Abstract
The antitumor ether lipid ET-18-OCH3 promotes apoptosis in tumor cells through intracellular activation of Fas/CD95. Results of this study showed that ET-18-OCH3 induces cocapping of Fas and membrane rafts, specialized plasma membrane regions involved in signaling, before the onset of apoptosis in human leukemic cells. Patches of membrane rafts accumulated Fas clusters in leukemic cells treated with ET-18-OCH3. Sucrose gradient centrifugation of Triton X-100 cell lysates showed that Fas translocated into membrane rafts following ET-18-OCH3 treatment of T-leukemic Jurkat cells. Disruption of membrane raft integrity by methyl-β-cyclodextrin or filipin inhibited ET-18-OCH3-induced apoptosis in leukemic primary cells and cell lines. Fas clustering was also inhibited by methyl-β-cyclodextrin. These data indicate that ET-18-OCH3 reorganizes membrane rafts to trigger apoptosis in human leukemic cells, and that Fas coaggregation with membrane rafts is required for ET-18-OCH3–induced apoptosis. This translocation of Fas into membrane rafts may provide a mechanism for amplifying Fas signaling by reorganization of membrane microdomains.
Introduction
The plasma membrane contains microdomains named membrane rafts, consisting of dynamic assemblies of cholesterol and sphingolipids.1-3 The presence of saturated hydrocarbon chains in sphingolipids allows for cholesterol to be tightly intercalated, leading to the presence of distinct liquid-ordered phases, membrane rafts, dispersed in the liquid-disordered matrix, and thereby more fluid, lipid bilayer.4 One key property of membrane rafts is that they can include or exclude proteins to varying degrees. Membrane rafts may serve as foci for recruitment and concentration of signaling molecules at the plasma membrane, and thus they have been implicated in signal transduction from cell surface receptors.3
Antitumor ether phospholipids constitute a novel class of promising cancer chemotherapeutic drugs.5-7 The ether lipid 1-O-octadecyl-2-O-methyl-rac-glycero-3-phosphocholine (ET-18-OCH3; edelfosine) exerts a selective cytotoxic action against transformed cells,5-7 and has become the effective standard and prototype of the antitumor ether phospholipids. Encouraging clinical research on the use of ET-18-OCH3 in purging leukemic bone marrow prior to autologous bone marrow transplantation has been reported.8,9ET-18-OCH3 is a potent inducer of apoptosis in tumor cells, especially in leukemic cells, sparing normal cells.7,10,11Recent evidence has shown that ET-18-OCH3–induced apoptosis is mediated by the intracellular activation of Fas/CD95 cell death receptor, independently of its ligand FasL, leading to clustering and subsequent capping of Fas.12 ET-18-OCH3must be incorporated into the cell to exert its apoptotic action,7 and the ether lipid has been reported to be accumulated in the plasma membrane.13 Because ET-18-OCH3 is a phospholipid and acts through plasma membrane–related processes, involving activation of cell surface Fas receptor, we investigated whether the Fas-mediated apoptotic effect of ET-18-OCH3 on leukemic cells involved lipid rafts.
Study design
Cell culture and apoptosis
The human leukemic cell lines HL-60 (acute myeloid leukemia) and Jurkat (acute T-cell leukemia) were grown as described previously in RPMI-1640 culture medium supplemented with 10% heat-inactivated fetal calf serum (FCS).7 Bone marrow aspirates, obtained from patients at the initial diagnosis and after signing informed consent, were kindly provided by the Hematology Department of the Rio Hortega Hospital (Valladolid, Spain). Mononuclear cells isolated by Ficoll-Hypaque density gradient centrifugation, consisting mostly of leukemia blasts (> 90%), were washed in phosphate-buffered saline (PBS), resuspended in cell culture medium, and used immediately for experimentation. ET-18-OCH3 (INKEYSA, Barcelona, Spain) was prepared as described previously.7 To disrupt lipid rafts, cells (5 × 105) were pretreated with methyl-β-cyclodextrin (MCD) (Sigma Chemical, St Louis, MO; 15 μg/mL) or filipin (Sigma; 1 μg/mL) for 1 hour at 37°C in serum-free medium before ether lipid addition.
Apoptosis was assessed by isolation of fragmented DNA as described previously.10,14 DNA was visualized after 1% agarose gel electrophoresis by ethidium bromide staining. For quantitative determination of apoptosis, cells (5 × 105) were fixed overnight in 70% ethanol at 4°C. Cells were then incubated for 1 hour with 1 mg/mL RNase A and 20 μg/mL propidium iodide at room temperature, and analyzed with a Becton Dickinson (San Jose, CA) FACScan flow cytometer as described previously.11Apoptotic cells were calculated as the percentage of cells in the sub-G1 region (hypodiploidy) in cell cycle analysis.
Confocal microscopy
Untreated and ET-18-OCH3–treated cells were settled onto slides coated with poly-l-lysine, fixed in 4% formaldehyde, stained with 8 μg/mL fluorescein isothiocyanate-labeled cholera toxin B subunit (FITC-CTx) (Sigma), and analyzed by confocal microscopy using a Zeiss LSM 310 laser scan confocal microscope (Oberkochen, Germany). For colocalization experiments FITC-CTx staining was performed as above, and then cells were incubated with 500 ng/mL antihuman Fas SM1/1 IgG2a mouse monoclonal antibody (mAb) (Bender Medsystems, Vienna, Austria) for 1 hour at room temperature. Samples were further processed using CY3-conjugated antimouse antibody (Pharmacia, Uppsala, Sweden) (diluted 1:200 in PBS) for 1 hour at room temperature, and analyzed by confocal microscopy. Negative controls were prepared by either omitting the primary antibody or by using an irrelevant antibody, showing no fluorescence staining of the samples. Colocalization of CTx and Fas was analyzed by excitation of both fluorochromes in the same section.
Isolation of lipid rafts and Western blotting
Lipid rafts were isolated by using lysis conditions and centrifugation on discontinuous sucrose gradients as previously reported.15 In brief, cells (3.5 × 107) were washed with ice-cold PBS and lysed for 30 minutes on ice in 1% Triton X-100 in TNEV buffer (10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM EDTA, 1 mM sodium orthovanadate) containing 1 mM phenylmethylsulfonyl fluoride. Cells were then homogenized with 10 strokes in a Potter-Elvehjem tissue grinder. Nuclei and cellular debris were pelleted by centrifugation at 1000 rpm for 8 minutes. Then, 1 mL cleared supernatant was mixed with 1 mL 85% sucrose in TNEV and transferred to the bottom of a Beckman 14 × 95-mm centrifuge tube. The diluted lysate was overlaid with 6 mL 35% sucrose in TNEV and finally 3.5 mL 5% sucrose in TNEV. The samples were centrifuged in an SW40 rotor at 38 000 rpm for 18 hours at 4°C in a Beckman Optima LE-80K ultracentrifuge (Beckman Instruments, Palo Alto, CA), and then 1-mL fractions were collected from the top of the gradient.
To determine the location of Fas and lipid rafts in the discontinuous sucrose gradient, 20 μL of the individual fractions was subjected to sodium dodecyl sulfate–12% polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted as described previously.15,16After blocking for 1 hour at room temperature with 5% powdered defatted milk in TBST (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.05% Tween-20), blots were incubated with anti-Fas rabbit polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at a dilution of 1:500 in TBST. Antibody reactivity was monitored with biotinylated anti–rabbit IgG, using an enhanced chemiluminescence detection (ECL) system (Amersham, Buckinghamshire, United Kingdom). The location of GM1-containing lipid rafts was determined using CTx B subunit conjugated to horseradish peroxidase (Sigma) and an ECL system as described previously.15
Results and discussion
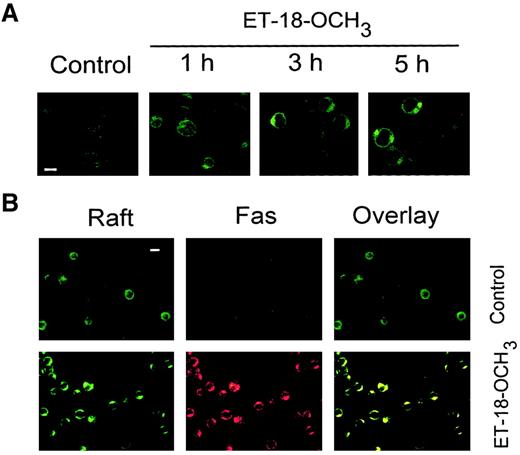
We tested whether ET-18-OCH3 affects the distribution of membrane rafts using the raft marker FITC-CTx. CTx B subunit binds the oligosaccharide portion of ganglioside GM1,17 which is thought to be mainly found in rafts.18 In unstimulated Jurkat cells, distribution of membrane rafts appeared homogeneous (Figure1A); however, after incubation of cells with ET-18-OCH3 lipid rafts were redistributed in a time-dependent way to form dense patches (Figure 1A), indicating that membrane rafts had aggregated. Because we have recently reported that ET-18-OCH3 induced clustering and capping of Fas in human leukemic Jurkat cells,12 we examined whether clustering of membrane rafts led to inclusion of Fas in the clustered rafts. As shown in Figure 1B, FITC-CTx staining colocalized with Fas in Jurkat cells treated with ET-18-OCH3. Untreated Jurkat cells showed an extremely weak diffuse staining of Fas, whereas incubation of cells with ET-18-OCH3 led to a dense patchy staining by confocal microscopy (Figure 1B), indicating clustering of Fas and corroborating our previous data.12 The Fas patches were colocalized with GM1 glycosphingolipid-lipid rafts, indicating that Fas accumulated in membrane rafts in ET-18-OCH3–treated Jurkat cells. This coclustering of membrane rafts and Fas occurred during the first 3 hours of ET-18-OCH3 treatment, whereas ET-18-OCH3–induced apoptosis of Jurkat cells was observed after 6 hours of incubation (2%, 3%, and 13% apoptotic cells after 3, 4, and 6 hours of ET-18-OCH3 treatment, respectively). Thus, clustering of Fas-associated membrane rafts preceded triggering of apoptosis. Similar data were obtained with HL-60 cells (data not shown).
Clustering of membrane rafts and Fas in ET-18-OCH3–treated Jurkat cells.
(A) Time course of the effect of ET-18-OCH3 on aggregation of membrane rafts. T-leukemic Jurkat cells, grown in 10% FCS-containing medium, were either untreated (Control) or treated with 5 μg/mL ET-18-OCH3 for the times indicated. Cells were then stained with FITC-CTx and analyzed by confocal microscopy. Bar indicates 7 μm. (B) Colocalization of membrane rafts (Raft) and Fas in ET-18-OCH3–treated Jurkat cells. Cells were either untreated (Control) or treated with 5 μg/mL ET-18-OCH3for 3 hours, and processed for confocal microscopy using FITC-CTx (green fluorescence for lipid rafts) and anti-Fas mAb, followed by CY3-conjugated antimouse antibody (red fluorescence for Fas). Areas of colocalization between membrane rafts and Fas in the overlay panels are yellow. Bar indicates 10 μm.
Clustering of membrane rafts and Fas in ET-18-OCH3–treated Jurkat cells.
(A) Time course of the effect of ET-18-OCH3 on aggregation of membrane rafts. T-leukemic Jurkat cells, grown in 10% FCS-containing medium, were either untreated (Control) or treated with 5 μg/mL ET-18-OCH3 for the times indicated. Cells were then stained with FITC-CTx and analyzed by confocal microscopy. Bar indicates 7 μm. (B) Colocalization of membrane rafts (Raft) and Fas in ET-18-OCH3–treated Jurkat cells. Cells were either untreated (Control) or treated with 5 μg/mL ET-18-OCH3for 3 hours, and processed for confocal microscopy using FITC-CTx (green fluorescence for lipid rafts) and anti-Fas mAb, followed by CY3-conjugated antimouse antibody (red fluorescence for Fas). Areas of colocalization between membrane rafts and Fas in the overlay panels are yellow. Bar indicates 10 μm.
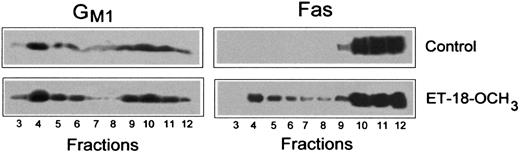
Because Fas labeling was negligible before treatment with ET-18-OCH3 (Figure 1B), it was not possible to discern whether Fas was already located in lipid rafts in untreated cells or Fas translocated into the membrane rafts on cell treatment with the ether lipid. To work out this issue we isolated lipid rafts in both untreated and ET-18-OCH3–treated Jurkat cells. Lipid rafts can be isolated based on their insolubility in Triton X-100 detergent and buoyant density on sucrose density gradients. Thus, Jurkat cells were lysed in 1% Triton X-100 lysis buffer and fractionated by discontinuous sucrose gradient centrifugation as previously described.15 The distinct fractions from the gradient were analyzed by SDS-PAGE and Western blotting. The position of the membrane rafts in the sucrose gradient was determined by the presence of the ganglioside GM1, detected using the GM1-specific ligand CTx B subunit (Figure2). GM1 was enriched in the upper part of the sucrose gradient (fractions 4-6), with a secondary localization at the bottom of the gradient (fractions 10-12), indicating a separation of the lipid rafts (fractions 4-6) from the Triton X-100–soluble membranes (Figure 2). By Western blot analysis using a specific anti-Fas antibody, we found that Fas was located in the soluble fractions of the sucrose gradient (fractions 10-12) and not in the detergent-insoluble lipid raft region in untreated Jurkat cells, indicating that Fas is excluded from the lipid rafts in untreated Jurkat cells (Figure 2). However, after ET-18-OCH3treatment, a significant portion of Fas was translocated into the lipid raft region (fractions 4-6) of the sucrose gradient (Figure 2). These data indicate that ET-18-OCH3 treatment induces translocation of Fas into lipid rafts.
ET-18-OCH3 induces translocation of Fas into GM1-containing lipid rafts.
Untreated Jurkat cells (Control) and Jurkat cells treated with 5 μg/mL ET-18-OCH3 for 3 hours were lysed in 1% Triton X-100 in TNEV buffer and subjected to discontinuous sucrose density gradient centrifugation. Individual fractions (20 μL) were subjected to SDS-PAGE and Western blotting. Location of GM1 and Fas was determined using CTx B subunit conjugated to horseradish peroxidase and anti-Fas polyclonal antibody, respectively, as described in “Materials and methods.” Representative blots of 3 separate experiments are shown.
ET-18-OCH3 induces translocation of Fas into GM1-containing lipid rafts.
Untreated Jurkat cells (Control) and Jurkat cells treated with 5 μg/mL ET-18-OCH3 for 3 hours were lysed in 1% Triton X-100 in TNEV buffer and subjected to discontinuous sucrose density gradient centrifugation. Individual fractions (20 μL) were subjected to SDS-PAGE and Western blotting. Location of GM1 and Fas was determined using CTx B subunit conjugated to horseradish peroxidase and anti-Fas polyclonal antibody, respectively, as described in “Materials and methods.” Representative blots of 3 separate experiments are shown.
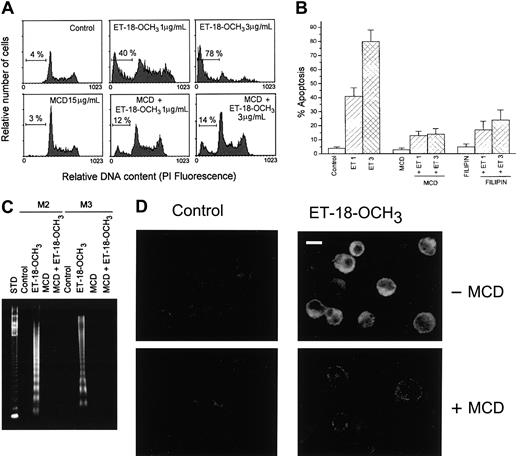
Membrane rafts are enriched in cholesterol, and depletion of cellular cholesterol can disrupt rafts and destroy function.3 To explore the role of membrane rafts in ET-18-OCH3–induced apoptosis, we disrupted membrane rafts by cell incubation with MCD or filipin in serum-free medium. MCD specifically removes cholesterol from the plasma membrane,19,20 and filipin is a polyene antibiotic that specifically complexes cholesterol and disrupts lipid raft function.21,22 HL-60 cells were used in these assays because they are prone to undergo ET-18-OCH3–induced apoptosis and can be cultured for longer times than Jurkat cells in serum-free medium.10,14 We found that MCD or filipin inhibited ET-18-OCH3–induced apoptosis in leukemic HL-60 cells (Figure 3A,B), indicating that ET-18-OCH3–induced apoptosis required membrane raft integrity. Bone marrow cells derived from patients with acute myeloblastic leukemia or acute promyelocytic leukemia (M2 or M3, respectively, following the FAB classification) were positive for Fas (> 60% positive cells), and underwent apoptosis after ET-18-OCH3 treatment (Figure 3C), corroborating our previous findings.7 In these M2 and M3 primary leukemic cells, Fas was clustered on ET-18-OCH3 treatment in a similar way to that of Jurkat and HL-60 cells (data not shown), and ET-18-OCH3–induced apoptosis of primary leukemic cells was also inhibited by MCD (Figure 3C). Furthermore, MCD-treated leukemic HL-60 cells did not show Fas clustering after ET-18-OCH3treatment (Figure 3D).
Effect of MCD and filipin on ET-18-OCH3–induced apoptosis and Fas clustering in human leukemic cells.
(A) HL-60 cells were pretreated with MCD (15 μg/mL) for 1 hour at 37°C in serum-free medium, and then ET-18-OCH3 was added at 1 or 3 μg/mL for 5 hours. Cells were stained with propidium iodide, and their DNA content was analyzed by fluorescence flow cytometry. The percentage of cells with a DNA content less than G1 (sub-G1) is indicated in each histogram. Control untreated cells and cells treated only with MCD were run in parallel. Data are representative of 3 experiments performed. (B) HL-60 cells were pretreated with MCD (15 μg/mL) or filipin (1 μg/mL) for 1 hour at 37°C in serum-free medium, and then ET-18-OCH3was added at 1 (ET 1) or 3 (ET 3) μg/mL for 5 hours. Percentage of apoptotic cells was determined as above. Control untreated cells and cells treated only with MCD or filipin were also run in parallel. Data shown are means of 3 independent experiments ± SD (C). Bone marrow leukemic primary cultures derived from patients with M2 or M3 leukemia were incubated in the absence (Control) or in the presence of 3 μg/mL ET-18-OCH3, MCD (15 μg/mL), or MCD plus ET-18-OCH3 for 3 hours. Fragmented DNA was extracted and analyzed as described previously.10 14 Fragmented DNA from 1.5 × 106 cells was loaded in each lane. A 123-bp DNA ladder was used as standard (STD). (D) HL-60 cells were untreated or pretreated with MCD (15 μg/mL) for 1 hour at 37°C in serum-free medium, and then ET-18-OCH3 was added at 1 μg/mL for 3 hours. Fas aggregation was then analyzed by confocal microscopy as indicated in Figure 1, using anti-Fas mAb and CY3-conjugated antimouse antibody. Bar indicates 8 μm.
Effect of MCD and filipin on ET-18-OCH3–induced apoptosis and Fas clustering in human leukemic cells.
(A) HL-60 cells were pretreated with MCD (15 μg/mL) for 1 hour at 37°C in serum-free medium, and then ET-18-OCH3 was added at 1 or 3 μg/mL for 5 hours. Cells were stained with propidium iodide, and their DNA content was analyzed by fluorescence flow cytometry. The percentage of cells with a DNA content less than G1 (sub-G1) is indicated in each histogram. Control untreated cells and cells treated only with MCD were run in parallel. Data are representative of 3 experiments performed. (B) HL-60 cells were pretreated with MCD (15 μg/mL) or filipin (1 μg/mL) for 1 hour at 37°C in serum-free medium, and then ET-18-OCH3was added at 1 (ET 1) or 3 (ET 3) μg/mL for 5 hours. Percentage of apoptotic cells was determined as above. Control untreated cells and cells treated only with MCD or filipin were also run in parallel. Data shown are means of 3 independent experiments ± SD (C). Bone marrow leukemic primary cultures derived from patients with M2 or M3 leukemia were incubated in the absence (Control) or in the presence of 3 μg/mL ET-18-OCH3, MCD (15 μg/mL), or MCD plus ET-18-OCH3 for 3 hours. Fragmented DNA was extracted and analyzed as described previously.10 14 Fragmented DNA from 1.5 × 106 cells was loaded in each lane. A 123-bp DNA ladder was used as standard (STD). (D) HL-60 cells were untreated or pretreated with MCD (15 μg/mL) for 1 hour at 37°C in serum-free medium, and then ET-18-OCH3 was added at 1 μg/mL for 3 hours. Fas aggregation was then analyzed by confocal microscopy as indicated in Figure 1, using anti-Fas mAb and CY3-conjugated antimouse antibody. Bar indicates 8 μm.
The present findings indicate that translocation of Fas into membrane rafts and clustering of membrane raft–associated Fas trigger the apoptotic signaling cascade in leukemic cells treated with ET-18-OCH3. The evidence for this conclusion is 3-fold: (1) Fas is translocated into membrane rafts following ET-18-OCH3 treatment; (2) Fas is accumulated in aggregated rafts on ET-18-OCH3 treatment; and (3) raft disruption inhibits both ET-18-OCH3–induced apoptosis and Fas clustering. Fas has been shown to be required for ET-18-OCH3–induced apoptosis because Fas+cells, but not Fas− cells, are susceptible to undergoing ET-18-OCH3–mediated apoptosis.12 Furthermore, Fas− cells become sensitive to ET-18-OCH3following Fas transfection.12 These data together with the results reported here indicate that clustering of membrane raft–associated Fas is required for antitumor ether lipid apoptosis. Clustered rafts are often bound to cytoskeleton.23 In this context, the association of Fas with ezrin24 and its capping during apoptosis have been reported recently.12,24The participation of membrane raft sphingolipid-cholesterol microdomains in ET-18-OCH3–induced apoptosis can explain previous reports showing that addition of exogenous cholesterol can affect the cytotoxic action of the ether lipid.25
The data reported herein showing that Fas is translocated into rafts on ET-18-OCH3 treatment and that reorganization of membrane raft-associated Fas is required for ET-18-OCH3–induced apoptosis indicate for the first time the association of Fas with membrane rafts and that antitumor ether lipids require membrane rafts for their proapoptotic activity. Thus, the present findings involve for the first time membrane rafts in cancer chemotherapy and Fas-mediated apoptosis. The generation of stabilized membrane lipid domains from a highly dispersed distribution may represent a general mode of regulating Fas activation. Membrane rafts could serve as platforms for coupling adaptor proteins required for Fas signaling.
We thank E. Del Canto-Jañez for excellent technical assistance and S. Callejo, E. Barbosa, and M. A. Ollacarizqueta for their help in confocal microscopy. We also thank Antonio Iglesias and Manuel Modolell for helpful discussions.
Supported by grants 1FD97-0622 and 1FD97-2018-C02-01 from the European Commission and Comisión Interministerial de Ciencia y Tecnologı́a, and grant CDTI 97-0355 from INKEYSA and Ministerio de Industria y Energı́a of Spain.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Faustino Mollinedo, Centro de Investigación del Cáncer, Instituto de Biologı́a Molecular y Celular del Cáncer, CSIC-Universidad de Salamanca, Campus Miguel de Unamuno, E-37007 Salamanca, Spain; e-mail: fmollin@usal.es.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal