Many patients receiving dose-intensive chemotherapy acquire thrombocytopenia and need platelet transfusions. A study was conducted to determine whether platelets harvested from healthy donors treated with thrombopoietin could provide larger increases in platelet counts and thereby delay time to next platelet transfusion compared to routinely available platelets given to thrombocytopenic patients. Community platelet donors received either 1 or 3 μg/kg pegylated recombinant human megakaryocyte growth and development factor (PEG-rHuMGDF) or placebo and then donated platelets 10 to 15 days later. One hundred sixty-six of these platelet concentrates were then transfused to 120 patients with platelets counts 25 × 109/L or lower. Pretransfusion platelet counts (11 × 109/L) were similar for recipients of placebo-derived and PEG-rHuMGDF–derived platelets. Early after transfusion, the median platelet count increment was higher in patients receiving PEG-rHuMGDF–derived platelets: 19 (range, −12-66) × 109/L, 41 (range, 5-133) × 109/L, and 82 (range, −4-188) × 109/L for placebo-, 1-μg/kg–, and 3-μ/kg–derived platelets, respectively. This difference was maintained 18 to 24 hours after transfusion. Transfusion-free intervals were 1.72, 2.64, and 3.80 days for the recipients of the placebo-, 1-μg/kg–, and 3-μ/kg–derived platelets, respectively. The rate of transfusion-related adverse events was not different in recipients of placebo-derived and PEG-rHuMGDF–derived platelets. Therefore, when transfused into patients with thrombocytopenia, platelets collected from healthy donors undergoing thrombopoietin therapy were safe and resulted in significantly greater platelet count increments and longer transfusion-free intervals than platelets obtained from donors treated with placebo.
Introduction
Platelet transfusions are increasing more rapidly than the transfusion of other components. Single-donor apheresis concentrates are used preferentially,1 driven in part by increasing needs from stem cell transplantation programs and by interest in minimizing allogeneic donor exposure for patients with thrombocytopenia.2 Today 50% to 80% of patients with leukemia or receiving stem cell transplantation are treated with apheresis platelets.3 4
Although hematopoietic growth factors were initially administered only to patients, these growth factors have been administered recently to healthy donors who have become an important resource for transfusion medicine support in stem cell transplantation. Erythropoietin has been used to stimulate red cell production for use by healthy allogeneic donors undergoing platelet apheresis to support transplant recipients.5 Granulocyte colony-stimulating factor (G-CSF) is administered to allogeneic stem cell donors6-8 and now to donors of granulocytes.9,10 The recent identification of the primary regulator of megakaryopoiesis, thrombopoietin, as the hematopoietic growth factor responsible for the regulation of platelet production,11,12 along with the demonstration of its biologic activity in persons with normal marrow reserve,13make the use of this hematopoietic growth factor a potential advance in the procurement of single donor platelet concentrates.
In a companion study, we determined the dose-response effect of the recombinant thrombopoietin, poly (ethylene glycol)-conjugated recombinant megakaryocyte growth and development factor (PEG-rHuMGDF), on donor platelet count and yield of apheresis platelets collected from healthy platelet donors (see accompanying article by Kuter et al,14 page 1339). We report here the safety and efficacy of PEG-rHuMGDF–derived platelet apheresis concentrates that were transfused prophylactically to patients with platelets counts lower than 25 × 109/L.
Patients, materials, and methods
Patients
The institutional review boards of the 5 participating centers approved this study, and all patients gave written, informed consent before study entry in accordance with the Helsinki protocol. Patients were eligible for transfusion of study platelets if they were 18 years of age or older, had chemotherapy-induced thrombocytopenia (platelets counts lower than 25 × 109/L), were to receive a prophylactic platelet transfusion, and had not received another platelet transfusion within the previous 24 hours. Patients were excluded if they were transfused because of bleeding; recovering from or were about to undergo surgery; had clinical evidence of a platelet consumptive disorder or splenomegaly; received antiplatelet agents or anticoagulation therapy within 24 hours; or were enrolled in another clinical trial of experimental drug therapy within the previous 4 weeks. No restrictions were placed on prior transfusion history, number or types of prior transfusions, type of malignancy, type of anticancer treatment, bone marrow transplantation or peripheral blood cell transplantation, refractoriness to platelet transfusions, or presence of HLA antibodies. The decision to transfuse was made by the patient's attending physician, based on platelet transfusion guidelines used routinely in each of the participating institutions.
Platelet components
One hundred sixty-six platelet concentrates were collected and transfused from healthy community platelet apheresis donors who met all the American Association of Blood Banks (AABB) and Food and Drug Administration (FDA) criteria for platelet donation. Study drug administration and platelet apheresis procedures are detailed in a companion paper14 that describes the collection of the first 110 apheresis products. Another 56 products were collected at the 3-μg/kg dose in an extension of that study, and this report describes the transfusion of all 166 apheresis products. Briefly, platelets were collected by apheresis 10 to 15 days after the administration of one subcutaneous dose of blinded study drug (placebo, 1 μg/kg or 3 μg/kg PEG-rHuMGDF). Apheresis procedures were performed using a 3.6 Spectra (COBE, Lakewood, CO) with or without an in-line leukocyte-reduction system, with a target to process 85% of the donor's calculated blood volume. All components underwent testing and release procedures that met AABB and FDA requirements and were stored at 20°C to 24°C on oscillating racks in standard platelet storage bags (COBE). A sample of the harvested platelets was obtained for platelet counting. Individual collections were aliquoted (by sterile methods) into 1, 2, or 3 bags, with no more than 6 × 1011 platelets placed in each bag, and the bags were retained together as a single transfusion dose. Platelets within the study drug or placebo cohorts were assigned to eligible patients based on out date on a first-in, first-out basis, ABO system and Rh specificity, and cytomegalovirus requirements. Patients were premedicated with 650 mg acetaminophen by mouth and 25 mg diphenhydramine by mouth within 30 minutes of platelet transfusion, at the discretion of the study institution.
Study design
This double-blinded study was designed to determine the safety and efficacy of the transfusion of platelet apheresis concentrates from healthy donors treated with PEG-rHuMGDF or placebo. Primary safety endpoints were the incidence and number of transfusion reactions. Transfusion reactions were defined according to established criteria present at each study site. Patients were monitored for hemorrhagic events with a daily hemostasis assessment while in the study. Primary efficacy endpoints were the absolute platelet increments and the corrected count increments obtained early (1-4 hours) and late (18-24 hours) after transfusion. Time to next platelet transfusion was also monitored. Patients were considered off the study when any of the following occurred: a subsequent platelet transfusion was given, the patient was discharged from the hospital, or 7 days elapsed since the last transfusion. Patients were allowed to be re-enrolled and to receive a subsequent study platelet transfusion if they were off-study (that is, patients gave informed consent again and were re-entered as study participants). Physicians and staff caring for the patients did not know whether platelets were collected from donors who had received PEG-rHuMGDF or donors who had received placebo.
Study drug
Recombinant human megakaryocyte growth and development factor (rHuMGDF; Amgen Inc, Thousand Oaks, CA) is a human thrombopoietin produced in Escherichia coli into which a plasmid containing the gene for a truncated form of thrombopoietin has been inserted. The rHuMGDF produced has an amino acid sequence identical to the first 163 amino acids of native human thrombopoietin and contains the entire receptor-binding domain. An approximately 20 000-d molecular weight polyethylene glycol molecule is covalently attached to the amino terminus of the protein to produce PEG-rHuMGDF.15
PEG-rHuMGDF is formulated as a sterile, clear, preservative-free liquid. Placebo and study drug (1 μg/kg or 3 μg/kg) were packaged in identical vials. Investigators, donors, patients, study staff, and study monitors were blinded to study drug assignment.
Statistical analysis
Transfusion of each study product was treated as an independent transfusion event. The absolute increment in platelet count was calculated as the posttransfusion platelet count minus the pretransfusion count. Corrected count increment (CCI) was calculated as the absolute increment times the body surface area per the number of platelets transfused (platelets/L × BSA m2/1011 platelets, where BSA is body surface area).
Results
Patients
One hundred twenty patients with chemotherapy-induced thrombocytopenia (platelets counts 25 × 109/L or lower) were consented and enrolled in the study. Demographic characteristics of the patients are detailed in Table 1. Gender, age, race distribution, body surface area, type of tumor (solid vs hematologic malignancy), and platelet count at study entry were similar for the 3 groups of patients.
Demographic characteristics of the 120 study patients
| . | Placebo (n = 59) . | PEG-rHuMGDF 1 μg/kg (n = 15) . | PEG-rHuMGDF 3 μg/kg (n = 46) . | PEG-rHuMGDF combined (n = 61) . |
|---|---|---|---|---|
| Age | ||||
| Median (range) | 46 (18-84) | 45 (20-61) | 52 (18-79) | 51 (18-79) |
| Gender | ||||
| Male (%) | 24 (41) | 9 (60) | 21 (46) | 30 (49) |
| Female (%) | 35 (59) | 6 (40) | 25 (54) | 31 (51) |
| Race | ||||
| White (%) | 54 (92) | 13 (87) | 46 (100) | 59 (97) |
| Body surface area (m2) | ||||
| Median (range) | 1.8 (1.5-2.5) | 2.0 (1.2-2.2) | 1.8 (1.5-2.4) | 1.9 (1.2-2.4) |
| Diagnosis | ||||
| Breast cancer (%) | 10 (17) | 2 (13) | 9 (20) | 11 (18) |
| Leukemia | ||||
| Acute (%) | 15 (25) | 3 (20) | 15 (34) | 18 (30) |
| Chronic (%) | 8 (14) | 0 | 6 (13) | 6 (10) |
| Lymphoma (%) | 11 (18) | 7 (47) | 6 (13) | 13 (21) |
| Study entry platelet | ||||
| count (× 109/L) | ||||
| Median (range) | 11 (2-25) | 12 (4-20) | 11 (11-22) | 11 (1-22) |
| . | Placebo (n = 59) . | PEG-rHuMGDF 1 μg/kg (n = 15) . | PEG-rHuMGDF 3 μg/kg (n = 46) . | PEG-rHuMGDF combined (n = 61) . |
|---|---|---|---|---|
| Age | ||||
| Median (range) | 46 (18-84) | 45 (20-61) | 52 (18-79) | 51 (18-79) |
| Gender | ||||
| Male (%) | 24 (41) | 9 (60) | 21 (46) | 30 (49) |
| Female (%) | 35 (59) | 6 (40) | 25 (54) | 31 (51) |
| Race | ||||
| White (%) | 54 (92) | 13 (87) | 46 (100) | 59 (97) |
| Body surface area (m2) | ||||
| Median (range) | 1.8 (1.5-2.5) | 2.0 (1.2-2.2) | 1.8 (1.5-2.4) | 1.9 (1.2-2.4) |
| Diagnosis | ||||
| Breast cancer (%) | 10 (17) | 2 (13) | 9 (20) | 11 (18) |
| Leukemia | ||||
| Acute (%) | 15 (25) | 3 (20) | 15 (34) | 18 (30) |
| Chronic (%) | 8 (14) | 0 | 6 (13) | 6 (10) |
| Lymphoma (%) | 11 (18) | 7 (47) | 6 (13) | 13 (21) |
| Study entry platelet | ||||
| count (× 109/L) | ||||
| Median (range) | 11 (2-25) | 12 (4-20) | 11 (11-22) | 11 (1-22) |
Platelet products
One hundred sixty-six apheresis components were transfused into 120 patients with thrombocytopenia. Eighty-three platelet concentrates were collected from placebo-treated donors, and 18 or 65 products were collected from donors administered either 1 μg/kg or 3 μg/kg PEG-rHuMGDF, respectively. Platelet product characteristics are detailed in Table 2. Median storage time (ie, interval from platelet apheresis completion to transfusion) was similar (2.1 to 2.9 days) in each group. Platelet concentrates (measured at the time of collection) contained a median of 3.4 × 1011, 5.7 × 1011, and 11.0 × 1011 platelets for the placebo, 1-μg/kg, and 3-μg/kg patient groups, respectively (P < .05). Median volumes of the platelet concentrates were 280, 403, and 565 mL, respectively (P < .001). Pretransfusion centrifugation to reduce transfused plasma volume was performed on platelet concentrates at one institution, according to the policy that limited plasma volume for ABO-incompatible units.
Platelet product characteristics
| . | Placebo (n = 83) . | PEG-rHuMGDF 1 μg/kg (n = 18) . | PEG-rHuMGDF 3 μg/kg (n = 65) . | PEG-rHuMGDF combined (n = 83) . |
|---|---|---|---|---|
| No. platelets collected (× 1011) | 3.4 (2.1-7.9) | 5.7 (3-13) | 11 (4.5-21) | 9.4 (3-21) |
| Plasma volume collected (mL) | 280 (183-513) | 403 (227-737) | 565 (279-722) | 524 (227-737) |
| Platelet concentration (× 109/mL) | 12 (7.7-20) | 14 (12-23) | 19.5 (13-37) | 18.4 (12-37) |
| Storage duration (d) | 2.5 (0.7-5.3) | 2.9 (1.1-5.3) | 2.1 (0.9-5.4) | 2.2 (0.9-5.4) |
| . | Placebo (n = 83) . | PEG-rHuMGDF 1 μg/kg (n = 18) . | PEG-rHuMGDF 3 μg/kg (n = 65) . | PEG-rHuMGDF combined (n = 83) . |
|---|---|---|---|---|
| No. platelets collected (× 1011) | 3.4 (2.1-7.9) | 5.7 (3-13) | 11 (4.5-21) | 9.4 (3-21) |
| Plasma volume collected (mL) | 280 (183-513) | 403 (227-737) | 565 (279-722) | 524 (227-737) |
| Platelet concentration (× 109/mL) | 12 (7.7-20) | 14 (12-23) | 19.5 (13-37) | 18.4 (12-37) |
| Storage duration (d) | 2.5 (0.7-5.3) | 2.9 (1.1-5.3) | 2.1 (0.9-5.4) | 2.2 (0.9-5.4) |
Ranges appear in parentheses.
Safety
Transfusion-associated adverse events.
Administration of platelet apheresis concentrates to patients with platelets counts equaling or lower than 25 × 109/L was associated with a low rate of transfusion-related events. Of the 166 transfusions, only 21 (12.6%) were associated with febrile transfusion reaction, and there was no difference between recipients of placebo-derived (7 of 83 [8.4%]) and PEG-rHuMGDF–derived (14 of 83 [16.9%]) platelets, respectively (P = .16). Similarly, 3 of 59 (5.1%) patients receiving placebo-derived platelets had afebrile transfusion reactions, similar to those of 6 of 61 (9.8%) patients who received PEG-rHuMGDF–derived platelets (P = .49).
Other adverse events.
The most frequent adverse events unrelated to transfusions were consistent with events that routinely occur in oncology patients and were similar among patient cohorts (data not shown). No recipient in either group reported serious adverse events, and most events were mild to moderate. Adverse events with more than a 5% difference between the treatment cohorts included increased stomatitis, oral pain, and facial flushing in recipients of PEG-rHuMGDF–derived platelets, but these were not considered related to the platelet transfusions and were not related to the dose of PEG-rHuMGDF.
Efficacy
Platelet count increments.
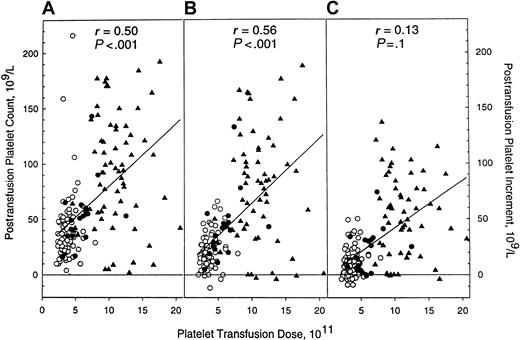
Hematologic evaluations before and after 166 platelet transfusions are detailed in Table 3. Hematocrit, white blood cell, and baseline platelet counts were similar among the 3 groups at the time of platelet transfusion. Absolute platelet counts and platelet increments, both early (1-4 hours) and late (18-24 hours), were greater in patients who received PEG-rHuMGDF–derived platelets (P < .001 for all comparisons vs placebo). Relationships between platelet transfusion dose, maximum posttransfusion platelet count, and posttransfusion platelet count increments (early and late) are illustrated in Figure 1. There was a statistically significant correlation between platelet transfusion dose, maximum posttransfusion platelet count, and early posttransfusion platelet—but not late posttransfusion platelet—increment.
Hematology values
| . | Placebo (n = 83) . | PEG-rHuMGDF 1 μg/kg (n = 18) . | PEG-rHuMGDF 3 μg/kg (n = 65) . | PEG-rHuMGDF combined (n = 83) . |
|---|---|---|---|---|
| Before transfusion | ||||
| Platelet count (× 109/L) | 11 (2-33) | 11 (4-20) | 11 (1-43) | 11 (1-43) |
| WBC (× 109/L) | 0.4 (0.1-44) | 0.8 (0.2-11) | 0.6 (0.05-40) | 0.6 (0.05-40) |
| Hematocrit (%) | 26 (17-35) | 26 (14-32) | 24 (20-33) | 25 (14-33) |
| Posttransfusion absolute platelet count increment (× 109/L) | ||||
| Early (1-4 h) | 19 (−12-66) | 41 (5-133) | 82 (−4-188) | 61 (−4-188) |
| Late (18-24 h) | 8 (−9-50) | 25 (−1-74) | 52 (−4-137) | 41 (−4-137) |
| Posttransfusion platelet count (× 109/L) | ||||
| Maximum | 32 (4-216) | 52 (16-143) | 93 (2-192) | 77 (2-192) |
| Early (1-4 h) | 31 (3-76) | 52 (14-143) | 92 (2-192) | 74 (2-192) |
| More than 50 × 109/L (%)3-150 | 18 | 56 | 77 | 72 |
| More than 75 × 109/L (%)3-150 | 1 | 11 | 60 | 49 |
| Late (18-24 h) | 22 (4-66) | 35 (10-84) | 66 (1-148) | 52 (1-148) |
| . | Placebo (n = 83) . | PEG-rHuMGDF 1 μg/kg (n = 18) . | PEG-rHuMGDF 3 μg/kg (n = 65) . | PEG-rHuMGDF combined (n = 83) . |
|---|---|---|---|---|
| Before transfusion | ||||
| Platelet count (× 109/L) | 11 (2-33) | 11 (4-20) | 11 (1-43) | 11 (1-43) |
| WBC (× 109/L) | 0.4 (0.1-44) | 0.8 (0.2-11) | 0.6 (0.05-40) | 0.6 (0.05-40) |
| Hematocrit (%) | 26 (17-35) | 26 (14-32) | 24 (20-33) | 25 (14-33) |
| Posttransfusion absolute platelet count increment (× 109/L) | ||||
| Early (1-4 h) | 19 (−12-66) | 41 (5-133) | 82 (−4-188) | 61 (−4-188) |
| Late (18-24 h) | 8 (−9-50) | 25 (−1-74) | 52 (−4-137) | 41 (−4-137) |
| Posttransfusion platelet count (× 109/L) | ||||
| Maximum | 32 (4-216) | 52 (16-143) | 93 (2-192) | 77 (2-192) |
| Early (1-4 h) | 31 (3-76) | 52 (14-143) | 92 (2-192) | 74 (2-192) |
| More than 50 × 109/L (%)3-150 | 18 | 56 | 77 | 72 |
| More than 75 × 109/L (%)3-150 | 1 | 11 | 60 | 49 |
| Late (18-24 h) | 22 (4-66) | 35 (10-84) | 66 (1-148) | 52 (1-148) |
Ranges appear in parentheses.
WBC indicates white blood cell count.
Transfusion percentages when posttransfusion platelet counts exceeded indicated values.
Posttransfusion platelet counts are directly related to the platelet dose transfused.
Relationship of recipient platelet counts to platelet dose after the transfusion of apheresis platelet products collected from donors administered either placebo (○) or PEG-rHuMGDF (1 μg/kg, ●; 3 μg/kg, ▴). (A) Maximum posttransfusion platelet count. (B) Early (1-4 hours) posttransfusion platelet count increment. (C) Late (18-24 hours) posttransfusion platelet count increment.
Posttransfusion platelet counts are directly related to the platelet dose transfused.
Relationship of recipient platelet counts to platelet dose after the transfusion of apheresis platelet products collected from donors administered either placebo (○) or PEG-rHuMGDF (1 μg/kg, ●; 3 μg/kg, ▴). (A) Maximum posttransfusion platelet count. (B) Early (1-4 hours) posttransfusion platelet count increment. (C) Late (18-24 hours) posttransfusion platelet count increment.
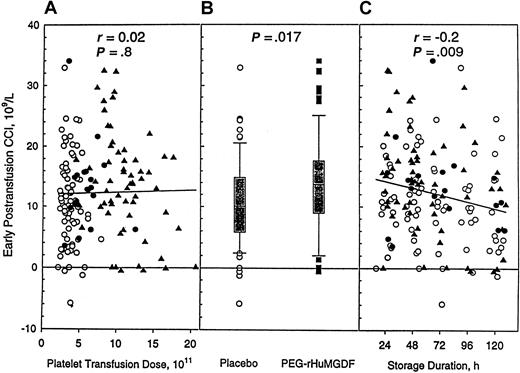
The CCI adjusts the posttransfusion platelet count for platelet dose and recipient body size, and no difference was seen in recipient cohorts when analyzed by platelet dose (Figure2A). It did reach statistical significance when placebo and the combined PEG-rHuMGDF groups were compared (Figure 2B). These results indicate that PEG-rHuMGDF–derived platelets demonstrate in vivo recoveries that are at least equivalent, and possibly superior, to control platelets after transfusion. When analyzed by storage time, the CCI did not differ among treatment groups (Figure 2C).
Recipient CCI is related to donor treatment and platelet storage duration but not to transfusion dose.
Recipient-corrected count increments (early, 1-4 hours after transfusion) after transfusion of apheresis platelet products. (A) Relationship to platelet dose. (B) Relationship to donor study drug. (C) Relationship to platelet product storage duration. Symbols for panels A and C are as described in Figure 1.
Recipient CCI is related to donor treatment and platelet storage duration but not to transfusion dose.
Recipient-corrected count increments (early, 1-4 hours after transfusion) after transfusion of apheresis platelet products. (A) Relationship to platelet dose. (B) Relationship to donor study drug. (C) Relationship to platelet product storage duration. Symbols for panels A and C are as described in Figure 1.
Time to next prophylactic platelet transfusion.
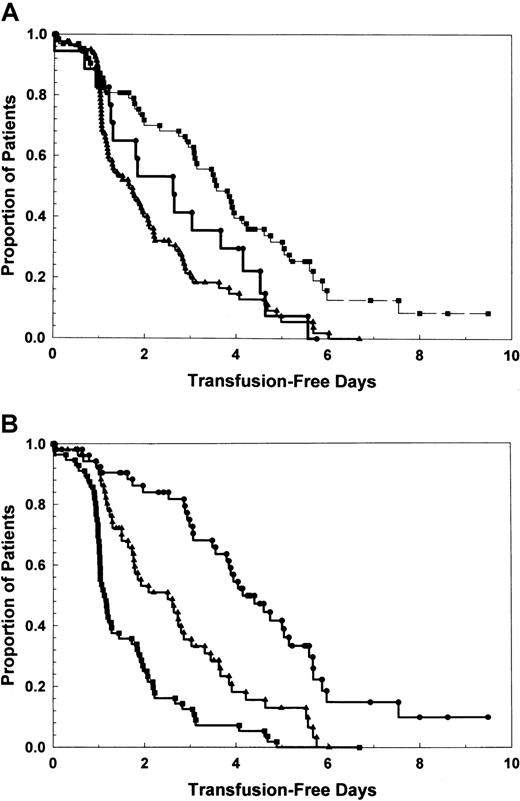
Data on intervals between study transfusion and time to next prophylactic platelet transfusion are illustrated in Figure3. Median intervals to next platelet transfusion were 1.72 days, 2.64 days, and 3.80 days for placebo, 1 μg/kg, and 3 μg/kg, respectively (P < .001 for all comparisons). When analyzed by posttransfusion platelet count tertile (3 patient cohorts with highest, middle, and lowest posttransfusion platelet counts) (Figure 3B), differences in time to next transfusion are also significantly different (P < .001).
Proportion of patients remaining transfusion free over time is related to platelet transfusion dose.
(A) By treatment cohort. ▴, placebo; ●, PEG-rHuMGDF, 1 μg/kg; ▪, PEG-rHuMGDF, 3 μg/kg. P < .001. (B) By posttransfusion platelet count—3 patient cohorts with highest (●), middle (▴), and lowest (▪) tertile posttransfusion platelet counts.P < .001.
Proportion of patients remaining transfusion free over time is related to platelet transfusion dose.
(A) By treatment cohort. ▴, placebo; ●, PEG-rHuMGDF, 1 μg/kg; ▪, PEG-rHuMGDF, 3 μg/kg. P < .001. (B) By posttransfusion platelet count—3 patient cohorts with highest (●), middle (▴), and lowest (▪) tertile posttransfusion platelet counts.P < .001.
Effect of platelet count increment on bleeding events.
Bleeding events are detailed in Table 4. Overall, 18 of 59 (31%) patients receiving placebo-derived platelets reported bleeding events, compared to 14 of 61 (23%) patients receiving platelets from PEG-rHuMGDF–derived platelets. Data indicate that the PEG-rHuMGDF–derived platelets provide clinically effective hemostasis.
Bleeding events
| Treatment group no. transfusion recipients4-150 no. transfusion recipients reporting bleeding events (%)4-151 . | Placebo n = 59 18 (31%) . | PEG-rHuMGDF . | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 μg/kg . | 3 μg/kg . | Combined . | ||||||
| n = 15 . | n = 46 . | N = 61 . | ||||||
| 2 (13%) . | 12 (26%) . | 14 (23%) . | ||||||
| Organ system . | N . | % . | N . | % . | N . | % . | N . | % . |
| Gastrointestinal‡ | 5 | 8 | 1 | 7 | 2 | 4 | 3 | 5 |
| Hematemesis | 3 | 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hematochezia | 2 | 3 | 1 | 7 | 2 | 4 | 3 | 5 |
| Hemorrhage | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cutaneous/mucosal‡ | 7 | 12 | 1 | 7 | 5 | 11 | 6 | 10 |
| Ecchymoses | 2 | 3 | 0 | 0 | 2 | 4 | 2 | 3 |
| Hematoma | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hemorrhage | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Petechiae | 4 | 7 | 1 | 7 | 4 | 9 | 5 | 8 |
| Respiratory‡ | 8 | 14 | 0 | 0 | 5 | 11 | 5 | 8 |
| Epistaxis | 7 | 12 | 0 | 0 | 5 | 11 | 5 | 8 |
| Hemoptysis | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Genitourinary | ||||||||
| Hematuria | 7 | 12 | 0 | 0 | 5 | 11 | 5 | 8 |
| Treatment group no. transfusion recipients4-150 no. transfusion recipients reporting bleeding events (%)4-151 . | Placebo n = 59 18 (31%) . | PEG-rHuMGDF . | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 μg/kg . | 3 μg/kg . | Combined . | ||||||
| n = 15 . | n = 46 . | N = 61 . | ||||||
| 2 (13%) . | 12 (26%) . | 14 (23%) . | ||||||
| Organ system . | N . | % . | N . | % . | N . | % . | N . | % . |
| Gastrointestinal‡ | 5 | 8 | 1 | 7 | 2 | 4 | 3 | 5 |
| Hematemesis | 3 | 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hematochezia | 2 | 3 | 1 | 7 | 2 | 4 | 3 | 5 |
| Hemorrhage | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cutaneous/mucosal‡ | 7 | 12 | 1 | 7 | 5 | 11 | 6 | 10 |
| Ecchymoses | 2 | 3 | 0 | 0 | 2 | 4 | 2 | 3 |
| Hematoma | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hemorrhage | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Petechiae | 4 | 7 | 1 | 7 | 4 | 9 | 5 | 8 |
| Respiratory‡ | 8 | 14 | 0 | 0 | 5 | 11 | 5 | 8 |
| Epistaxis | 7 | 12 | 0 | 0 | 5 | 11 | 5 | 8 |
| Hemoptysis | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Genitourinary | ||||||||
| Hematuria | 7 | 12 | 0 | 0 | 5 | 11 | 5 | 8 |
Data are for unique, first study transfusion only recipients.
Total number of subjects affected for any of the indicated organ systems—ie, some patients had both hematologic and respiratory bleeding.
Total number of subjects affected for the indicated organ system—ie, some patients had both ecchymoses and petechiae.
Discussion
The administration of PEG-rHuMGDF to healthy platelet apheresis donors produces a predictable increase in platelet count on day 15 that allows the routine collection of large numbers of platelets (on average, 3-fold greater than standard) from a platelet apheresis collection.14 Our report demonstrated that the transfusion of these platelet concentrates was safe and effective in producing substantially (and statistically significantly) higher platelet counts when compared to placebo-derived platelets. Moreover, the interval to the next transfusion was prolonged with increasing platelet dose.
Transfusing patients with thrombocytopenia to higher platelet counts has several potential benefits. One potential benefit is to reduce the prevalence of hemorrhagic events; however, our study did not find any relationship between patient cohort and likelihood of bleeding (Table4). A recent multicenter audit showed that platelet hemorrhagic complications correlated poorly with the degree of thrombocytopenia.4
Another potential benefit to higher platelet counts is that platelet survival is directly related to platelet count at platelet counts of 100 × 109/L or less because there is a fixed platelet hemostatic use that is progressively more important in determining the lifespan of platelets in patients with progressively lower platelet counts.16,17 A previously reported controlled trial of very high-dose, high-dose, and standard-dose platelet products found that across a similar 3-fold dose range, the posttransfusion platelet half-disappearance times were indistinguishable from each other.18 Our results indicate that hypertransfusion to platelet counts greater than 100 × 109/L is realistic because a median 3-fold increase in platelet dosage resulted in a median maximum posttransfusion platelet count of 93 × 109/L, with a range of up to 192 × 109/L. Our results support the contention that maintenance of higher platelet counts is associated with prolonged intervals to next platelet transfusion. Additional studies comparing the merits of low-dose and high-dose platelet therapy in patients with and without clinical platelet refractoriness are needed.
The generation of high-dose apheresis concentrates has economic implications for transfusion services and blood centers. The AABB standards mandate that 70% of apheresis products exceed 3 × 1011 platelets; platelet products that exceed 6 × l011 can be “split” into 2 transfusion products.19 Apheresis products containing 7 × 1011 to 18 × 1011 platelets have the potential to be split into 2 to 6 platelet transfusion products, so that many patients could potentially benefit from one apheresis procedure; costs per apheresis platelet product would be reduced substantially.20 However, if transfusion of a very large number of platelets results in an increased interval between transfusions and, most important, fewer total platelet transfusion events during a treatment course, it might be a preferable strategy to frequent transfusions at lower doses. A recent prospective, randomized trial found that when compared to the administration of lower dose platelets (mean, 3.1 × 1011 platelets), higher dose platelets (mean, 5.0 × 1011 platelets) were associated with longer transfusion-free intervals and lower relative risk per day for additional transfusions.21 However, neither this study21 nor a second study18 analyzed cumulative platelet dosage administered over the duration of the study period to assess whether platelet dose strategies can affect this clinical outcome; additional controlled clinical trials are needed to answer this important question.
Since the completion of this study, clinical development of PEG-rHuMGDF has been halted; multiple subcutaneous injections of PEG-rHuMGDF in volunteer control subjects and in chemotherapy patients produced neutralizing antibodies and consequent thrombocytopenia.22,23 Transient, low-titer antibody partially inhibitory on bioassay developed in 1 in 400 patients receiving multiple doses of recombinant human thrombopoietin (rhTPO; Genentech, San Francisco, CA).24 None of the apheresis donors in this study received more than one injection of PEG-rHuMGDF, and none developed antibodies.
In conclusion, platelets collected from healthy platelet apheresis donors given a single injection of PEG-rHuMGDF are safer and result in significantly greater posttransfusion platelet counts when transfused into patients with thrombocytopenia than platelets obtained from apheresis donors given placebo.
Supported by Amgen, Thousand Oaks, CA.
S.A. and D.T. are employees of Amgen, whose product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Lawrence T. Goodnough, Department of Medicine and Pathology, Washington University School of Medicine, Box 8118, 660 S Euclid Ave, St Louis, MO 63110-1093; e-mail:goodnough@labmed.wustl.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal