS binding to monocytes in the presence of severe sepsis sera and healthy control sera.
(A) Mean fluorescence of monocytes incubated with FITC-LPS in the presence of 15% serum from patients with severe sepsis or from healthy controls. Data are presented as 25th (box), 50th (median), and 75th percentiles (box) and range (-). Statistical analysis was performed with the Mann-Whitney U test; *P < .05. (B) Patient example of the FITC-LPS FACS experiment: Monocytes were incubated with FITC-LPS in the presence of 15% serum from a healthy volunteer (light gray graph) and serum from a randomly chosen patient with severe sepsis (dark gray graph).
FITC-LPS binding to monocytes in the presence of severe sepsis sera and healthy control sera.
(A) Mean fluorescence of monocytes incubated with FITC-LPS in the presence of 15% serum from patients with severe sepsis or from healthy controls. Data are presented as 25th (box), 50th (median), and 75th percentiles (box) and range (-). Statistical analysis was performed with the Mann-Whitney U test; *P < .05. (B) Patient example of the FITC-LPS FACS experiment: Monocytes were incubated with FITC-LPS in the presence of 15% serum from a healthy volunteer (light gray graph) and serum from a randomly chosen patient with severe sepsis (dark gray graph).
FITC-LPS binding to monocytes in the presence of rhLBP-supplemented control sera and partially LBP-depleted severe sepsis sera
When rhLBP was added to serum of healthy controls leading to total LBP concentrations comparable to peak serum concentrations of the severe sepsis/septic shock cohort, the LPS transfer activity of these sera was diminished, comparable to the results obtained with severe sepsis sera (Figure 4A). When increasing concentrations of rhLBP were added, the transfer activity was slightly enhanced for LBP concentrations up to 50 μg/mL (Figure 4B). However, higher rhLBP concentrations led to a marked down-regulation of LPS binding. Next, we depleted severe sepsis sera from LBP by using an anti-LBP antibody resulting in serum LBP concentrations found in healthy controls. This partial LBP depletion of severe sepsis sera led to a mean LBP concentration of 16.7± 10.1 μg/mL. LBP depletion significantly enhanced the LPS transfer activity and the LPS-induced TNF-α secretion of this serum (Table2). A representative patient's example of this effect is shown in Figure 4C.
FITC-LPS binding to monocytes in the presence of healthy control sera, rhLBP-supplemented control sera, and LBP-depleted severe sepsis sera.
(A) Mean fluorescence of monocytes incubated with FITC-LPS in the presence of control sera and control sera supplemented with 100 μg/mL rhLBP. Data are presented as 25th (box), 50th (median), and 75th percentiles (box) and range (-). Statistical analysis was performed with the Mann-Whitney U test; *P < .05. (B) Mean fluorescence of monocytes incubated with FITC-LPS in the presence of control sera supplemented with increasing concentrations of rhLBP assessed by FACS analysis. Data are presented as mean ± SEM of 5 independent experiments. (C) Patient example of the FITC-LPS FACS experiment: mean fluorescence of monocytes incubated with FITC-LPS in the presence of severe sepsis serum (dark gray graph) and partially LBP-depleted severe sepsis serum (light gray graph).
FITC-LPS binding to monocytes in the presence of healthy control sera, rhLBP-supplemented control sera, and LBP-depleted severe sepsis sera.
(A) Mean fluorescence of monocytes incubated with FITC-LPS in the presence of control sera and control sera supplemented with 100 μg/mL rhLBP. Data are presented as 25th (box), 50th (median), and 75th percentiles (box) and range (-). Statistical analysis was performed with the Mann-Whitney U test; *P < .05. (B) Mean fluorescence of monocytes incubated with FITC-LPS in the presence of control sera supplemented with increasing concentrations of rhLBP assessed by FACS analysis. Data are presented as mean ± SEM of 5 independent experiments. (C) Patient example of the FITC-LPS FACS experiment: mean fluorescence of monocytes incubated with FITC-LPS in the presence of severe sepsis serum (dark gray graph) and partially LBP-depleted severe sepsis serum (light gray graph).
Enhancement of LPS-induced monocytic response by LBP depletion of severe sepsis sera
| . | LBP concentration, mg/L* . | LPS binding, FU† . | TNF-α secretion, pg/mL‡ . |
|---|---|---|---|
| Severe sepsis sera | |||
| Native | 60.5 ± 38.2 | 36.7 ± 17.9 | 544 ± 228 |
| Partially LBP-depleted | 16.7 ± 10.1 | 65.1 ± 28.02-153 | 991 ± 2452-153 |
| . | LBP concentration, mg/L* . | LPS binding, FU† . | TNF-α secretion, pg/mL‡ . |
|---|---|---|---|
| Severe sepsis sera | |||
| Native | 60.5 ± 38.2 | 36.7 ± 17.9 | 544 ± 228 |
| Partially LBP-depleted | 16.7 ± 10.1 | 65.1 ± 28.02-153 | 991 ± 2452-153 |
Data are presented as mean ± SD of 8 independent experiments with randomly chosen sera of severe sepsis patients.
LBP serum concentrations were assessed by ELISA.
Mean fluorescence units were obtained by FACS analysis of gated monocytes incubated with FITC-LPS in the presence of 15% native or partially LBP-depleted severe sepsis sera.
Monocytes were stimulated with 10 ng/mL LPS in the presence of 15% native or partially LBP-depleted severe sepsis sera, and TNF-α concentrations in the supernatants were assessed by ELISA.
The statistical significance of the difference between groups was assessed by the Mann-Whitney U test;P < .05.
FITC-LPS binding to monocytes of healthy volunteers in the presence of different concentrations of severe sepsis sera and sera of healthy controls
To obtain information on whether other serum compounds in addition to LBP might be responsible for LPS-inhibitory activity, increasing concentrations of serum were used in the FITC-LPS FACS assay. The addition of severe sepsis and control sera in concentrations up to 5% of the total volume per well enhanced binding of FITC-LPS to monocytes. While acute-phase sera showed an inhibitory activity when applied in concentrations above 7.5%, the inhibitory activity of control sera could be demonstrated at a concentration above 30% (Figure5).
FITC-LPS binding to monocytes of healthy volunteers in the presence of different concentrations of severe sepsis sera and sera of healthy controls.
Binding of FITC-LPS to monocytes was assessed employing a FACS-based method as described in “Patients, materials, and methods.” Five independent experiments with sera of 5 healthy controls (●; LBP serum concentration, 7.98 ± 1.03 μg/mL) and of 5 randomly chosen severe sepsis patients (▴; LBP serum concentration, 107 ± 32 μg/mL) were performed. Different volumes of serum were added as indicated. Data are presented as mean ± SEM. Differences were analyzed by the Mann-Whitney U test; *P < .05.
FITC-LPS binding to monocytes of healthy volunteers in the presence of different concentrations of severe sepsis sera and sera of healthy controls.
Binding of FITC-LPS to monocytes was assessed employing a FACS-based method as described in “Patients, materials, and methods.” Five independent experiments with sera of 5 healthy controls (●; LBP serum concentration, 7.98 ± 1.03 μg/mL) and of 5 randomly chosen severe sepsis patients (▴; LBP serum concentration, 107 ± 32 μg/mL) were performed. Different volumes of serum were added as indicated. Data are presented as mean ± SEM. Differences were analyzed by the Mann-Whitney U test; *P < .05.
Endotoxin-neutralizing capacity of sera measured by the LAL assay
Because LPS transfer to monocytes was significantly reduced by severe sepsis sera, we next investigated whether these sera would display an increased LPS binding resulting in a reduced concentration of “free” LPS. The median baseline LPS concentration found in the sera of 8 randomly chosen patients was 86.5 pg/mL (range 36-150 pg/mL) as compared with a median concentration of 35 pg/mL (range 17-37 pg/mL) in sera of 6 healthy controls. While the sera of sepsis patients thus exhibit elevated LPS levels, it can be clearly ruled out that these concentrations would affect baseline responsiveness in our biological assays.
Sera of the study cohort and of healthy controls were incubated with 10 ng/mL LPS without heating and subjected to a modified LAL assay as described in detail in “Patients, materials, and methods” (Figure6). Endotoxin concentrations measured in the absence of serum were taken as 100%. Sera from healthy controls were able to reduce LPS detected by LAL to a median value of 60%. Sera from severe sepsis patients, however, exhibited a significantly more pronounced ability to bind LPS, resulting in a median endotoxin concentration of 20% as measured by the LAL assay (Mann-WhitneyU test, P < .05).
Endotoxin-neutralizing capacity of severe sepsis and control sera measured by the LAL assay.
The LAL assay was performed as described in “Patients, materials, and methods,” employing either sera of healthy controls or sera of patients with severe sepsis/septic shock. The concentration of LPS measured in the absence of serum was set as 100%. Presented are the median; the 10th, 25th, 75th, and 90th percentiles; and the range. Statistical analysis was performed using the Mann-Whitney Utest; *P < .05.
Endotoxin-neutralizing capacity of severe sepsis and control sera measured by the LAL assay.
The LAL assay was performed as described in “Patients, materials, and methods,” employing either sera of healthy controls or sera of patients with severe sepsis/septic shock. The concentration of LPS measured in the absence of serum was set as 100%. Presented are the median; the 10th, 25th, 75th, and 90th percentiles; and the range. Statistical analysis was performed using the Mann-Whitney Utest; *P < .05.
LPS-induced TNF-α secretion in the presence of severe sepsis sera, healthy control sera, LBP-depleted severe sepsis sera, and rhLBP-supplemented control and LBP-depleted severe sepsis sera
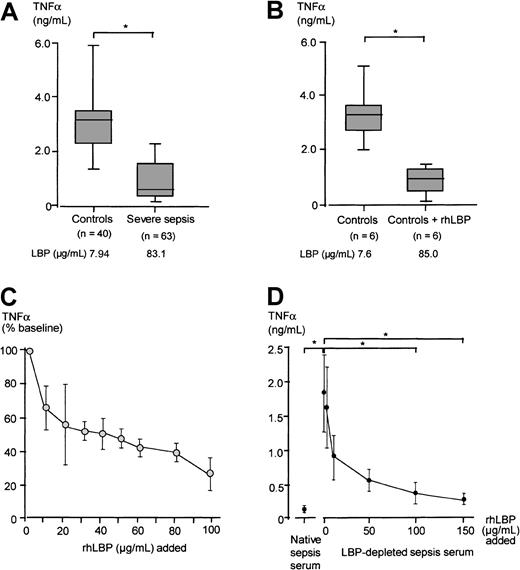
We next investigated whether sera from severe sepsis patients would influence LPS-induced TNF-α secretion of monocytes. To this end freshly isolated PBMCs of healthy donors were stimulated with 10 ng/mL LPS preincubated with severe sepsis or control sera. LPS-induced monocytic TNF-α secretion was significantly reduced in the presence of severe sepsis sera as compared with control sera (Mann-WhitneyU test, P < .05; Figure7A). When rhLBP was added to control sera, resulting in acute-phase concentrations, a similar reduction in TNF-α secretion was observed (Figure 7B).
LPS-induced TNF-α secretion in the presence of severe sepsis sera, control sera, LBP-depleted severe sepsis sera, and rhLBP-supplemented sera.
Monocytic TNF-α secretion in the presence of different sera preincubated with LPS. (A) LPS-induced TNF-α secretion in the presence of 15% sera from patients with severe sepsis and from healthy controls. (B) LPS-induced TNF-α secretion in the presence of 15% control sera and control sera supplemented with 100 μg/mL rhLBP, resulting in LBP concentrations comparable to those found in severe sepsis. Data of panels A and B are presented as 25th (box), 50th (median), and 75th percentiles (box) and range (-). Statistical analysis was performed with the Mann-Whitney U test; *P < .05. (C) Monocytes were stimulated with 10 ng/mL LPS preincubated in the presence of sera of healthy controls supplemented with increasing amounts of rhLBP. LPS-induced TNF-α concentration in the presence of nonsupplemented serum of the healthy volunteers was set as 100%. Presented is the mean ± SEM of 6 independent experiments. (D) Monocytes were stimulated with 10 ng/mL LPS preincubated with native severe sepsis sera, LBP-depleted severe sepsis sera, and LBP-depleted severe sepsis sera supplemented with increasing concentrations of rhLBP starting with 1μg/mL. Each experiment was performed in duplicate employing 6 different randomly chosen severe sepsis sera. Data are presented as mean ± SEM. Statistical analysis was performed with the Wilcoxon test; *P < .05.
LPS-induced TNF-α secretion in the presence of severe sepsis sera, control sera, LBP-depleted severe sepsis sera, and rhLBP-supplemented sera.
Monocytic TNF-α secretion in the presence of different sera preincubated with LPS. (A) LPS-induced TNF-α secretion in the presence of 15% sera from patients with severe sepsis and from healthy controls. (B) LPS-induced TNF-α secretion in the presence of 15% control sera and control sera supplemented with 100 μg/mL rhLBP, resulting in LBP concentrations comparable to those found in severe sepsis. Data of panels A and B are presented as 25th (box), 50th (median), and 75th percentiles (box) and range (-). Statistical analysis was performed with the Mann-Whitney U test; *P < .05. (C) Monocytes were stimulated with 10 ng/mL LPS preincubated in the presence of sera of healthy controls supplemented with increasing amounts of rhLBP. LPS-induced TNF-α concentration in the presence of nonsupplemented serum of the healthy volunteers was set as 100%. Presented is the mean ± SEM of 6 independent experiments. (D) Monocytes were stimulated with 10 ng/mL LPS preincubated with native severe sepsis sera, LBP-depleted severe sepsis sera, and LBP-depleted severe sepsis sera supplemented with increasing concentrations of rhLBP starting with 1μg/mL. Each experiment was performed in duplicate employing 6 different randomly chosen severe sepsis sera. Data are presented as mean ± SEM. Statistical analysis was performed with the Wilcoxon test; *P < .05.
To evaluate the dose-dependent effect of LBP, rhLBP was added in increasing amounts to control sera, and the TNF-α secretion induced by 10 ng/mL LPS preincubated with these sera was assessed (Figure 7C). Increasing LBP serum concentrations led to a clearly reduced LPS-induced TNF-α secretion in an LBP dose-dependent manner (Friedman test, P < .05). We also performed this experiment using an LPS concentration of 1 ng/mL to stimulate the monocytes and found that a 5-fold lesser concentration of rhLBP was able to achieve a similar reduction of TNF-α secretion as compared with stimulation with 10 ng/mL (data not shown).
To prove the specificity of LBP in inhibiting the LPS-induced effects on monocytes, 6 randomly chosen severe sepsis sera were depleted of LBP. Employing an anti-LBP antibody, we were able to reduce LBP concentrations of severe sepsis sera to a concentration below the lower detection limit of the LBP ELISA. Following this depletion procedure, these sera were supplemented stepwise with rhLBP. These sera were incubated with 10 ng/mL LPS, and the stimulation experiment with monocytes was repeated. We found that sera containing only traces of LBP, as well as those sera reconstituted with rhLBP up to 1 μg/mL, were enhancing LPS-induced TNF-α secretion as compared with the sepsis sera. Adding back rhLBP up to acute-phase concentrations restored the LPS-inhibiting activity comparable to native severe sepsis sera (Figure 7D).
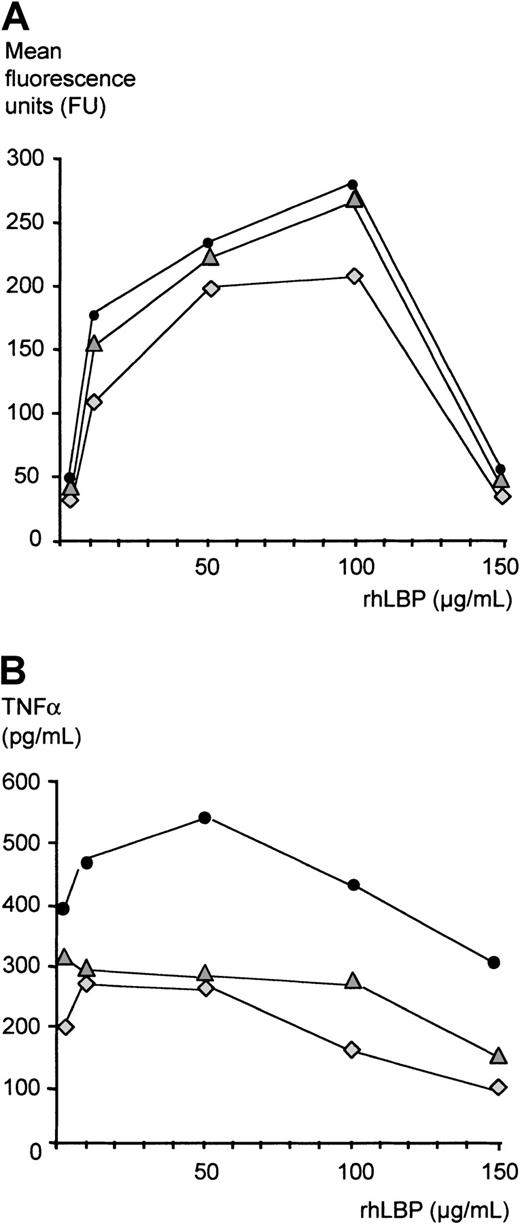
Inhibition of LPS effects in the absence of serum
Finally, to address whether LBP can inhibit LPS activity independently of other serum factors, we established a serum-free in vitro system: Monocytes were incubated with FITC-LPS in the presence of increasing concentrations of rhLBP, and binding of LPS was assessed by FACS as described above (Figure 8A). While concentrations of up to 100 μg/mL gradually increased LPS binding to monocytes, the addition of rhLBP resulting in a concentration of 150 μg/mL significantly reduced LPS binding as measured by fluorescence intensity. LPS bioactivity measured by LPS-induced TNF-α secretion of monocytes was studied as described above. As has been shown by others, low concentrations of LBP (10-100 ng/mL) increased LPS-induced TNF-α secretion of monocytes as compared with stimulation in the absence of LBP (data not shown). Addition of 1, 10, and 50 μg/mL rhLBP also enhanced TNF-α secretion, while acute-phase concentrations of more than 50 μg/mL led to a gradual decrease of LPS-induced TNF-α secretion (Figure 8B).
Inhibition of LPS effects in the absence of serum.
(A) LPS transfer was assessed by a FACS-based method as described in “Patients, materials, and methods.” Monocytes were incubated with increasing concentrations of rhLBP starting with 1μg/mL as indicated and with 1 μg/mL FITC-LPS. Presented are the values of 3 independent experiments. (B) Monocytes were incubated with increasing concentrations of rhLBP starting with 1μg/mL as indicated and with 10 ng/mL LPS. TNF-α concentrations were measured by ELISA. Presented are the values of 3 independent experiments.
Inhibition of LPS effects in the absence of serum.
(A) LPS transfer was assessed by a FACS-based method as described in “Patients, materials, and methods.” Monocytes were incubated with increasing concentrations of rhLBP starting with 1μg/mL as indicated and with 1 μg/mL FITC-LPS. Presented are the values of 3 independent experiments. (B) Monocytes were incubated with increasing concentrations of rhLBP starting with 1μg/mL as indicated and with 10 ng/mL LPS. TNF-α concentrations were measured by ELISA. Presented are the values of 3 independent experiments.
Discussion
Several lines of evidence are presented here indicating that increased LBP concentrations found in serum of severe sepsis patients inhibit proinflammatory activity of LPS. The acute-phase increase of LBP concentrations therefore may represent an important part of the antimicrobial defense system of the host. A down-regulation of proinflammatory cytokine release upon in vitro LPS stimulation has been demonstrated by others in whole blood obtained from severe sepsis patients.26-28 The mechanism of this down-regulation, however, has remained unclear. In severe sepsis it has been demonstrated that IL-10, IL-4, and transforming growth factor β have pronounced anti-inflammatory effects on mononuclear cells.34 It has remained unclear, however, whether hepatic acute-phase proteins can modulate the monocytic secretion of proinflammatory cytokines. In the present study we provide evidence that LBP has this ability.
Several studies have shown that LBP at low concentrations activates and amplifies the inflammatory host response to LPS, thus potentially serving as a critical component in the initiation of the innate immune response.18 Blocking LBP with a polyclonal antibody led to protection of mice after LPS application.35,36 First experiments with LBP-deficient mice supported these findings, because deletion of the LBP gene was associated with suppression of TNF-α induction and lethality in an LPS sepsis model.23,37 After injection of whole Gram-negative bacteria, however, an enhanced mortality in LBP knock-out mice was observed due to an overwhelming spread of bacteria.24 This may have been caused either by a lack of the early and effective activation of the innate immune system or by a lack of the inhibitory function of LBP, as suggested by the data shown here.
The mechanisms of the LPS-inhibitory activity of LBP are currently not clear. LBP has been found to transfer LPS into lipoproteins, thus inhibiting LPS effects.21,22,38,39 We have recently shown in a mouse sepsis model that high concentrations of LBP generated by application of recombinant LBP can protect mice against a lethal intraperitoneal injection of LPS or vital Gram-negative bacteria.24 The ability of LBP to transfer LPS to lipoproteins may be the key mechanism for this protective role and for the LPS-inhibitory activity of severe sepsis sera described in the present paper. Although the early recognition of LPS may be crucial for host defense, the spread of LPS monomers from a site of infection via the bloodstream may be prevented by this mechanism.21,40In addition, our results in a serum-free system point to a second mechanism of inhibitory activity of high-dose LBP. We and others have proposed a “silent uptake” of LPS that potentially is mediated by higher concentrations of LBP.41,42 Recent observations by us suggest that these cellular mechanisms may occur both CD14-dependently and CD14-independently (unpublished results, 2001), which is in line with recent observations by others on CD14-independent effects of Gram-negative bacteria and LPS.43-45
We demonstrate here that acute-phase sera of severe sepsis patients are able to reduce LPS binding to monocytes and their subsequent activation. The LBP depletion and reconstitution experiments strongly suggest that these inhibitory effects can be mainly attributed to LBP. Besides LBP, other serum proteins are known to bind LPS and modulate LPS-induced monocytic activity: sCD14 is released from neutrophil membranes by shedding and has been shown to act as a coligand in the LPS-induced activation of CD14− cells such as endothelial and epithelial cells.46,47 However, sCD14 is also able to inhibit LPS-induced TNF-α synthesis by monocytes in the presence of LBP and high-density lipoprotein.48,49 The median of the peak serum sCD14 concentration measured in the study cohort with severe sepsis was 14.7 μg/mL (range 5.18-39.4 μg/mL). These concentrations are well below the inhibiting concentrations observed in the study by Haziot et al,49 and sCD14 therefore can be ruled out as being mainly responsible for the observed inhibitory effects of the severe sepsis sera. Nevertheless, sCD14 and LBP might act synergistic. Another inhibitory LPS binding protein is the bactericidal/permeability-increasing protein (BPI) usually not detectable in plasma of healthy volunteers.17,50,51 It has been demonstrated that in experimental endotoxemia as well as in patients with severe sepsis, the peak LBP serum concentrations were approximately 250-fold to 3000-fold higher than the peak plasma BPI concentrations.17,50,51 Although we did not determine BPI plasma concentrations in the present study, we assume that the BPI concentrations in severe sepsis sera were not sufficient enough to be responsible for the modulatory effects on the LPS activity observed. Other serum factors, such as serum amyloid A and P, albumin, transferrin, and LDL, have been shown to have the ability to bind LPS.2,19,39 52 The composition of the acute-phase sera is quite complex, and we cannot exclude these factors from also being responsible for the LPS modulating effects observed here. On the other hand, our LBP depletion and reconstitution experiments presented here present clear evidence that LBP is a major factor for LPS detoxification during the acute-phase response.
Results of others up to now mainly indicated LPS-enhancing effects of LBP.18,53 54 In these studies, however, always low concentrations of LBP were employed. Enhancement of LPS effects was demonstrated by the addition of either 0.1% to 1% acute-phase serum of severe sepsis patients or by using up to 10% of control serum. In the present study we confirmed these results for the addition of low amounts of serum (Figure 5); however, increasing volumes of serum, thus increasing LBP concentrations, clearly down-regulated LPS transfer and monocytic activation. Endotoxin present in patients' sera, although elevated, can be ruled out as influencing our in vitro results, because the LPS concentrations added experimentally were always in at least 10-fold to 100-fold excess.
In the serum-free assay described in this study, higher concentrations of rhLBP were needed to achieve a decrease of LPS binding and a reduced TNF-α secretion as compared with the assays employing severe sepsis sera. Furthermore, we obtained evidence that small concentrations of LPS are more easily inhibited by LBP as compared with higher concentrations, in line with results obtained with a murine macrophage cell line.24 Our different assays exhibited a different degree of inhibition, with the FITC-LPS binding assay giving rise to lesser inhibition. This also may very well be related to the higher LPS concentrations employed in this assay. A more detailed analysis of serum components, their concentrations, and LPS inhibition is subject of a separate ongoing investigation in our laboratory. As the major mechanism of the effects observed here in severe sepsis sera, we propose that high serum LBP concentrations were able to down-regulate LPS activity in the presence of lipoproteins. Several studies have demonstrated reduced lipoprotein concentrations in severe sepsis.55 56 It is tempting to speculate that sera from patients with elevated LBP, yet higher lipoprotein concentrations, are even more efficient in LPS neutralization. Experiments addressing the synergistic role of lipoproteins and LBP in inhibiting LPS activity are also currently under way in our laboratory.
In the present prospective clinical study, we observed that the up-regulation of LBP takes place both in Gram-negative as well as in Gram-positive severe sepsis and is thus not specific for infections with Gram-negative microorganisms. We could not confirm in our study the findings of Opal et al that serum LBP concentrations of nonsurvivors were significantly lower as compared with survivors.16 In the present study with a clearly defined onset of severe sepsis and serial measurements, we did not find a correlation between initial or peak serum LBP concentrations and outcome in patients with severe sepsis or septic shock. This is consistent with other reports.17,18 50
In summary, our experiments with human severe sepsis sera demonstrate an inhibitory role of high LBP concentrations in the LPS-induced inflammatory response. Further experiments are needed to completely elucidate this novel defense mechanism. A complete understanding of the innate immune system during the acute-phase response in severe sepsis or septic shock and its regulation may provide a basis for new therapeutic approaches in patients suffering from an uncontrolled systemic inflammation.
We are very grateful to Fränzi Creutzburg for outstanding technical support and for performing LBP depletion and reconstitution experiments. Nicole Siegemund is acknowledged for excellent technical support. We are furthermore grateful to Ariane Asmus and Naser Qedra for assistance in blood sample collection and clinical evaluation of the patients. We also thank Peter Germain for the critical reading of this manuscript.
Supported in part by grants from the German Research Foundation (DFG, grant no. Schu 828/1-5) and by the Bundesministerium für Bildung und Forschung (BMBF, grants no. 01KV98067 and 01KI9855/0).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ralf R. Schumann, Institut für Mikrobiologie und Hygiene, Universitätsklinikum Charité, Dorotheenstr 96, D-10117 Berlin, Germany; e-mail: ralf.schumann@charite.de.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal