Hematologic and immunologic functions were examined in 19 HIV-negative infants of HIV-positive mothers and 19 control infants of HIV-negative mothers. Control infants were selected to match for gestational age, weight, and mode of delivery. Cord blood was obtained from all infants and used for flow cytometric determination of lymphocyte subsets, including the naive CD4 count. Furthermore, to determine thymic output, cord blood mononuclear cells were used for determination of T-cell receptor excision circles (TRECs). Evaluation of progenitor cell function was done by means of colony-forming cell assay and fetal thymic organ cultures (FTOCs). Lower naive CD4 counts (459.3 ± 68.9 vs 1128.9 ± 146.8 cells/μL,P < .001) and reduced thymic output in infants of HIV-positive mothers were found (frequency of CD4+cells with TRECs was 3.6% ± 0.7% compared with 14.3% ± 2.2% in controls, P < .001). In combination with lower red blood cell counts in infants of HIV-positive mothers, this finding suggested impairment of progenitor cell function. Indeed, progenitors from infants of HIV-positive mothers had decreased cloning efficiency (15.7% ± 2.6% vs 55.8% ± 15.9%,P = .009) and seemed to generate fewer T cells in FTOCs. In conclusion, lower numbers of naive CD4+ cells and reduced thymic output in HIV-negative infants of HIV-positive mothers may be due to impaired progenitor cell function.
Introduction
Vertical transmission of HIV from an HIV-positive mother to her infant occurs in 15% to 25% of pregnancies if no precautions are taken. However, the risk of vertical transmission of HIV has been dramatically reduced with the introduction of antiretroviral treatment in combination with delivery by elective cesarean section and avoidance of breast-feeding.1 2
Although infants of HIV-positive mothers are rarely HIV-infected, they may have been exposed to HIV proteins or even HIV particles during fetal life, as indicated by the presence of HIV-specific T cells, immune activation, and positive HIV polymerase chain reaction (PCR) found in HIV-exposed infants.3-7 Thus, a recent study demonstrated high frequencies of HIV-specific CD4+ cells and a lower frequency of HIV-specific CD8+ cells, indicating transplacental diffusion of HIV-soluble proteins.8 HIV particles as well as HIV proteins are known to inhibit progenitor cell function and to cause progenitor cell apoptosis which, in turn, would lead to both hematologic and immunologic deficiencies in the infants.9-18 Furthermore, cytokine imbalance between Th1- and Th2-type cytokines has been suggested in HIV-positive individuals.19-21 Such an imbalance in pregnant HIV-positive women might also cause cytokine imbalance in the fetus, resulting in immunologic deficiencies. Finally, pregnant HIV-positive women are commonly treated with antiretroviral therapy including zidovudine (AZT), and AZT is known to inhibit bone marrow functions.22
The present study was conducted to determine if HIV-negative infants of HIV-positive mothers have immune deficiencies as determined by CD4 and CD8 counts in cord blood. Furthermore, thymic output was evaluated by determination of CD4+ and CD8+ cells with naive phenotype (coexpression of CD45RA) and determination of T-cell receptor excision circles (TRECs). Evidence of reduced thymic output was found and, to determine if impaired progenitor cell function might contribute to this, colony-forming cell (CFC) assays were performed to examine the function of myeloid progenitors, and fetal thymic organ cultures (FTOCs) were done to examine the function of T-cell progenitors. Recently, correlation between lymphocyte proliferation and expression of the early activation marker CD69 has been shown.23 24To determine if immune activation in infants of HIV-positive mothers might contribute to the lower level of naive CD4+cells and TRECs, coexpression of activation markers CD69 and CD25 on CD4+ and CD8+ cells was measured. Finally, cytokine imbalances might contribute to the immune deficiencies observed; therefore, the concentration of Th1 cytokines (ie, interleukin [IL]-2 and interferon [IFN]-γ) and Th2-type cytokines (ie, IL-4) in cord blood plasma was determined.
Patients, materials, and methods
Patients and study design
This study was approved by the local ethics committee, and informed consent was obtained from all patients after the nature and consequences of the study had been fully explained.
A total of 20 infants of HIV-positive mothers were included in this study from August 1996 to March 2000. The study population included one pair of twins (patients No. 2 and 3). The clinical characteristics of the 19 mothers are presented in Table 1. The 19 HIV-positive women included 3 Danes (including 1 former intravenous drug abuser), 13 women from Africa, and 3 women from Asia. Antiretroviral treatment antepartum and/or intrapartum was accepted by 18 of the HIV-positive mothers, and all but 2 of the women chose to give birth by cesarean section. None of the infants were infected with HIV as detected by PCR at the age of 6 months. As controls, 90 infants born to HIV-negative mothers were included. However, data proved to be confounded by gestational age (GA, ie, the age of the infant determined as the number of weeks after the first day in the last menstruation). Therefore, matched controls were selected for further comparison. Controls were selected to match for GA, birth weight, and mode of delivery (Table 2). One infant born to an HIV-positive mother had a GA of 31 weeks (Table 1), and a suitable matched control was not available. The final analysis therefore included 19 infants of HIV-positive mothers and 19 matched controls. It was not possible to match for ethnicity, and the controls included 15 Danes and 4 women from Asia.
Clinical characteristics of 19 HIV-positive mothers and their infants
| Patient No. . | CD4 count, cells/μL . | HIV RNA, copies/mL . | Antiretroviral treatment, Antepartum (from week) . | Antiretroviral treatment, intrapartum . | Antiretroviral treatment, postpartum . | GA, wk . | Mode of delivery . |
|---|---|---|---|---|---|---|---|
| 1 | 445 | ND | AZT (14) | AZT | AZT | 38 | Cesarean section |
| 2, 3 | 187 | ND | AZT (17) | AZT | AZT | 34 | Cesarean section |
| 4 | 858 | ND | AZT (20) | AZT | AZT | 38 | Cesarean section |
| 5 | 379 | ND | AZT (14) | AZT | AZT | 37 | Cesarean section |
| 6 | 227 | 14 000 | AZT, ddI (18) | AZT | AZT | 31 | Cesarean section |
| 7 | 221 | 34 000 | AZT, 3TC (16) | AZT | AZT | 37 | Cesarean section |
| 8 | 209 | < 100 | AZT (27) | AZT | AZT | 41 | Vaginal |
| 9 | 478 | 28 | AZT (20) | AZT | AZT | 38 | Cesarean section |
| 10 | 598 | 1 886 | AZT, 3TC (14) | AZT | AZT | 38 | Cesarean section |
| 11 | 165 | 291 | AZT, 3TC, IND (0) | AZT | AZT | 38 | Cesarean section |
| 12 | 80 | 1 301 | AZT, 3TC, IND (32) | AZT | AZT | 38 | Cesarean section |
| 13 | 385 | 119 | AZT (14) | AZT | AZT | 38 | Cesarean section |
| 14 | 348 | < 20 | D4T, 3TC, NEV (0) | AZT | AZT | 38 | Cesarean section |
| 15 | 330 | < 20 | AZT, 3TC, NEV (24) | AZT | AZT | 38 | Cesarean section |
| 16 | 299 | < 20 | AZT, 3TC, ddI, NEV (14) | AZT | AZT | 38 | Cesarean section |
| 17 | 352 | 1 173 | None | None | None | 40 | Vaginal |
| 18 | 336 | < 20 | AZT, 3TC, NFV (16) | AZT | AZT | 38 | Cesarean section |
| 19 | 621 | < 200 | AZT, 3TC (30) | AZT | AZT | 38 | Cesarean section |
| 20 | 430 | < 20 | AZT, 3TC, NEV (24) | AZT | AZT | 38 | Cesarean section |
| Patient No. . | CD4 count, cells/μL . | HIV RNA, copies/mL . | Antiretroviral treatment, Antepartum (from week) . | Antiretroviral treatment, intrapartum . | Antiretroviral treatment, postpartum . | GA, wk . | Mode of delivery . |
|---|---|---|---|---|---|---|---|
| 1 | 445 | ND | AZT (14) | AZT | AZT | 38 | Cesarean section |
| 2, 3 | 187 | ND | AZT (17) | AZT | AZT | 34 | Cesarean section |
| 4 | 858 | ND | AZT (20) | AZT | AZT | 38 | Cesarean section |
| 5 | 379 | ND | AZT (14) | AZT | AZT | 37 | Cesarean section |
| 6 | 227 | 14 000 | AZT, ddI (18) | AZT | AZT | 31 | Cesarean section |
| 7 | 221 | 34 000 | AZT, 3TC (16) | AZT | AZT | 37 | Cesarean section |
| 8 | 209 | < 100 | AZT (27) | AZT | AZT | 41 | Vaginal |
| 9 | 478 | 28 | AZT (20) | AZT | AZT | 38 | Cesarean section |
| 10 | 598 | 1 886 | AZT, 3TC (14) | AZT | AZT | 38 | Cesarean section |
| 11 | 165 | 291 | AZT, 3TC, IND (0) | AZT | AZT | 38 | Cesarean section |
| 12 | 80 | 1 301 | AZT, 3TC, IND (32) | AZT | AZT | 38 | Cesarean section |
| 13 | 385 | 119 | AZT (14) | AZT | AZT | 38 | Cesarean section |
| 14 | 348 | < 20 | D4T, 3TC, NEV (0) | AZT | AZT | 38 | Cesarean section |
| 15 | 330 | < 20 | AZT, 3TC, NEV (24) | AZT | AZT | 38 | Cesarean section |
| 16 | 299 | < 20 | AZT, 3TC, ddI, NEV (14) | AZT | AZT | 38 | Cesarean section |
| 17 | 352 | 1 173 | None | None | None | 40 | Vaginal |
| 18 | 336 | < 20 | AZT, 3TC, NFV (16) | AZT | AZT | 38 | Cesarean section |
| 19 | 621 | < 200 | AZT, 3TC (30) | AZT | AZT | 38 | Cesarean section |
| 20 | 430 | < 20 | AZT, 3TC, NEV (24) | AZT | AZT | 38 | Cesarean section |
3TC indicates lamivudine; ddI, dideoxynosine; D4T, stavudine; IND, indinavir; NEV, nevirapine; NFV, nelfinavir; GA, gestational age; and AZT, antiviral therapy including zidovudine.
Clinical characteristics of 19 infants with HIV-positive mothers and the 19 matched controls with HIV-negative mothers
| . | Infants with HIV-positive mothers . | Controls (infants with HIV-negative mothers) . |
|---|---|---|
| GA, wk | 37.7 ± 0.4 | 37.9 ± 0.3 |
| Birth weight, g | 3122.4 ± 146.2 | 3164.8 ± 95.9 |
| Mode of delivery (vaginal, cesarean section) | 2, 17 | 3, 16 |
| . | Infants with HIV-positive mothers . | Controls (infants with HIV-negative mothers) . |
|---|---|---|
| GA, wk | 37.7 ± 0.4 | 37.9 ± 0.3 |
| Birth weight, g | 3122.4 ± 146.2 | 3164.8 ± 95.9 |
| Mode of delivery (vaginal, cesarean section) | 2, 17 | 3, 16 |
Data are given as means ± SEM.
CA indicates gestational age.
Cord blood collected from the umbilical vein was obtained from all infants and used to obtain a full blood count and for flow cytometry. Cord blood samples drawn into tubes containing heparin were used to obtain cord blood mononuclear cells (CBMCs) by means of density gradient centrifugation.25 26 CBMCs were used for determination of TRECs and for determination of progenitor cell function by means of CFC assay and FTOCs. Finally, cord blood plasma was obtained and used to determine the concentration of the cytokines IL-2, IL-4, and IFN-γ.
Flow cytometry
Flow cytometry was performed as described previously.11,25 Briefly, 100 μL blood was incubated with 10 μL fluorescence-conjugated monoclonal antibodies at room temperature for 15 minutes. Erythrocytes were lysed with 2 mL NH4Cl buffer at room temperature for 10 minutes, and the samples were washed and resuspended in phosphate-buffered saline supplemented with 10% CellFix (Becton Dickinson Immunocytometry Systems, San Jose, CA). All samples were analyzed using a FACScan (Becton Dickinson) equipped with a 488-nm argon-ion laser. Data were processed using CellQuest software (Becton Dickinson). Monoclonal antibodies used to determine phenotypes were isotype controls, CD34 (anti–HPCA-2), CD3 (Leu-4), CD4 (Leu-3a), CD8 (Leu-2a), and CD19 (Leu). The fraction of lymphocytes with naive and memory phenotype were determined using CD45RA (Leu-18) and CD45RO (Leu 45RO, UCHL-1), respectively. Finally, CD25 (Leu) and CD69 (Leu-23) were used as markers of early immune activation. All antibodies were purchased from Becton Dickinson. To determine the absolute number of CD34+cells or lymphocyte subsets in peripheral blood, the percentage of cells expressing CD34 was multiplied by the white blood count, and the percentage of a lymphocyte subset was multiplied by the lymphocyte count.11
Enrichment of CD4+ and CD8+ cells for determination of TRECs
Frozen CBMCs were carefully thawed and separated into CD4+ and CD8+ cells using a magnetic cell separator (MACS; Miltenyi Biotec, Bergisch Gladbach, Germany) as described previously.26 Viability of frozen CBMCs was always more than 90% and comparable in the 2 groups. Briefly, CBMCs were washed twice in phosphate-buffered saline supplemented with 5% fetal calf serum (Gibco, Paisley, Scotland). CBMCs were then incubated with CD4 microbeads or CD8 microbeads (Miltenyi Biotec) for 15 minutes at 4°C and then washed prior to separation. Separation was performed using a mini-MACS column (Miltenyi Biotec). The column was placed in the magnetic separator, magnetically labeled cells were passed down the column, and the column was washed extensively. The column was removed from the magnetic separator, and cells retained were eluted. Separation of CD4+ cells and CD8+ cells was done in 15 infants with HIV-positive mothers and 15 controls. The purity of sorted populations was determined by flow cytometry and was always more than 90%.
Quantification of signal-joint (sj) TRECs in enriched CD4+and CD8+ cells was done by real-time quantitative PCR with the 5′-nuclease (TaqMan) assay. DNA was extracted from CD4+and CD8+ cells using a salting out procedure,27 and the DNA concentration was determined by spectrophotometry (Shimadzu, Kyoto, Japan) prior to further analysis. A multiplex assay was used to quantify sj TREC value and a manan binding lectin (MBL) coding sequence to measure cell equivalents in the input DNA. Sequences of the sj primers were 5′-CACATCCCTTTCAACCATGCT-3′ and 5′-GCCAGCTGCAGGGTTTAGG-3′, and the probe FAM′ACACCTCTGGTTTTTGTAAA-GGTGCCCACT′TAMRA (DNA Technology, Aarhus, Denmark) was used.28 The sequences of the MBL primers were 5′-TGGCAGCGTCTTACTCAGAA-3′ and 5′-ATCACTGCAGGGCAGGTC-3′ and probe VIC′CTGTGACCTGTGAGGATGCCCAA′TAMRA (DNA Technology). Each PCR reaction mixture contained 10 000 or 30 000 copies of genomic DNA, 0.2 μM sj probe, 0.3 μM each sj primer, 0.1 μM MBL probe, 0.05 μM each MBL primer, and TaqMan universal mastermix (Applied Biosystems, Branchburg, NJ). The PCR reactions were run in an ABI prism 7700 (Applied Biosystems), and conditions were 50°C for 2 minutes, 95°C for 10 minutes, and then 50 cycles of 95°C for 12 seconds and 60°C for 1 minute. A standard curve was plotted, and sj TREC values for samples were calculated using the ABI 7700 software (Applied Biosystems). Samples were analyzed in triplicates that never varied by more than 10%, and the results were averaged.
Colony assays for progenitor cells
Colony assays were done using CBMCs from all infants. CFCs were grown in methylcellulose medium using the Stem Cell CFU Kit (Baxter Healthcare, Deerfield, IL) according to manufacturer's instructions. Briefly, 8 × 105 CBMCs in 1 mL dilution medium were mixed with 3 mL colony-forming unit (CFU) culture medium to allow plating at a concentration of 2 × 105 CBMCs per milliliter. Stem cell factor, 100 ng/mL, (Genzyme, Cambridge, MA) was added, and the cell suspension was aliquoted in triplicates of 1 mL in 35-mm culture plates (Nunc, Roskilde, Denmark). The plates were incubated for 14 days in a humidified incubator at 37°C and 5% CO2. Then colonies (CFU–granulocyte macrophage) of more than 50 cells were counted using an inverted microscope. Thus, the median number of colonies counted in triplicate was the number of CFCs per 2 × 105 CBMCs. The number of CFCs per milliliter of peripheral blood was calculated using the equation: CFC/mL = 5 × (CFC/2 × 105 CBMC) × (number of mononuclear cells [monocytes and lymphocytes] × 106/mL peripheral blood). Finally, we determined the cloning efficiency of CD34+ cells using the equation: cloning efficiency (%) = (CFCs/mL)/(number of CD34+ cells/mL) × 100.11
Thymic organ cultures
Progenitor cells in CBMC preparations from 3 infants with HIV-positive mothers (patients No. 10, 11, and 18) and 3 controls were analyzed for ability to differentiate into T cells in thymic organ cultures.
RAG-1 knockout mice were maintained and bred in microisolator cages in the animal care facility at Netherlands Cancer Institute. Fetal thymus lobes were removed from 14- to 15-day-gestation mouse fetuses and placed in organ cultures as described previously.9 10Briefly, fetal murine thymus lobes were placed in an organ culture system on filters 25-μm thick with 0.45-μm pore size (Millipore, Bedford, MA) supported on surgical Gelfoam (Upjohn, Kalamazoo, MI). Organ cultures were grown in Dulbecco modified Eagle medium (Sigma, St Louis, MO) with 20% fetal bovine serum (Life Technologies, Paisley, Scotland), 1 mg/mL penicillin, 1 U/mL streptomycin, and 3.4 g/L sodium bicarbonate and maintained at 37°C with 5% CO2. Cryopreserved CBMCs were thawed, counted, and viability assessed by trypan blue dye exclusion. Then, 1 × 106 total viable CBMCs were placed on each of the lobes by direct application of broken pellets of cells in 0.2-μL aliquots until the designated total number of donor cells per lobe was reached. After 14 days in culture, lobes were enzymatically digested in collagenase, and harvested cells were counted and stained for flow cytometry.
Cells from collagenase digestion were stained with monoclonal antibodies CD3, CD4, CD8, CD45, MHCI, MHCII, and isotype controls. All antibodies were from Caltag (Burlingame, CA). Cells were analyzed on a FACScan (Becton Dickinson) using CellQuest software (Becton Dickinson). Cells were analyzed for the expression of CD4+ and CD8+ cell subsets: CD4+CD8−, CD4−CD8+, CD4+CD8+, and CD4−CD8−. Each subset was further analyzed for the expression of CD3. Total human cells were calculated by multiplying cell number by percentage CD45+MHC+ cells. Flow cytometry data from thymic organ cultures were calculated on a per lobe basis by multiplying the frequency of a given T-cell subset by the total number of human cells produced per lobe as assessed by CD45 and MHC staining.
Cytokine enzyme-linked immunosorbent assay
Determination of the concentration of cytokines in cord blood plasma was done using Opteia IL-2, IL-4, and IFN-γ enzyme-linked immunosorbent assay (Pharmingen, San Diego, CA) according to instructions of the manufacturer. Briefly, 100 μL cord blood plasma was added to a microtiter well coated with monoclonal antibodies to the cytokine of choice. After incubation for 2 hours, the wells were washed and 100 μL biotinylated antibody to the cytokine of choice and avidin–horseradish peroxidase was added to each well, and the plates were incubated for 1 hour. Finally, the plates were washed, and 100 μL working substrate solution containing hydrogen peroxide was added. The reaction was stopped with phosphoric acid, and the plates were read at 450 nm. Included on each plate was a cytokine standard allowing determination of the concentration of the cytokine in the supernatants.
Statistical methods
Data are given as means (± SEM). Due to a skewed distribution of some variables, logarithmic transformations of these measurements were done prior to further statistical analyses. Differences between infants with HIV-positive mothers and controls were evaluated using a t test. The correlation between measurements was calculated using Pearson correlation coefficient. A 5% significance level was used.
Results
Lower red blood cell counts in infants of HIV-positive mothers
To evaluate hematologic functions, the red blood cell count was determined. Compared with controls, a lower concentration of hemoglobin was found (8.3 ± 0.2 vs 9.7 ± 0.2 mM,P < .01). In contrast, although the white blood cell counts tended to be lower in infants of HIV-positive mothers, this did not reach significance. No difference was found when the platelet counts were compared (263.6 ± 34.4 vs 279.4 ± 17.9 × 109/L,P = .657).
Lower naive CD4+ cell counts and thymic output in infants of HIV-positive mothers
To evaluate if immunologic deficiencies occurred in infants of HIV-positive mothers, the lymphocyte counts were determined. Interestingly, a major difference in CD3 count was found (1808.4 ± 178.5 vs 2604.4 ± 193.5 CD3+ cells/μL,P = .005), primarily due to lower CD4 count in infants of HIV-positive mothers (553.1 ± 76.7 vs 1279.9 ± 162.8 CD4+ cells/μL, P < .001). The lower CD4 count in infants of HIV-positive mothers was due to a lower fraction of CD4+ cells (26.2% ± 2.2% vs 40.3% ± 2.7%,P < .001). In contrast, no difference in total lymphocyte count was found between the 2 groups. The difference in CD4 count, in turn, resulted in lower naive CD4 count (459.3 ± 68.9 vs 1128.9 ± 146.8 cells/μL, P < .001) and lower memory CD4 count in infants of HIV-positive mothers (Figure1). The fraction of naive CD4+ cells was 88.3% ± 2.3% in infants of HIV-positive mothers versus 92.3% ± 0.9% in controls (P = .094). Differences in lymphocyte counts related to ethnicity have been reported,29 and in the present study it was not possible to match for ethnicity. However, the 3 infants of HIV-positive mothers with Danish origin had a mean CD4 count of 783.9 ± 188.0 cells/μL and a mean naive CD4 count of 412.9 ± 57.9 CD4+ cells per microliter. The former intravenous drug abuser gave birth to an infant with a CD4 count of 1109.6 CD4+ cells per microliter. No differences were found between infants of HIV-positive mothers and controls when the CD8 and CD19 counts were compared (Figure 1).
Lower CD4 counts in infants of HIV-infected mothers.
The CD4 and CD8 counts in infants of HIV-infected mothers (white bars) and control infants of HIV-negative mothers (hatched bars) are shown. CD45RA+ represents naive cells, and CD45RO+represents memory cells. Data are given as means ± SEM.
Lower CD4 counts in infants of HIV-infected mothers.
The CD4 and CD8 counts in infants of HIV-infected mothers (white bars) and control infants of HIV-negative mothers (hatched bars) are shown. CD45RA+ represents naive cells, and CD45RO+represents memory cells. Data are given as means ± SEM.
To evaluate the thymic output, the frequency of CD4+ and CD8+ cells with sj TRECs was determined using real-time PCR. The frequency of CD4+ cells with sj TRECs was 3.6% ± 0.7% in infants of HIV-positive mothers compared with 14.3% ± 2.2% in controls (P < .001). Significant correlation was found between the naive CD4 count and the frequency of CD4+ cells with sj TRECs (r = 0.78,P < .001). The frequency of CD8+ cells with sj TRECs was also reduced in infants of HIV-positive mothers (2.8% ± 0.6% vs 9.0% ± 1.7%, P = .001).
Finally, to determine if the fraction of naive CD4+ cells was lower in infants of HIV-positive mothers due to immune activation, the percentage of CD4+ and CD8+ cells coexpressing CD25 and CD69 was determined. Significant differences indicating immune activation between the 2 groups was not found (Table3).
Immune activation in infants with HIV-positive mothers and the 19 matched controls with HIV-negative mothers measured as percentage of CD4+ and CD8+ cells coexpressing CD25 and CD69
| . | Infants with HIV-positive mothers . | Controls (infants with HIV-negative mothers) . | P . |
|---|---|---|---|
| CD4+CD25+, % | 9.2 ± 0.6 | 11.3 ± 0.5 | .013 |
| CD8+CD25+, % | 1.5 ± 0.3 | 1.3 ± 0.3 | .676 |
| CD4+CD69+, % | 6.0 ± 1.1 | 4.9 ± 0.7 | .422 |
| CD8+CD69+, % | 7.3 ± 1.1 | 7.7 ± 0.7 | .758 |
| . | Infants with HIV-positive mothers . | Controls (infants with HIV-negative mothers) . | P . |
|---|---|---|---|
| CD4+CD25+, % | 9.2 ± 0.6 | 11.3 ± 0.5 | .013 |
| CD8+CD25+, % | 1.5 ± 0.3 | 1.3 ± 0.3 | .676 |
| CD4+CD69+, % | 6.0 ± 1.1 | 4.9 ± 0.7 | .422 |
| CD8+CD69+, % | 7.3 ± 1.1 | 7.7 ± 0.7 | .758 |
Data are given as means ± SEM.
Numbers and functions of progenitor cells in cord blood
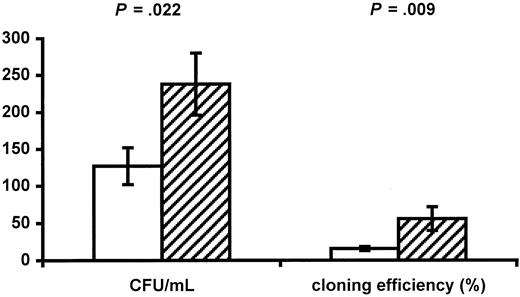
Infants of HIV-positive mothers have decreased numbers of red blood cells and CD4+ cells, suggesting impaired function of progenitor cells. The numbers and function of progenitors were therefore determined. No difference was found between the 2 groups in numbers of circulating CD34+ progenitors (38.8 ± 5.4 cells/μL in infants of HIV-positive mothers vs 29.5 ± 3.6 cells/μL in controls, P = .157). The function of progenitors was evaluated using a CFC assay, and infants of HIV-positive mothers had significantly reduced numbers of CFCs per milliliter (126.6 ± 24.5/mL vs 238.1 ± 42.1/mL,P = .022; Figure 2) as well as a decreased cloning efficiency (15.7% ± 2.6% vs 55.8% ± 15.9%, P = .009; Figure 2). AZT may be the cause of impaired progenitor cell function. Only 2 infants were not exposed to AZT during fetal life (patients No. 14 and 17), and these infants had cloning efficiencies of 31.1% and 0%, respectively.
Impaired progenitor cell function in infants of HIV-infected mothers.
The CFUs per milliliter and the cloning efficiency of CD34+ progenitor cells in infants of HIV-infected mothers (white bars) and control infants of HIV-negative mothers (hatched bars) are shown. Data are given as means ± SEM.
Impaired progenitor cell function in infants of HIV-infected mothers.
The CFUs per milliliter and the cloning efficiency of CD34+ progenitor cells in infants of HIV-infected mothers (white bars) and control infants of HIV-negative mothers (hatched bars) are shown. Data are given as means ± SEM.
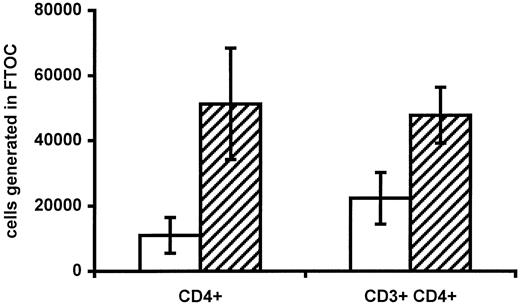
To further evaluate if progenitor cell function was impaired in infants of HIV-positive mothers, progenitors harvested from cord blood from 3 infants of HIV-positive mothers and 3 controls were allowed to differentiate into lymphocytes in FTOCs. A total of 1 × 106 viable CBMCs were placed on a fetal thymus lobe. The fraction of CD34+ cells in CBMCs was comparable in the 2 groups (0.68% ± 0.09% in infants of HIV-positive mothers vs 0.59% ± 0.08% in controls). The number of immature single-positive CD4+ cells and mature CD3+CD4+cells generated was then determined (Figure3). Interestingly, progenitors from infants of HIV-positive mothers seemed to generate fewer CD4+ cells per lobe (1.1 × 104 ± 0.5 × 104 vs 5.1 × 104 ± 1.7 × 104,P = .088, and 2.2 × 104 ± 0.8 vs 4.8 × 104 ± 0.9, P = .094, for immature and mature CD4+ cells, respectively). Because the number of CD34+ cells was equal in the 2 groups and a murine thymus is unaffected by HIV, this finding indicates that impaired progenitor cell function may be responsible for the low number of CD4+cells in HIV-negative infants of HIV-positive mothers.
Generation of CD4+ T cells in FTOCs.
The absolute numbers of immature (CD4+ single-positive) and mature (CD3+CD4+) CD4+ cells generated per fetal thymus lobe in infants of HIV-infected mothers (white bars) and control infants of HIV-negative mothers (hatched bars) are shown. In each case, 1 × 106 total viable CBMCs were placed on each lobe. Data are given as means ± SEM.
Generation of CD4+ T cells in FTOCs.
The absolute numbers of immature (CD4+ single-positive) and mature (CD3+CD4+) CD4+ cells generated per fetal thymus lobe in infants of HIV-infected mothers (white bars) and control infants of HIV-negative mothers (hatched bars) are shown. In each case, 1 × 106 total viable CBMCs were placed on each lobe. Data are given as means ± SEM.
Cytokines in cord blood
Previously, the hypothesis that HIV-positive individuals have an imbalance between Th1- and Th2-type cytokines has been stated.19-21 The hypothesis is based on the finding that progression to acquired immunodeficiency syndrome is characterized by loss of IL-2 and IFN-γ production concomitant with increases in IL-4 and IL-10 production.19-21 Such an imbalance in HIV-positive pregnant women may cause a similar imbalance in their fetuses. Cord blood plasma was therefore used to determine the concentration of the cytokines IL-2, IL-4, and IFN-γ (Figure4). A lower concentration of IFN-γ was found in infants of HIV-positive mothers (0.75 ± 0.75 vs 8.63 ± 3.12 pg/mL in controls, P = .028). In contrast, no significant differences were found between the 2 groups when the concentrations of IL-2 and IL-4 were compared.
Cytokines in cord blood.
The concentration of the cytokines IFN-γ, IL-2, and IL-4 in cord blood plasma from infants of HIV-infected mothers (white bars) and control infants of HIV-negative mothers (hatched bars) is shown. Data are given as means ± SEM.
Cytokines in cord blood.
The concentration of the cytokines IFN-γ, IL-2, and IL-4 in cord blood plasma from infants of HIV-infected mothers (white bars) and control infants of HIV-negative mothers (hatched bars) is shown. Data are given as means ± SEM.
Discussion
Low CD4 counts in HIV-positive and HIV-exposed children have previously been reported.4,5,8 30 In this study, this finding was confirmed because lower CD4 counts in cord blood from HIV-negative infants of HIV-positive mothers were found when compared with unexposed controls. Furthermore, we also report reduced thymic output as demonstrated by lower naive CD4 counts and lower TREC frequency. To determine if reduced thymic output was caused by impaired progenitor cell function, the progenitor cell function was examined using both a CFC assay and FTOCs, and in both cases progenitors from infants of HIV-positive mothers had impaired function. We therefore suggest that impaired progenitor cell function in HIV-negative infants of HIV-positive mothers results in decreased CD4 counts.
Generation of CD4+ cells involves both a thymus-dependent pathway (the generation of naive CD4+ cells from progenitor cells) and a thymus-independent pathway (peripheral expansion of preexisting memory CD4+ cells).31,32 To determine thymic output, most studies rely on surface molecules such as isoforms of CD45.33 The decrease in naive CD4+cells found in the present study would be interpreted as a reduced thymic output. However, T cells expressing the naive phenotype are not necessarily accurate surrogate markers of thymic output. Thus, naive T-cell markers may be obtained by memory cells.34,35 To measure thymic output more directly, quantification of TRECs has been proposed.30,36 In the present study, both the numbers of naive CD4+ cells and TREC frequency were lower in infants of HIV-positive mothers, strongly suggesting a reduced thymic output. However, decrease in TREC frequency may be due to increased cell division.37 38 The level of immune activation was therefore determined, and no evidence of immune activation was found. In contrast, in infants of HIV-positive mothers, lower coexpression of CD25 on CD4+ cells was found—a finding that does not seem to explain the lower naive CD4 counts and TREC frequency in these infants.
Evidence of reduced thymic output in HIV-negative infants of HIV-positive mothers found in the present study is in agreement with reduced thymic output reported in HIV-positive children.39The importance of contribution of the thymus to reconstitution of T cells in HIV-infected children has been demonstrated recently.40 Thymic output, in turn, reflects the function of the thymus and the function of T-cell progenitors. Thymic abnormalities due to HIV are common41 and may be due to HIV infection of T-cell precursors or thymic stromal cells. Furthermore, thymic abnormalities are described in fetuses aborted from HIV-positive women even in the absence of thymic HIV infection.42Impaired progenitor cell function in HIV-positive patients has been reported,11-18 the number of immature progenitors is decreased,11,15 and progenitors from HIV-infected patients display diminished T-cell generation capacity when examined in FTOCs.9 10 In the present study, the decreased red blood cell count and the lower CD4 counts indicated that progenitor cell function was impaired in infants of HIV-positive mothers. To test this hypothesis, progenitor cell function was examined in CFC assay and FTOCs, both of which indicated impaired progenitor cell function in infants of HIV-positive mothers. Thus, impaired progenitor cell function in infants of HIV-positive mothers seemed to be at least partly responsible for the low naive CD4 counts. It is, however, possible that thymic function is impaired as well.
The mechanisms that could lead to impaired progenitor cell function in HIV-negative infants of HIV-positive mothers include exposure to HIV particles or HIV proteins.3-8 HIV proteins have been demonstrated in cord blood plasma from HIV-negative infants of HIV-positive mothers6,43 (also S.D.N., unpublished data, 2000). Glycoprotein 120 has been described to induce progenitor cell apoptosis14,17 and glycoprotein 160 to induce IL-3 and IL-6 secretion from cord blood T cells, resulting in T-cell–mediated stimulation of myelopoiesis,44,45 both of which could cause diminished red blood cell and T-cell generation. Another possible cause of impaired progenitor cell function is the use of antiretroviral treatment. AZT is a potent inhibitor of bone marrow function22 and, as for the finding of anemia in infants of HIV-positive mothers, administration of AZT is likely to be responsible.46 However, multipotent progenitor cells, including early T-cell progenitors, are less sensitive to the effect of AZT.46 In addition, in a trial comparing stavudine plus lamivudine (seldom associated with bone marrow toxicity) versus AZT plus lamivudine (commonly associated with bone marrow toxicity) in antiretroviral therapy-naive patients, no difference in CD4 count increase was observed.47 Thus, at present evidence does not point toward antiretroviral treatment being responsible for the low thymic output in infants of HIV-positive mothers. Finally, children from Africa have lower CD4+ cell percentages compared with children from developed countries,29 and it is possible that differences in hematologic parameters related to ethnicity exit.48 In the present study it was not possible to match for ethnicity, and some of the hematologic and immunologic differences observed may be explained by ethnic differences. However, in the study comparing African children with children from developed countries,29 it seems reasonable to assume unequal access to nutritional products in the 2 groups. In the present study, however, patients and controls had equal access to nutritional products. In light of the small number of patients included in the present study, it is not possible to rule out that ethnicity may be in part responsible for the observed hematologic and immunologic differences observed, but the finding that 3 infants of HIV-positive mothers with Danish origin did not seem to differ in CD4 count when compared with infants of HIV-positive mothers with African or Asian background does indicate that ethnic difference is not the sole reason.
At present, the implications of the immunologic and hematologic impairments reported here are unknown. Immune abnormalities observed in children of HIV-infected mothers persisted over time and were still present at the age of 7, indicating that the physiologic development of the bone marrow/immune system was impaired.8 A follow-up study to the present study will be conducted to determine if these HIV-negative infants of HIV-positive mothers are more prone to infectious diseases. In addition, the results presented here may have implications for the design of gene therapy to treat infants infected by HIV. Successful progenitor cell gene therapy of infants with severe combined immunodeficiency-X1 has recently been reported.49 Progenitor cell gene therapy for HIV-infected infants before the immune system is severely compromised has been suggested.50 The ultimate goal of gene therapy would be to reconstitute the immune system with genetically altered cells that are resistant to HIV. However, if there were intrinsic functional defects in CD34+ cells from HIV-exposed or infected infants, such a strategy would not succeed.
In conclusion, lower naive CD4 counts and thymic output in combination with a reduced number of red blood cells was found in HIV-negative infants of HIV-positive mothers, indicating that progenitor cell function was impaired. Progenitor cell function was therefore examined using CFC assay and FTOCs, and in both cases progenitors from infants of HIV-positive mothers had impaired function. The implications of these hematologic and immunologic deficiencies are at present unknown, but a recent study suggested that immune abnormalities observed in children of HIV-infected mothers persisted for several years.8 Larger studies including longitudinal studies are needed to clarify if HIV-negative children of HIV-positive mothers are prone to infections or indeed have lasting impairment of their immune system.
We gratefully acknowledge the patients who made this study possible. We thank Tonni Hansen and Anna-Louise Sørensen for excellent technical assistance, and John-Erik S. Hansen, Jens Lundgren, and Hergen Spits for helpful discussion and for providing the necessary laboratory facilities.
Supported by the 17-12-1981 Foundation.
Part of the information in this paper was presented at the 13th World AIDS Conference, Durban, South Africa, July 2000.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Susanne D. Nielsen, Dept of Infectious Diseases, 144, Hvidovre Hospital, 2650 Hvidovre, Denmark; e-mail:sdn@dadlnet.dk.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal