Huët first observed an abnormal hyposegmented neutrophil granulocyte in 1928, and a year later Pelger reported an additional case. Such a cell later became known as Pelger-Huët anomaly. In 1932 Huët pointed out its hereditary and systemic character.1 The Pelger-Huët anomaly is a familial anomaly that is transmitted as a Mendelian dominant trait but that is unaccompanied by any pathologic phenomenon.2 It is found in a heterozygous state in about one of every 5000 individuals. It is characterized by hyposegmentation of the granulocyte nuclei. Almost all nuclei are rod-shaped or have only 2 lobes, and their chromatin structure is coarser than that of a normal neutrophil.
Anomalies resembling Pelger-Huët anomaly that are acquired rather than familial have been described as pseudo Pelger-Huët anomaly.3 4 The morphologic characteristic seen in pseudo Pelger-Huët anomaly is similar to Pelger-Huët anomaly. The acquired pseudo Pelger-Huët anomaly has been associated with pathologic conditions including myelodysplastic syndromes (MDS).
MDS are a closely related group of bone marrow disorders seen in elderly populations characterized by peripheral cytopenias and dysplastic hematopoiesis. But dysplastic hematopoiesis is a feature not confined only to MDS: this feature is also seen during the administration of certain drugs and in megaloblastic anemia.5 On the other hand, some abnormalities such as pseudo Pelger-Huët anomaly and micromegakaryocytes are considered to be highly characteristic and highly pathognomonic of MDS. One or the other of these abnormalities occurs in a high percentage of MDS patients. From its first recognition until now, the presence of pseudo Pelger-Huët anomaly is considered to be the most specific dysplastic marker for the diagnosis of MDS without any bias.6 Recent studies have also included this morphologic abnormality as a prognostic marker in MDS.7,8 In the past our group has reported excessive intramedullary apoptosis of hematopoietic cells in MDS.9 Apoptosis was found to be pronounced in maturing/matured cells in the high-density fraction of the bone marrow (BM) aspirate and biopsy.10
In light of the above findings in MDS and the current knowledge of morphology of apoptotic cells, we decided to evaluate whether the Pelger-Huët cells are apoptotic. BM aspirates and peripheral blood (PB) specimens from 56 MDS patients were examined for apoptosis using light microscopy (LM), electron microscopy (EM), and in situ end labeling (ISEL) of DNA, as described earlier.10 Of these, 25 patients had refractory anemia (RA), 12 were RA with ring sideroblasts (RARS), 13 were RA with excessive blasts (RAEB), 4 were RAEB in tranformation (RAEB-t), and 2 were chronic myelomonocytic leukemia (CMMoL). The patient population consisted of 34 males and 22 females, and the median age was 68 years (range, 26-85 years).
On examining the BM aspirate and PB of the MDS patients, 78% (44 of 56) of patients belonging to different FAB types showed the presence of characteristic Pelger-Huët anomaly by LM and EM (Figure1). They were seen in both the PB and the BM aspirates of these patients. The median frequency of the pseudo Pelger-Huët cells was 3.8% (range, 1-34) in the PB and 4.6% (range, 2-38) in the BM aspirate. The incidence of these cells reported in this study was comparable to those reported earlier.6Interestingly, both the high density (HD) and the low density (LD) fractions of the PB and BM aspirate compartments showed the presence of pseudo Pelger-Huët anomaly. In addition, a higher number of these cells were seen in the HD fraction than in the LD fraction (9.6% versus 4.2%; P = .0001). Morphologically, 98% of these cells looked like mature granulocytes undergoing apoptosis. The electron microscopic morphology showed that both unilobed and bilobed cells were undergoing apoptosis. The cells showed characteristic apoptotic features in both the nucleus and the cytoplasm. The nucleus showed compact and segregated chromatin sharply delineated toward the periphery of the nucleus. Marked condensation of the cytoplasm with distorted organelles and mild convolution of the cellular outlines were noted. These findings were further confirmed by ISEL (Figure 1).
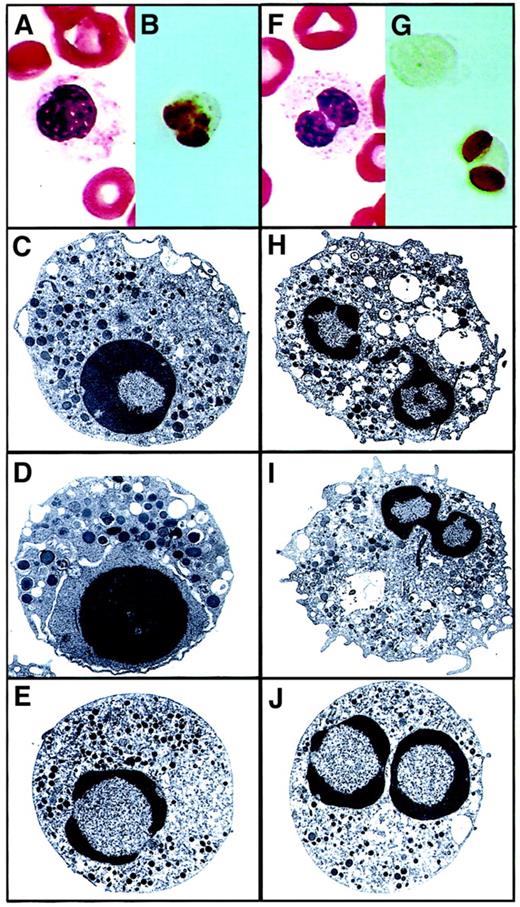
Pseudo Pelger-Huët anomaly forms.
Panels A through E depict the Stodtmeister, or unilobed, form. Early granulocyte with coarse chromatin segregated toward the periphery of the nucleus. (A) PB Giemsa from an MDS patient (magnification × 100). (B) ISEL showing a pseudo Pelger-Huët cell undergoing apoptosis (magnification × 100). (C-E) Electron micrographs showing the classical apoptotic features (uranyl acetate and lead citrate staining; original magnifications, × 6600, × 10 000, and × 6600, respectively). Panels F through J depict the bilobed form. Mature neutrophil showing bilobed nucleus with marked chromatin condensation and altered cytoplasm. (F) PB Giemsa from an MDS patient (magnification × 100). (G) ISEL showing a pseudo Pelger-Huët cell undergoing apoptosis (magnification × 100). (H- J) Electron micrographs showing the classical apoptotic features (uranyl acetate and lead citrate staining; original magnifications, × 8300, × 6600, and × 6600, respectively).
Pseudo Pelger-Huët anomaly forms.
Panels A through E depict the Stodtmeister, or unilobed, form. Early granulocyte with coarse chromatin segregated toward the periphery of the nucleus. (A) PB Giemsa from an MDS patient (magnification × 100). (B) ISEL showing a pseudo Pelger-Huët cell undergoing apoptosis (magnification × 100). (C-E) Electron micrographs showing the classical apoptotic features (uranyl acetate and lead citrate staining; original magnifications, × 6600, × 10 000, and × 6600, respectively). Panels F through J depict the bilobed form. Mature neutrophil showing bilobed nucleus with marked chromatin condensation and altered cytoplasm. (F) PB Giemsa from an MDS patient (magnification × 100). (G) ISEL showing a pseudo Pelger-Huët cell undergoing apoptosis (magnification × 100). (H- J) Electron micrographs showing the classical apoptotic features (uranyl acetate and lead citrate staining; original magnifications, × 8300, × 6600, and × 6600, respectively).
The above finding raises the question whether the pseudo Pelger-Huët cell is an anomaly or a manifestation of apoptosis. A normal neutrophil attains its matured multilobulated nuclear morphology subsequent to the mononuclear (pro/meta/myelocyte) and band-form stages. The mononuclear pseudo Pelger-Huët cells shown by different techniques in the figure thus may resemble an early myeloid cell undergoing apoptosis. Similarly, it is possible that the bilobulated pseudo Pelger-Huët cells may also be an apoptotic manifestation of the band cells or later stages prior to culminating in to multilobulated form. Pseudo Pelger-Huët cells thus may represent different stages of neutrophil differentiation undergoing apoptosis. The death of pseudo Pelger-Huët cells in the BM of MDS patients may be inflicted by the BM microenvironment that has been shown to have high amounts of proapoptotic cytokines such as tumor necrosis factor alpha (TNF-α) and interferon gamma (IFN-γ).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal