Natural interferon-α producing cells (IPCs) are a newly characterized blood cell type, which is the major source of type I interferons in antiviral innate immune responses. The relationship between the number of circulating IPCs, HIV disease progression, and the occurrence of HIV-related complications was investigated. The study of 25 healthy donors and 54 HIV-infected subjects demonstrated a direct correlation between blood IPC number, interferon-α production, and clinical state of HIV-infected subjects. Asymptomatic long-term survivors had increased IPC number and function relative to uninfected controls and infected individuals with progressive disease. IPC numbers were markedly reduced in AIDS patients developing opportunistic infections and cancer. A negative correlation was found between the IPC number in the blood and the HIV viral load, suggesting that IPCs are important in controlling HIV replication. This study provides the first evidence that IPCs are being affected during the course of HIV infection and suggests that these cells can play a vital role in the protection against opportunistic pathogens and cancer.
Introduction
A progressive reduction in CD4+T-helper lymphocytes is the main feature of HIV infection and leads to a depression in adaptive immunity.1 Innate immunity is also important in the host response to HIV infection and can be impaired during the course of this infection. Dendritic cells (DCs) can promote HIV transmission,2-5 and DC function6and number7 decline with HIV infection. The effector functions of monocytes and macrophages, including phagocytosis and intracellular oxidative responses, can be found decreased in HIV-infected subjects8,9 and in cultured cells in the presence of HIV.10-13 Superoxide production by neutrophils14 as well as natural killer cell function as measured by the lymphokine-activated killer activity and responsiveness to interferon-α (IFN-α)15 16 have been shown to be defective in HIV-infected subjects.
An important part of the innate defense against virus is the production of the type I IFNs, IFN-α, and IFN-β.17 IFN-α/β not only directly inhibit HIV replication,18-20 but also have important adjuvant effects on a variety of immune cell types, such as monocytes, natural killer cells,21 and T cells.22-26 The in vitro type I IFN production by total peripheral blood mononuclear cells (PBMCs) was shown to be impaired during the course of HIV infection, and this impairment was associated with the occurrence of opportunistic infections.27 28
CD4+CD11c− lineage marker− type 2 DC precursors (pre-DC2) were recently shown to be the natural IFN-α/β–producing cells in human blood.29,30 IPCs produce up to 1000 times more IFN-α than any other blood cell type in response to viral stimulation.29 Whether this impairment of IFN-α/β production in HIV-infected individuals is due to a functional defect or to a reduction in number of IPCs is not known.
In this study, we show that blood IPCs are severely decreased in AIDS patients but increased in asymptomatic long-term survivors (LTSs). The drop in IPC number and a decrease in their induced IFN production are associated with the presence of opportunistic infections and active Kaposi sarcoma. Our findings bring a new insight into the physiopathology of HIV infection and identify the IPC count as a new parameter to monitor the status of the immune system of HIV-infected subjects.
Patients, materials, and methods
HIV-infected subjects
Fifty-four HIV-infected subjects were recruited from 3 centers: the University of California at San Francisco (UCSF), the San Francisco General Hospital, and the Hospices Civils de Lyon, France. This study was approved by the Committee for Human Research, UCSF. Subjects were enrolled consecutively, and the only inclusion criterion was a confirmed HIV-positive serology and a written informed consent. The following conditions, which can nonspecifically affect blood cell counts, were used as exclusion criteria: previous cytotoxic chemotherapy, splenectomy, hypersplenism, and blood transfusion within the past 4 weeks. After inclusion, a full medical history was taken and physical examination was performed. The following biological parameters were obtained: IPC count, complete blood count (CBC) and differential, CD4+ and CD8+ T-cell counts, and HIV RNA level. HIV-infected subjects were classified into 3 clinical stages. The first stage was LTS and included subjects with persistent CD4+T-cell counts more than 400 cells/μL, no antiretroviral therapy, and no clinical sign of disease for at least 10 years. The second stage was progressors and included patients who were either (1) undergoing antiretroviral therapy and presently had no AIDS-defining condition or (2) had CD4+ T-cell counts less than 400 cells/μL and no AIDS-defining condition. For progressors receiving antiretroviral drugs, therapy was initiated when their CD4+ T-cell count dropped below 400 cells/μL. Three progressor patients were not treated despite CD4+ T-cell counts less than 400 cells/μL because of personal reasons or toxic side effects of the therapy. The third stage was AIDS and consisted of patients with an AIDS-defining condition according to the World Health Organization classification, including CD4+ T cells less than 200 cells/μL, present or previous opportunistic infections, or HIV-related neoplasms. The median age of the study population was 47 years (range: 34-61), and the majority of the subjects (93%) were male homosexuals. Other characteristics are summarized in Table1.
Characteristics of the 54 HIV-infected subjects
| Characteristic . | LTS . | Progressors . | AIDS . |
|---|---|---|---|
| Patients, n | 23 | 12 | 19 |
| Age, median (range) | 47 (35-59) | 47 (38-57) | 44.5 (34-61) |
| Ethnicity | |||
| Caucasian, n | 22 (96%) | 11 (92%) | 15 (79%) |
| Other, n | 1 (4%) | 1 (8%) | 4 (21%) |
| Medical history | |||
| Recurrent HSV, n | 2 (87%) | 3 (25%) | 3 (16%) |
| Chronic HBV, n | 0 | 3 (25%) | 1 (5%) |
| Chronic HCV, n | 0 | 2 (17%) | 2 (10%) |
| Risk group | |||
| Homosexual | 23 (100%) | 12 (100%) | 16 (85%) |
| Bisexual | 0 | 0 | 2 (10%) |
| IV drug use | 0 | 0 | 1 (5%) |
| Years from seroconversion | 14 (1-19) | 14 (2-20) | 10 (1-18) |
| Therapy | |||
| Antiretroviral | 0 | 9 (75%) | 12 (63%) |
| Acyclovir | 1 (4%) | 2 (17%) | 4 (21%) |
| Corticosteroids | 0 | 0 | 3 (16%) |
| IFN-α | 0 | 1 (8%) | 2 (10%) |
| Characteristic . | LTS . | Progressors . | AIDS . |
|---|---|---|---|
| Patients, n | 23 | 12 | 19 |
| Age, median (range) | 47 (35-59) | 47 (38-57) | 44.5 (34-61) |
| Ethnicity | |||
| Caucasian, n | 22 (96%) | 11 (92%) | 15 (79%) |
| Other, n | 1 (4%) | 1 (8%) | 4 (21%) |
| Medical history | |||
| Recurrent HSV, n | 2 (87%) | 3 (25%) | 3 (16%) |
| Chronic HBV, n | 0 | 3 (25%) | 1 (5%) |
| Chronic HCV, n | 0 | 2 (17%) | 2 (10%) |
| Risk group | |||
| Homosexual | 23 (100%) | 12 (100%) | 16 (85%) |
| Bisexual | 0 | 0 | 2 (10%) |
| IV drug use | 0 | 0 | 1 (5%) |
| Years from seroconversion | 14 (1-19) | 14 (2-20) | 10 (1-18) |
| Therapy | |||
| Antiretroviral | 0 | 9 (75%) | 12 (63%) |
| Acyclovir | 1 (4%) | 2 (17%) | 4 (21%) |
| Corticosteroids | 0 | 0 | 3 (16%) |
| IFN-α | 0 | 1 (8%) | 2 (10%) |
LTS, long-term survivors; HSV, herpes simplex virus; HBV, hepatitis B virus; HCV, hepatitis C virus; IV, intravenous; IFN, interferon.
Healthy controls
Blood donors from the Stanford Medical School Blood Center were used as healthy controls. They were randomly enrolled and then matched for age and gender with the subject population. Twenty-five subjects met the matching criteria. They were all male, with a median age of 45 years (range: 34-60).
Quantification of blood IPCs
Blood was drawn from each study subject in an EDTA- or heparin-coated tube and analyzed within 3 hours. PBMCs were obtained by Ficoll Hypaque separation, washed twice, and resuspended in phosphate-buffered saline containing 2% fetal calf serum and 0.5 mM EDTA. PBMCs (106) were stained for 25 minutes with the following monoclonal antibodies: CD4-PE, CD3, CD14, CD16, CD20, all fluorescein isothiocyanate (FITC) conjugated (Becton Dickinson, San Jose, CA), and CD11c-FITC (Caltag, South San Francisco, CA). Cells were fixed in 1% paraformaldehyde and a 2-color flow cytometric analysis (FACS) was performed. After setting the R1 gate on total viable PBMCs, 105 events were acquired through R1 and the percentage of CD4+FITC− IPCs was determined as the upper-left population by using the quadrant-stat function (Cellquest, Becton Dickinson, San Jose, CA) (Figure1A). The absolute number of blood IPCs was calculated by using the percentage of cells in relation to the lymphocyte and monocyte count as determined by the automated differential blood count. For each sample, the FACS analysis to determine the percentage of IPCs was performed blinded to the subject's clinical status. A second analysis was performed independently by another investigator and gave comparable results (data not shown).
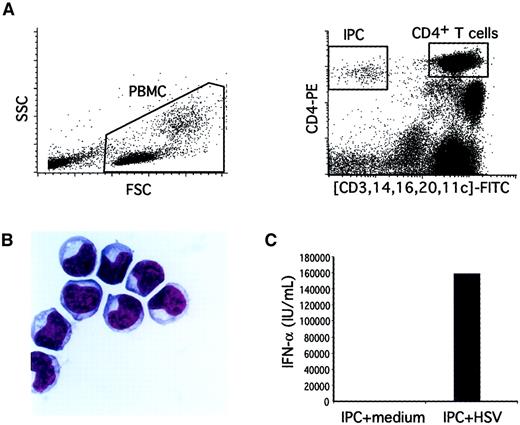
Validation of the flow cytometry method to quantify blood IPCs.
(A) Total viable PBMCs were gated based on their forward and side scatter (left panel). After a 2-color staining with anti–CD4-PE and (CD3, CD14, CD16, CD20, CD11c)-FITC, IPCs were identified as CD4+FITC− cells (right panel). CD4+ T cells were identified as CD4highFITChigh cells (right panel). For the IPC quantification, 105 PBMCs were analyzed and the percentage of cells was determined by using the quadrant-stat function (CellQuest). For purification of IPCs, CD4+FITC− cells were sorted. (B) Purified IPCs display a typical plasmacytoid morphology on cytospin preparation after Giemsa staining (×100). (C) Purified IPCs produce high amounts of type I IFN after 24 hours' stimulation with herpes simplex virus-1 as compared to cultures in medium alone.
Validation of the flow cytometry method to quantify blood IPCs.
(A) Total viable PBMCs were gated based on their forward and side scatter (left panel). After a 2-color staining with anti–CD4-PE and (CD3, CD14, CD16, CD20, CD11c)-FITC, IPCs were identified as CD4+FITC− cells (right panel). CD4+ T cells were identified as CD4highFITChigh cells (right panel). For the IPC quantification, 105 PBMCs were analyzed and the percentage of cells was determined by using the quadrant-stat function (CellQuest). For purification of IPCs, CD4+FITC− cells were sorted. (B) Purified IPCs display a typical plasmacytoid morphology on cytospin preparation after Giemsa staining (×100). (C) Purified IPCs produce high amounts of type I IFN after 24 hours' stimulation with herpes simplex virus-1 as compared to cultures in medium alone.
Giemsa staining of purified IPCs
The same staining procedure as for IPC quantification was used to sort the CD4+FITC− cells from undepleted PBMCs. Cytospins of the sorted cells were air dried, fixed in methanol, and stained with Giemsa for cytological analysis.
IFN-α production
The capacity of total PBMCs to produce IFN-α was assessed by culturing 2 × 105 freshly separated PBMCs with 1 plaque-forming unit (PFU)/cell herpes simplex virus type 1 (HSV-1; Kos strain, γ-irradiated; obtained from Robert Chase, Schering-Plough, Kenilworth, NJ) in 96-well round-bottom plates. For purified IPCs, cells were cultured at 4 × 104 cells/well with 10 PFU/cell of HSV-1. Culture supernatants were harvested after 24 hours and stored at −80°C until analyzed. IFN-α protein was measured by sandwich enzyme-linked immunosorbent analysis (Biosource, Camarillo, CA) in duplicate. Type-I IFN activity was measured by a bioassay that quantified the capacity of either the supernatant or an IFN-α standard (Schering-Plough) to protect A549 cells (American Type Culture Collection [ATCC], Manassas, VA) from the lysis induced by mouse encephalomyocarditis virus (ATCC). For this bioassay, each study sample was serially diluted. The viable A549 cells were quantified by using MTS staining (Promega, Madison, WI) and compared with an IFN-α standard curve.
HIV viral load
The HIV RNA level was measured in the serum from each subject sample, using the QUANTIPLEX bDNA method, version 3.0 (Bayer Diagnostics, Emeryville, CA). Results are expressed as the number of viral RNA copies/mL. The lower detection limit of the assay was 50 copies/mL. When statistical analysis was performed, values below 50 were assumed to be equivalent to 50 copies/mL.
Differential blood cell count
The complete differential blood cell count included red cell number, hemoglobin, total leukocytes, granulocytes, lymphocytes, monocytes, and platelets. The CBC for healthy donors was performed on a cell counter that had different specifications from the instruments used for the HIV-infected subjects. A correction factor was therefore calculated to adjust for instrument-related discrepancies.
Lymphocyte subpopulations
The CD4+ and CD8+ T-cell counts were obtained for the subject samples by CD3/CD4+ and CD3/CD8+ double staining of lysed whole blood followed by flow cytometric analysis after gating on the lymphocyte population. For the healthy donors, CD4+ T cells were quantified as the percentage of CD4highFITChigh cells on the IPC staining profile (Figure 1A). The 2 methods gave comparable results.
Statistical analysis
Univariate one-way analyses of variance were performed on the data. The dependent variables were IPC count, CD4+ cell number, HIV RNA level (log10), granulocyte, monocyte, and platelet counts. The group variable was HIV clinical stage. For each of these variables, all pairwise mean comparisons among HIV-infected groups were conducted by using stepdown Bonferroni tests. SAS statistical package (SAS Institute, Cary, NC) was used for all computations. The P values are based on comparisons of group means throughout the study.
Results
Validation of a simple method to quantify blood IPCs
Our previous method for isolating IPCs from human tonsils or blood involved 3-color immunofluorescence flow cytometry cell sorting.29,31 For clinical applications we modified this method to simplify quantification of IPCs in a small blood sample (2 mL). Undepleted PBMCs were directly analyzed for IPC number by 2-color immunofluorescence flow cytometry, consisting of anti–CD4-PE and a cocktail of FITC-conjugated antibodies to lineage markers (CD3, CD14, CD16, and CD20) and the DC marker CD11c (Figure 1A). To confirm that the CD4+lineage−CD11c− cells identified by this method were indeed IPCs, they were isolated by cell sorting and further characterized. First, the CD4+lineage−CD11c− cells displayed a typical phenotype (IL-3Rα++, CD45RA+, HLA-DR+) and size profile (between lymphocyte and monocyte) of IPCs by flow cytometry (not shown). Second, the CD4+lineage−CD11c− cells on cytospin preparations all had a plasmacytoid morphology by Giemsa staining (Figure 1B).29 30 Third, CD4+lineage−CD11c− cells produced high amounts of type-I IFN following stimulation with herpes simplex virus (Figure 1C) (see below).
To establish the range and distribution of IPC numbers in a control population, we analyzed 25 healthy blood donors who were age- and gender-matched with the study HIV-infected subjects. The median blood IPC count was 5.5 cells/μL (range: 2.15-16.0). The blood IPC numbers display a normal distribution on a logarithmic scale with an estimated mean of 6.28 cells/μL and an estimated SD of 3.29. Thus, the method described here is simple, highly specific for IPC quantification, and gives a normal distribution of IPC number in a healthy control population.
Blood IPCs are increased in asymptomatic LTSs but severely reduced in AIDS patients
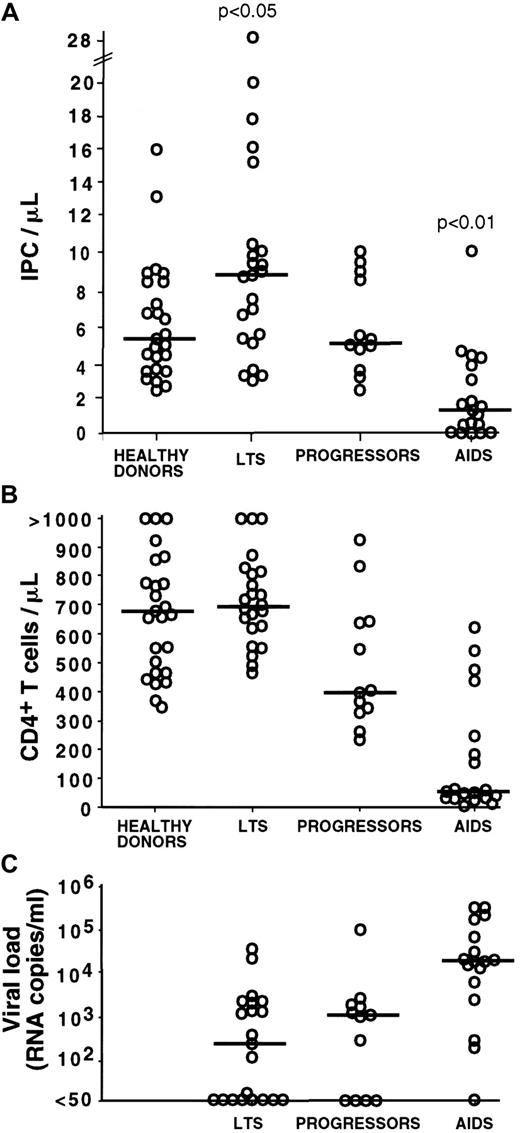
With the use of the above procedure, the blood IPC number was quantified in 54 HIV-infected subjects, including 23 LTSs, 12 progressors, and 19 AIDS patients (Figure2A). LTSs are HIV-infected subjects with normal CD4+ T-cell counts and no clinical sign of disease for at least 10 years following seroconversion. In this particular population, we found that the IPC count was higher (median: 8.89 cells/μL; range: 3.04-28.3) as compared to healthy controls (median: 5.5 cells/μL; range: 2.15-16.0) (Table2). The difference was statistically significant (P < .05). Strikingly, 3 LTS subjects had very high IPC counts of 28.28, 20.08, and 17.9 cells/μL, respectively (Figure 2A, Figure 5, subject 2). Such high values were not observed among the healthy donors, progressors, and AIDS subjects analyzed. In contrast to the IPC number, similar CD4+ T-cell counts were observed in healthy donors (median: 680 cells/μL; range 347-1926) and LTSs (median: 699 cells/μL; range 460-1350; P = .58) (Table 2 and Figure 2B).
Blood IPC numbers, CD4+ T-cell numbers, and HIV RNA levels by clinical subgroup.
Each open circle represents a value for a different study subject. Horizontal bars indicate the median. The P values are based on the comparison of the group means. (A) The number of blood IPCs is increased in LTSs (P < .05 for all group comparisons versus LTS) and decreased in AIDS patients (P < .01 for all group comparisons versus AIDS). Progressors and healthy donors have comparable IPC numbers (P > .05). Most of the progressors had received antiretroviral therapy for several months. No substantial difference in IPC number was observed between subjects who were treated and those who were untreated; therefore, all subjects were considered together in each clinical group. (B) CD4+ T-cell numbers are comparable in healthy donors and LTSs (P > .05) and follow a stage-specific decrease from LTSs to progressors and AIDS (P < .05 for the comparison of LTS versus progressors and LTS versus AIDS). (C) HIV viral load is comparable in LTSs and progressors (P > .05) and increased in AIDS subjects as compared with the 2 other groups (P < .05 for each of these comparisons).
Blood IPC numbers, CD4+ T-cell numbers, and HIV RNA levels by clinical subgroup.
Each open circle represents a value for a different study subject. Horizontal bars indicate the median. The P values are based on the comparison of the group means. (A) The number of blood IPCs is increased in LTSs (P < .05 for all group comparisons versus LTS) and decreased in AIDS patients (P < .01 for all group comparisons versus AIDS). Progressors and healthy donors have comparable IPC numbers (P > .05). Most of the progressors had received antiretroviral therapy for several months. No substantial difference in IPC number was observed between subjects who were treated and those who were untreated; therefore, all subjects were considered together in each clinical group. (B) CD4+ T-cell numbers are comparable in healthy donors and LTSs (P > .05) and follow a stage-specific decrease from LTSs to progressors and AIDS (P < .05 for the comparison of LTS versus progressors and LTS versus AIDS). (C) HIV viral load is comparable in LTSs and progressors (P > .05) and increased in AIDS subjects as compared with the 2 other groups (P < .05 for each of these comparisons).
Circulating IPC, CD4+, and CD8+T-cell numbers, and HIV RNA levels for each clinical subgroup
| Variable . | Healthy . | LTS . | Progressors . | AIDS . |
|---|---|---|---|---|
| IPC count (μL) | 5.5 (2.15-16) | 8.9 (3.04-8.3) | 5.4 (2.4-10.1) | 1.38 (0-10.8) |
| CD4 T cells (μL) | 680 (347-1 926) | 699 (460-1 350) | 400 (231-925) | 47 (12-619) |
| CD8 T cells (μL) | ND | 974 (222-3 623) | 909 (561-4 377) | 683 (147-1 798) |
| Viral load (copies/mL) | ND | 440 (< 50-62 961) | 1 145 (< 50-107 188) | 21 366 (< 50-500 000) |
| Variable . | Healthy . | LTS . | Progressors . | AIDS . |
|---|---|---|---|---|
| IPC count (μL) | 5.5 (2.15-16) | 8.9 (3.04-8.3) | 5.4 (2.4-10.1) | 1.38 (0-10.8) |
| CD4 T cells (μL) | 680 (347-1 926) | 699 (460-1 350) | 400 (231-925) | 47 (12-619) |
| CD8 T cells (μL) | ND | 974 (222-3 623) | 909 (561-4 377) | 683 (147-1 798) |
| Viral load (copies/mL) | ND | 440 (< 50-62 961) | 1 145 (< 50-107 188) | 21 366 (< 50-500 000) |
Results are indicated as median (range). IPC, type-1 interferon-producing cells; LTS, long-term survivors; ND, not determined.
The IPC count was comparable in progressors (median: 5.4 cells/μL; range: 2.4-10.09) to that noted in healthy controls (median: 5.5 cells/μL; range: 2.15-16; P = .77) (Table 2). The CD4+ T-cell count in progressors (median: 400 cells/μL; range: 231-925) was significantly lower than that of healthy controls (median: 680 cells/μL; range: 347-1926) (Figure 2B).
In the AIDS patients, the IPC count was found markedly decreased, with a median of 1.38 cells/μL (range: 0-10.8) (Figure 2A). It was significantly lower than in healthy donors, progressors, and LTSs (P < .001 for all pairwise comparisons) (Table 2). As expected, the AIDS group also had the lowest CD4+ T-cell count (median: 47 cells/μL; range 12-619; P < .001 for comparison of each group versus AIDS) (Figure 2B).
In the progressor and AIDS groups, 25% and 27% of the subjects, respectively, were not undergoing antiretroviral therapy. Because therapy could have an effect on IPC numbers, we performed a subgroup analysis to compare IPC numbers in subjects receiving or not receiving antiretroviral therapy. The mean IPC number was not statistically different between the treated and untreated subjects studied (6.75 and 5.11 IPC/μL, respectively, in progressors; 2.80 and 1.03 IPC/μL, respectively, in AIDS patients; P > .05). Nevertheless, the potential role of antiretroviral therapy on IPC number requires longitudinal studies that are in progress.
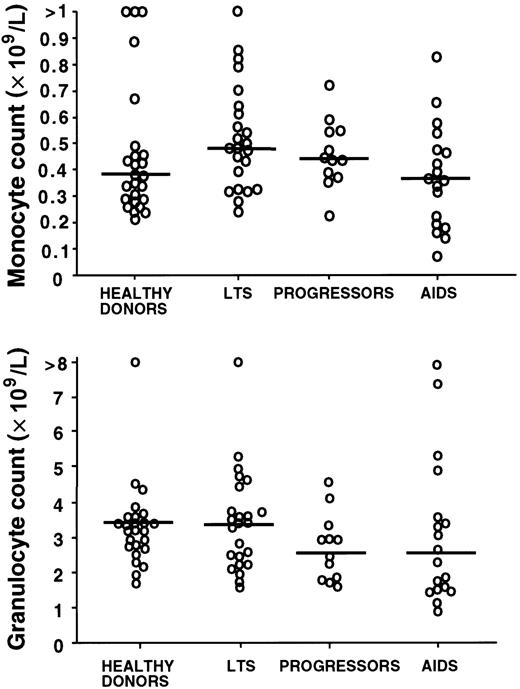
Reduction of blood IPC number in AIDS patients is not due to a generalized hematopoietic failure
To confirm that the stage-specific decrease in blood IPC number was not part of a generalized hematopoietic failure, we compared the complete differential blood counts in the clinical groups of subjects. The monocyte and granulocyte counts (Figure3) as well as platelet and hemoglobin counts (not shown) were not significantly different among the healthy controls and the 3 HIV-infected groups (P > .05).
Blood monocyte and granulocyte counts by clinical subgroup.
Blood monocyte and granulocyte counts are not significantly different in HIV-infected subjects (LTSs, progressors, and AIDS) as compared with healthy donors (P > .05 for all comparisons of the mean). Each open circle represents a value for a different study subject. Horizontal bars indicate the median.
Blood monocyte and granulocyte counts by clinical subgroup.
Blood monocyte and granulocyte counts are not significantly different in HIV-infected subjects (LTSs, progressors, and AIDS) as compared with healthy donors (P > .05 for all comparisons of the mean). Each open circle represents a value for a different study subject. Horizontal bars indicate the median.
Blood IPC count correlates negatively with the HIV viral load
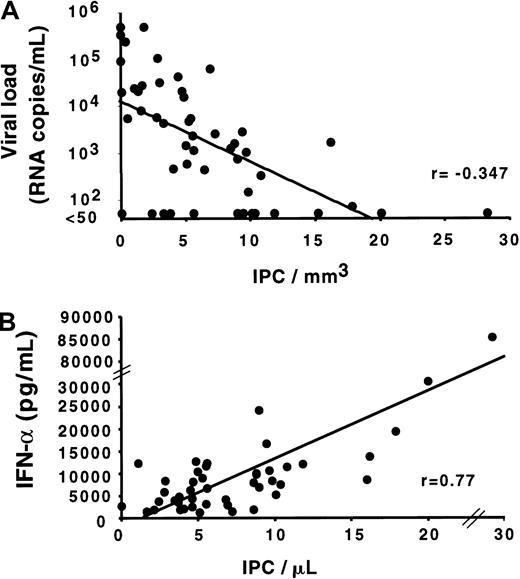
As shown by the quantification of IPCs in the different groups of HIV-infected subjects, IPCs are increased in the LTS group, which has the lower HIV viral load levels (median: 440 copies/mL; range: < 50-62 961), and are decreased in AIDS, where the higher viral loads are observed (median: 21 366 copies/mL; range: < 50-500 000;P < .01 for comparison of each group versus AIDS) (Figure2C). We therefore determined the relationship between IPC counts and HIV viral load in the 54 HIV-infected subjects (Figure4A). A statistically significant negative correlation was found between those 2 parameters (r = −0.347;P < .05), suggesting a role of IPCs in the control of HIV replication.
Correlation of the IPC number with HIV viral load and IFN-α production by PBMCs.
(A) Direct negative correlation between the blood IPC number and the HIV viral load in the plasma (correlation coefficient = −0.347;P < .05). For the convenience of the analysis, RNA levels less than 50 copies/mL were considered as 50 copies/mL. (B) Direct positive correlation of the blood IPC number with the capacity of PBMCs to produce IFN-alpha (correlation coefficient = 0.77;P < .01). Each dot represents a different study subject. Total PBMCs were cultured with HSV-1 the same day as the IPC quantification. Culture supernatants were harvested after 24 hours, frozen, and assayed later for IFN-alpha by enzyme-linked immunosorbent assay. Comparable results were obtained by using the IFN-α bioassay (data not shown).
Correlation of the IPC number with HIV viral load and IFN-α production by PBMCs.
(A) Direct negative correlation between the blood IPC number and the HIV viral load in the plasma (correlation coefficient = −0.347;P < .05). For the convenience of the analysis, RNA levels less than 50 copies/mL were considered as 50 copies/mL. (B) Direct positive correlation of the blood IPC number with the capacity of PBMCs to produce IFN-alpha (correlation coefficient = 0.77;P < .01). Each dot represents a different study subject. Total PBMCs were cultured with HSV-1 the same day as the IPC quantification. Culture supernatants were harvested after 24 hours, frozen, and assayed later for IFN-alpha by enzyme-linked immunosorbent assay. Comparable results were obtained by using the IFN-α bioassay (data not shown).
Because antiretroviral therapy can substantially affect the viral load independently of IPCs, we performed a separate analysis on the LTS group in which the subjects had not received antiretroviral therapy. LTS subjects with IPC counts greater than 10/μL had lower viral loads (median: 50 RNA copies/mL; range: 50-1632) as compared with LTS subjects with IPC counts less than 10/μL (median: 1357.5 RNA copies/mL; range: 50-62 961). The difference was statistically significant (P = .014), suggesting that IPCs could play a role in controlling HIV replication independent of any antiretroviral therapy.
Blood IPC count correlates positively with the capacity of PBMCs to produce IFN-α
Within the total 42 subjects studied for IFN-α production by PBMCs, a strong positive correlation was found between the IPC count and the in vitro IFN-α production by PBMCs in response to HSV (r = .77; P < .01) (Figure 4B). Importantly, subjects in the LTS group with very high IPC counts also had the highest IFN-α production in response to virus. This finding adds a functional basis to the high IPC counts observed in LTSs.
Reduction in blood IPC count is associated with the presence of opportunistic infections
Because of the key role of IPCs in innate immunity, we questioned whether the loss of this cell type was more specifically associated with the presence of opportunistic infections. Among the 19 patients in the AIDS group, 8 had an evolving opportunistic infection (4Pneumocystis carinii; 2 disseminated cytomegalovirus [CMV]; 1 CMV retinitis; and 1 progressive multifocal leukoencephalopathy) (Figure 5, subject 4). The IPC count of the AIDS patients with an opportunistic infection (median: 0.052/μL; range: 0-1.83) was lower than the IPC count of AIDS patients without opportunistic infection (median: 3.2/μL; range: 0.33-10.8). The difference was found highly significant (P < .01), suggesting that the decrease in circulating IPCs could be a predisposing factor for the development of infection by opportunistic pathogens.
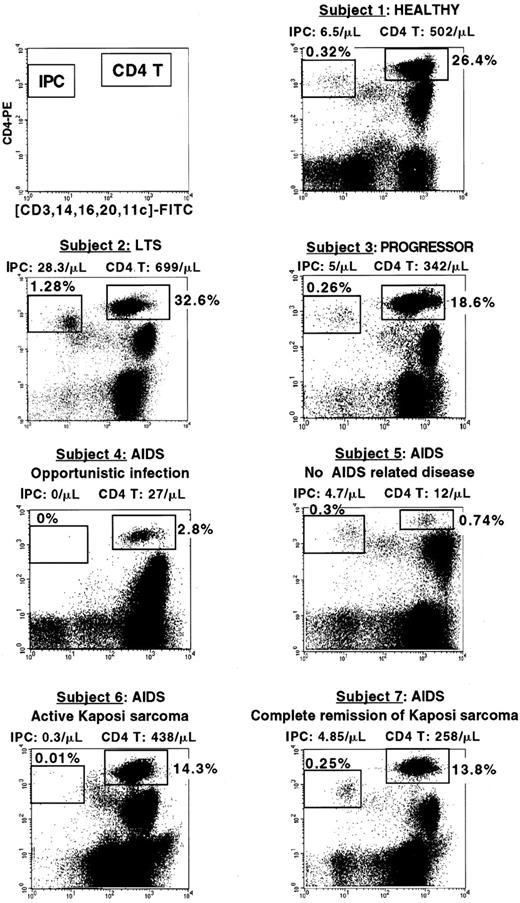
Percentage of IPCs and CD4+ T cells in HIV-infected subjects at different clinical stages.
The clinical status, IPC, and CD4+ T-cell count of each subject are indicated. Subject 1 (healthy) and subject 3 (progressor) have comparable percentage and number of IPCs (0.32%, 6.5 cells/μL versus 0.26%, 5 cells/μL) and T cells (26.4%, 502 cells/μL versus 18.6%, 342 cells/μL). Subject 2 (LTS) has a very high IPC percentage and number (1.28%, 28.8 cells/μL). Subject 4 (AIDS with opportunistic Pneumocystis carinii infection) has undetectable IPCs and very low CD4+ T cells (2.8%, 27 cells/μL). Subject 5 who was diagnosed as AIDS because of very low CD4+ T cells (0.74%, 12 cells/μL) has conserved IPCs (0.3%, 4.7 cells/μL) and no AIDS-related disease. Subject 6 (AIDS with active Kaposi sarcoma) has low IPCs (0.01%, 0.3 cells/μL) but normal CD4 T cells (14.3%, 438 cells/μL). Subject 7 (had a history of Kaposi sarcoma but had been in complete tumor remission for the past 7 years) has both conserved IPC number (0.25%, 4.85 cells/μL) and CD4+ T cells (13.8%, 258 cells/μL).
Percentage of IPCs and CD4+ T cells in HIV-infected subjects at different clinical stages.
The clinical status, IPC, and CD4+ T-cell count of each subject are indicated. Subject 1 (healthy) and subject 3 (progressor) have comparable percentage and number of IPCs (0.32%, 6.5 cells/μL versus 0.26%, 5 cells/μL) and T cells (26.4%, 502 cells/μL versus 18.6%, 342 cells/μL). Subject 2 (LTS) has a very high IPC percentage and number (1.28%, 28.8 cells/μL). Subject 4 (AIDS with opportunistic Pneumocystis carinii infection) has undetectable IPCs and very low CD4+ T cells (2.8%, 27 cells/μL). Subject 5 who was diagnosed as AIDS because of very low CD4+ T cells (0.74%, 12 cells/μL) has conserved IPCs (0.3%, 4.7 cells/μL) and no AIDS-related disease. Subject 6 (AIDS with active Kaposi sarcoma) has low IPCs (0.01%, 0.3 cells/μL) but normal CD4 T cells (14.3%, 438 cells/μL). Subject 7 (had a history of Kaposi sarcoma but had been in complete tumor remission for the past 7 years) has both conserved IPC number (0.25%, 4.85 cells/μL) and CD4+ T cells (13.8%, 258 cells/μL).
Next, we questioned whether a cut-off value with a clinical relevance could be identified to define a low IPC number. On the basis of the distribution of the blood IPC number in the healthy control population, a value of 2 cells/μL was selected. Healthy donors all had blood IPC numbers greater than 2 cells/μL. Among HIV-infected subjects, 13 had less than 2 cells/μL of IPCs and were all found within the AIDS group (total of 19 patients), confirming the validity of the cut-off value. Of these 13 patients, 8 had an evolving opportunistic infection (Figure5, subject 4), 3 had active Kaposi sarcoma (Figure 5, subject 6), and 2 had CD4+ cell counts less than 200 cells/μL (Table 3). Thus, 84.6% of the patients who had less than 2 cells/μL IPCs in their blood had either opportunistic infection or active Kaposi sarcoma. Importantly, whereas the 8 patients with opportunistic infection all had CD4+ T cells less than 100 cells/μL, the 3 patients with active Kaposi sarcoma had normal CD4+ T-cell counts (438, 547, and 482 cells/μL) (Table 3).
Association between low IPC counts (fewer than 2 IPCs/μL) and low CD4+ T-cell counts (fewer than 100/μL) with the presence of AIDS-related complications at the time of the study
| . | IPCs (/μL) . | CD4 T cells (/μL) . | ||
|---|---|---|---|---|
| Fewer than 2 . | More than 2 . | Fewer than 100 . | More than 100 . | |
| Opportunistic infection | 8 | 0 | 8 | 0 |
| Kaposi sarcoma | 3 | 3 | 0 | 6 |
| No AIDS-defining disease | 2 | 38 | 4 | 36 |
| . | IPCs (/μL) . | CD4 T cells (/μL) . | ||
|---|---|---|---|---|
| Fewer than 2 . | More than 2 . | Fewer than 100 . | More than 100 . | |
| Opportunistic infection | 8 | 0 | 8 | 0 |
| Kaposi sarcoma | 3 | 3 | 0 | 6 |
| No AIDS-defining disease | 2 | 38 | 4 | 36 |
Good clinical condition means no current clinical abnormalities. IPC, type-1 interferon-producing cells.
Among the remaining 41 HIV-infected subjects who had an IPC number of more than 2 cells/μL, no opportunistic infection was observed (Table3). Three of these subjects, who were included in the AIDS group because of the previous history of Kaposi sarcoma, had normal IPC counts (10.8, 4.85, and 4.81 cells/μL) and were in complete remission of the Kaposi sarcoma for the last 5, 6, and 7 years (Figure 5, subject 7). Two other subjects had CD4+ T-cell counts less than 100 cells/μL (47 and 12 cells/μL) and yet did not develop AIDS-related complications. They both had blood IPCs greater than 2 cells/μL (3.2 and 4.7 cells/μL) (Figure 5, subject 5). One was in good clinical condition. The other was admitted for lung infection with a strong suspicion of Pneumocystis carinii infection because of the low CD4+ T-cell count (47 cells/μL). He was finally diagnosed with a bacterial infection caused by Pneumococcus pneumoniae, not an AIDS-defining disease.
From our series, an IPC count less than 2 cells/μL is highly specific for patients with AIDS. Longitudinal studies are now needed to define the predictive value of such a cut-off for the development of HIV-related complications.
Discussion
CD4+ T-helper lymphocytes are the major cells directly affected by HIV and are routinely used to monitor HIV-infected subjects. IPCs share 2 important characteristics with CD4+T cells: (1) they express high levels of surface CD431 and (2) they can be found in blood, thymus, and secondary lymphoid organs32 where HIV is able to replicate actively.33 Moreover, they constitutively express the chemokine receptors CCR5 and CXCR4 (H Kanzler, unpublished data, 1999) that are the main coreceptors for HIV infection of a cell.34 These characteristics make IPC a potential target for HIV. In this study, using a rapid flow cytometric method (Figure 1), we show that the number of circulating IPCs can be used to monitor the immune system of HIV-infected subjects. The IPCs in the blood are markedly reduced in AIDS patients but increased in asymptomatic LTSs (Figure 2). The number of circulating IPCs correlates negatively with the HIV viral load and a decrease in IPC numbers is associated with the presence of opportunistic infections (Figure 2, Table 3).
LTSs (or long-term nonprogressors) form a particular group of HIV-infected subjects who remain asymptomatic for many years without any biological sign of disease.35,36 Several factors, both host and virus related, have been implicated in the resistance to disease progression. These include CCR537 and CCR5 promoter38 genotypes, heterozygosity (HLA class I loci),39 the presence of the HLA B*5701 allele,40 and CD8+ cytotoxic41,42and noncytotoxic cellular immune responses43-45 Besides the known antiviral and adjuvant effects of type I IFN,17,21,23,24,26,46 these cytokines inhibit HIV infection.18-20 47 In this study, we show that (1) the circulating IPC number and their function are increased in LTSs and (2) LTSs with a high IPC number have significantly lower viral loads than those with a low IPC number (Figures 2,4). These findings strongly suggest that IPCs and the type I IFN response represent a key factor for the control of HIV replication in this particular population.
In AIDS patients, a low CD4+ T-cell count, reflecting impaired adaptive immunity, is thought to be the main factor favoring infection with opportunistic pathogens. In the present study, all the AIDS patients with opportunistic infections have a severe reduction of both blood IPCs and CD4+ T cell (Table 3). This observation is in accordance with early reports suggesting that concomitant impairment of the innate and adaptive immune system is responsible for the development of opportunistic infections.27 28 However, the IPC count and CD4+ T-cell count were found to be different in 3 situations. First, asymptomatic LTSs had higher IPC numbers than normal donors (P < .05) but similar CD4+ T-cell counts (Figure 2 and Table 2). Second, 3 HIV-infected subjects who had active Kaposi sarcoma were found to have normal CD4+ T-cell counts but a depletion of IPCs (< 2/μL) (Figure 5). Third, 2 HIV-infected subjects who were classified as having AIDS because of a marked depletion of CD4+ T cells were found to have normal IPC counts (> 2/μL) and were in a healthy clinical condition (Table 2and Figures 2,5). These 2 last cases represent rare individuals, and the results indicate important exceptions that merit further studies. Thus, the monitoring of both CD4+ cells and IPCs enable a more accurate evaluation of the immune system in HIV infection.
The fact that IPC loss is associated with the occurrence of various types of opportunistic infections suggests that the function of IPCs is not restricted to antiviral immunity. In our series of patients with IPC counts less than 2 cells/μL, 3 had active CMV infection, one had progressive multifocal leukoencephalopathy, and 4 hadPneumocystis carinii infection. These clinical observations fit with recent results (N Kadowaki, unpublished data, 2001) showing that nonviral stimuli, such as gram-positive bacteria and mycobacteria, can strongly induce IPCs to produce IFN-α in vitro. The results suggest that type I IFN responses can be crucial in other types of infection besides those caused by viruses.
The identification of IPCs as being severely affected during HIV infection brings a new insight into the pathophysiology of HIV infection and could have important diagnostic and therapeutic implications. The combination of the IPCs with the CD4+T-cell count should provide an optimal means to evaluate the immune function and to predict the occurrence of AIDS-related complications. Cell therapy or drugs increasing the number and/or function of IPCs48 49 could become an option in the future to control both HIV replication and HIV-related clinical conditions.
The authors thank Dr Robert Chase (Schering-Plough Research Institute, Kenilworth, NJ) for providing HSV-1; Dr Edgar Engelman and Karin Kealoha (Stanford Medical School Blood Center) for assistance with the study of healthy blood donors; and Michel Gilliet, Holger Kanzler, Franck Barrat, and Bernhard Homey for helpful suggestions and critical reading of the manuscript.
Supported in part by a fellowship from the Association pour la Recherche contre le Cancer (ARC) (to V.S.) and supported by a grant from the National Institutes of Health, UCSF-Gladstone Institute of Virology & Immunology Center for AIDS Research, P30 MH59037 (to L.H.).
V.S. and I.S. contributed equally to this work.
Correspondence:Jay A. Levy, University of California, School of Medicine, San Francisco, CA 94143-1270; e-mail:jalevy@itsa.ucsf.edu; or Yong-Jun Liu, DNAX, 901 California Ave, Palo Alto, CA 94304; e-mail: yong-jun.liu@dnax.org.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal