Lipid rafts are plasma membrane microdomains characterized by a unique lipid environment enriched in gangliosides and cholesterol, leading to their insolubility in nonionic detergents. Many receptors are constitutively or inducibly localized in lipid rafts, which have been shown to function as platforms coordinating the induction of signaling pathways. In this report, the first evidence is provided for a role of these lipid microdomains in regulating interleukin-2 receptor (IL-2R) signaling. It is demonstrated that antibody- or ligand-mediated immobilization of components of lipid rafts, glycosyl-phosphatidyl-inositol–anchored proteins, and the GM1 ganglioside, respectively, inhibit IL-2–induced proliferation in T cells. IL-2Rα is shown to be constitutively enriched in rafts and further enriched in the presence of immobilized anti–Thy-1. In contrast, IL-2Rβ and IL-2Rγ, as well as JAK1 and JAK3, are found in soluble membrane fractions, and their localization is not altered by anti–Thy-1. IL-2–mediated heterotrimerization of IL-2R chains is shown to occur within soluble membrane fractions, exclusively, as is the activation of JAK1 and JAK3. As predicted by these results, the disruption of lipid raft integrity did not impair IL-2–induced signaling. Thus, the sequestration of IL-2Rα within lipid microdomains restricts its intermolecular interactions and regulates IL-2R signaling through impeding its association with IL-2Rβ and IL-2Rγ.
Introduction
Lipid rafts are plasma membrane microdomains postulated to function in signaling and membrane trafficking.1-3 Lipid rafts are enriched in gangliosides (glycosphingolipids) and cholesterol, which form liquid-ordered domains of decreased membrane fluidity. The long, saturated acyl chains of gangliosides impart a high degree of order further stabilized by intercalating cholesterol molecules, leading to the insolubility of lipid rafts in nonionic detergents. Lipid rafts can be isolated based on their detergent insolubility and low-buoyancy density using discontinuous sucrose gradient ultracentrifugation of nonionic detergent lysates. Lipid rafts are not artifacts of detergent extraction. They have been detected in living cells using chemical cross-linking and fluorescence resonance energy transfer.4 5
The modification of proteins with saturated acyl groups can result in their localization within lipid rafts. Thus, these microdomains are enriched in glycosyl-phosphatidyl-inositol anchored proteins (GPI-AP) and in many signaling molecules such as Src family protein tyrosine kinases (PTKs), the adaptor protein LAT, heterotrimeric and small G-proteins, and phosphoinositides.3 In addition, several transmembrane receptors are inducibly recruited to or stabilized within lipid rafts, including T-cell receptor (TCR), B-cell receptor (BCR), and FcεRI.2 Subsequent activation of signaling molecules in lipid rafts may facilitate signaling through these immunoreceptors. Lipid rafts may act to segregate molecules in the plasma membrane and to regulate signaling through the spatial coordination of intermolecular associations.
Stimulation of T cells through TCR results in the activation of multiple signaling pathways, leading to interleukin-2 (IL-2) responsiveness and the secretion of IL-2 and resulting in autocrine cell growth.6 The high-affinity receptor for IL-2 is composed of the IL-2Rα chain, which functions solely in IL-2 binding, and IL-2Rβ and IL-2Rγc, which contribute to IL-2 binding and mediate signal transduction.7 IL-2–induced proliferation requires activation of the Janus family kinases JAK1 and JAK3, which are constitutively associated with IL-2Rβ and IL-2Rγ, respectively.8,9 Ligand-induced IL-2R aggregation leads to the juxtaposition of JAK1 and JAK3, resulting in their phosphorylation and activation. Subsequent phosphorylation of tyrosine residues in receptor chains leads to the SH2-domain–mediated recruitment of signal transducer and activator of transcription (STAT) proteins STAT5a and STAT5b.7 JAK-mediated phosphorylation of STAT proteins leads to their dimerization by SH2 domain-phosphotyrosine interactions and their translocation to the nucleus, where STAT proteins regulate gene transcription. Signaling through the IL-2R also induces the recruitment and activation of phosphatidylinositol 3 kinase (PI3K), which is implicated in IL-2–mediated proliferation and survival through its downstream effector protein kinase B/Akt.10,11In addition, the tyrosine phosphorylation of IL-2Rβ results in the recruitment of Shc and Grb2 and activation of the Ras/MAPK pathway. Lck, Syk, and Pyk-2 also associate with IL-2Rβ; however, the functional outcome of these interactions remains unclear.7
Similar to many other receptors, IL-2R is not randomly distributed in the lipid bilayer. IL-2Rα, IL-2Rβ, and IL-2Rγ chains appear to form pre-existing complexes on the surfaces of T cells, brought closer by ligand binding,12 resulting in the aggregation of IL-2Rβ and IL-2Rγ required for signaling.13,14 In addition, IL-2Rα has been found in cell surface clusters that appear to correspond to lipid rafts.15 In this study, we demonstrate that components of lipid rafts modify signaling through IL-2R and characterize the involvement of lipid rafts in IL-2R signaling.
Materials and methods
Cells, antibodies, and flow cytometry
It has been reported that 2.10 is an IL-2–dependent, CD4− T-cell clone.16 Stable 2.10 clonal variants expressing or lacking GPI-AP were isolated through the sorting of Thy-1+ and Thy-1− cells on a FACStar Plus (Becton Dickinson, Mountain View, CA). The CTLL-2 T-cell line was obtained from American Type Culture Collection (Rockville, MD). Primary CD8+ T cells were purified from the lymph nodes of C57Bl/6 mice as described previously.17 The CD8+ T-cell preparations were consistently found to be greater than 95% CD8+TCRαβ+ when assessed by flow cytometry.
Antibodies used in this study and described previously18include monoclonal antibodies (mAbs) specific for Thy-1 (30H12, M5/49, and 5-3.2.1), CD4 (GK1.5, rat IgG2b mAb isotype control and H129, rat IgG2a mAb isotype control), Ly6A/E (D7), TcR-Cβ (H57-597), and CD48 (5-8A10). In addition, anti-CD45 [M18919] and anti-CD5 [53-7.320] were used. Normal hamster immunoglobulin G (IgG) (Jackson Immunoresearch, Westgrove, PA) was used as an isotype control for anti-CD48. Unless otherwise indicated, anti–Thy-1 refers to the 30H12 mAb.
Flow cytometric analysis was performed by labeling cells with the indicated antibodies for 10 minutes, followed by 3 washes. Expression of GPI-AP was determined using anti–Thy-1 or anti-Ly6A/E followed by fluorescein isothiocyanate (FITC)–mouse anti–rat κ or FITC-conjugated anti-CD48. Before analysis on a FACScalibur (Becton Dickinson), cells were resuspended in 1 μg/mL 7-amino-actinomycin-D (7AAD; Sigma, St Louis, MO). Plots shown exclude dead cells based on forward- versus side-scattering profiles and on positive staining with 7AAD.
Cellular DNA content was determined by the Vindelov method.21 Cells cultured as indicated were harvested, pelleted, and resuspended in Vindelov solution (3.4 mM Tris HCl, pH 7.6, 10 mM NaCl, 0.1% vol/vol NP-40, 50 μg/mL propidium iodide [Sigma], and 20 μg/mL RNase A [Boehringer Mannheim Laval, QC, Canada]). The proportion of cells with subdiploid DNA content was assessed by flow cytometry on a FACScalibur, using doublet discrimination in the FL2 channel.
Proliferation assays
Purified cholera toxin β subunit (CT; Sigma) and all mAbs used in this study were diluted to 10 μg/mL in Hanks balanced salt solution without CaCl2 or MgCl2, except anti-TCR, which was used at 1 μg/mL. CT or antibody solutions were incubated in wells of 96-well plates for 1 hour at 37°C. After 2 washes with Hanks balanced salt solution, 2 × 104 2.10 or CTLL-2 cells or 5 × 104 primary T cells were added in serum-free medium.16 After 20 hours, each culture was pulsed for 6 hours with 1 μCi of 3H-thymidine and thymidine uptake assessed using a Topcount Microplate scintillation counter (Canberra Packard, Meriden, CT). Where indicated, primary CD8+ T cells were stimulated for 20 hours with anti-TCR. Viable cells were isolated on a Lympholyte M gradient (Cedarlane, ON, Canada) and cultured as described above. Supernatant from the X630 hybridoma transfected with complementary DNA (cDNA) encoding IL-2 was used as a source of cytokine.22 The activity of IL-2–containing supernatant was quantitated by bioassay, and 1 U was defined as resulting in half-maximal proliferation of 5 × 103 CTLL-2 cells cultured for 48 hours.
Isolation of lipid rafts
Lipid rafts were isolated by discontinuous sucrose density gradient ultracentrifugation. Cells were lysed at 2 × 107 cells/mL in TKM buffer (50 mM Tris, pH 7.4, 25 mM KCl, 5 mM MgCl2, and 1 mM EDTA) containing 0.5% wt/vol Brij58 and protease inhibitors leupeptin (2.5 μg/mL), aprotinin (2.5 μg/mL), and Pefabloc (1 mM), all from Boehringer Mannheim. Lysates were incubated on ice for 30 minutes, mixed with an equal volume of 80% wt/vol sucrose in TKM, and overlaid with 5.5 mL 36% sucrose followed by 2.5 mL 5% sucrose. The gradients were subjected to ultracentrifugation at 250 000g for 16 to 18 hours in an SW41 rotor (Becton Dickinson), and 1-mL fractions were collected from the top of the gradient.
Immunoprecipitations and immunoblotting
Protein or glycolipid content of fractions isolated from sucrose density gradients was determined by immunoblotting. GM1 was detected using CT conjugated to horseradish-peroxidase (HRP). Fyn, Thy-1, and CD45 were detected using anti–Thy-1 and anti-CD45 followed by rabbit anti–rat IgG-HRP (Sigma) and anti-Fyn serum (provided by Dr A. Veillette, IRCM, Montreal, QC, Canada) followed by protein-A–HRP (ICN, Costa Mesa, CA).
The localization of IL-2R chains was assessed in CTLL-2 cells, which were starved for 16 hours in 1.25 U/mL IL-2 (unstimulated) or after stimulation with 400 U/mL IL-2. Cells were pelleted and lysed in TKM/Brij58, and lipid rafts were isolated. To assess the effect of immobilized anti–Thy-1 on the localization of IL-2R chains, rafts were isolated from CTLL-2 cells cultured for 16 hours with 15 U/mL IL-2 in flasks coated with anti–Thy-1 or control mAbs. As a positive control for immunoblotting, immunoprecipitation of IL-2Rα, IL-2Rβ, and IL-2Rγ was performed as described previously.18Immunoprecipitates or 150 μL-fractions from sucrose gradients were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Western blot analysis was performed using polyclonal rabbit anti–IL-2Rα, IL-2Rβ, and IL-2Rγ (Santa Cruz Biotechnology, Santa Cruz, CA) followed by Protein-A–HRP.
To determine the localization of JAK1 and JAK3, equal amounts of pooled lipid raft and soluble fractions—as assessed by GM1 immunoblotting—were diluted 4-fold in TX-100 buffer containing 50 mM HEPES, pH 7.5, 1% Triton X-100, 150 mM NaCl, 10% glycerol, 1.5 mM MgCl2, 1 mM Na2VO4, and protease inhibitors. Immunoprecipitations were performed using anti-JAK1 (Transduction, Lexington, KY) and JAK3-specific antisera (UBI, Lake Placid, NY) and were collected using Protein-A– or Protein-G–Sepharose beads, respectively. Proteins were resolved on SDS-PAGE gels run in parallel, and immunoblot analysis was performed using antiphosphotyrosine [4G1023] and anti-JAK1 followed by HRP–goat, anti–mouse IgG (GaMIg-HRP; Sigma), or JAK3-specific antisera followed by Protein-A–HRP.
To determine the effect of disrupting lipid rafts on IL-2R–induced signaling, 107 cells/mL were incubated in 10 mM methyl-β-cyclodextrin (MCD; Aldrich, Milwaukee, WI) for 20 minutes at 37°C.24 Where indicated, 400 U/mL IL-2 was added, and cells were incubated for an additional 10 minutes. Cells were pelleted and lysed in TX-100 buffer, and immunoprecipitation and immunoblot analyses of JAK1 and JAK3 from postnuclear lysates were performed as described above. To assess the effect of MCD on TCR-induced signaling, MCD-treated 2.10 were pelleted and incubated at 107cells/mL with 2.5 μg/mL biotin-labeled anti–TCR-Cβ and 10 mM MCD for 45 minutes on ice. Cells were pelleted, resuspended to 4 × 106 cells/mL, and warmed to 37°C before stimulation with 20 μg/mL streptavidin for 30 seconds. Phospholipase C (PLC)γ1 was immunoprecipitated from post-nuclear lysates using a mixture of mouse mAbs (UBI). Immunoblot analysis was performed on gels run in parallel using antiphosphotyrosine or anti-PLCγ1 revealed by GaMIg-HRP.
Sodium iodide 125–IL-2 binding assays
CTLL-2 cells maintained in IL-2 were harvested, and IL-2 bound to its receptor was dissociated by a 1-minute incubation in 10 mM sodium citrate, 150 mM NaCl, pH 4.0. Cells were washed twice in phosphate-buffered saline (PBS) with 3% fetal calf serum and 0.1% azide before the addition of 5 × 10−10 M sodium iodide 125 (125I)–labeled IL-2 (NEN, Boston, MA). After 30 minutes on ice, cells were washed twice in PBS with 0.1% azide and incubated for 10 minutes in 2 mM disuccinimidyl suberate (DSS) (Pierce, Rockford, IL). Cells were then incubated in 5 mM ammonium acetate for 1 minute to quench unreacted DSS, washed twice in PBS with 0.1% azide, and lysed in TKM/Brij58. Sucrose density gradient centrifugation was performed, and proteins from fractions corresponding to lipid rafts and soluble membranes, as assessed by GM1 immunoblotting, were resolved by SDS-PAGE. Gels were fixed in 40% methanol and 10% acetic acid, dried, and autoradiographed at −70°C.
Results
Immobilized mAbs specific for GPI-AP inhibit IL-2–induced proliferation
We recently demonstrated that in the presence of immobilized mAbs specific for GPI-AP, anti-TCR–induced proliferation was inhibited, despite the production of IL-2.18 In addition, IL-2–induced signaling was inhibited in these circumstances. These results were consistent with a signaling defect in the responsiveness of T cells to endogenously produced IL-2. To confirm that GPI-AP inhibited signaling through IL-2R, we determined the effect of mAb specific for GPI-AP on T-cell proliferation in response to exogenous IL-2.
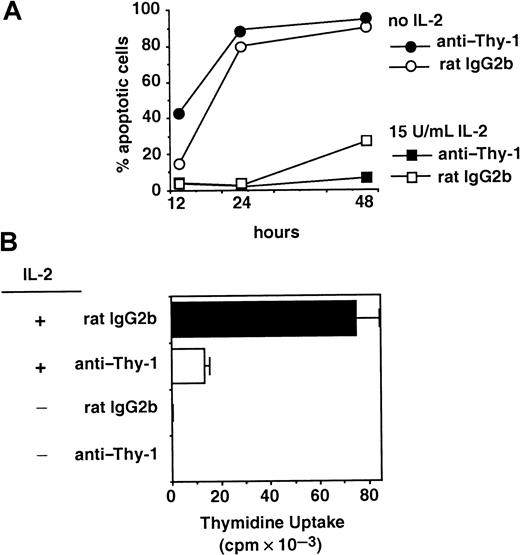
The IL-2–dependent 2.10 T-cell clone (Figure1A) and the CTLL-2 T-cell line (Figure1B) were cultured in wells coated with anti–Thy-1 or an isotype-matched control mAb. Immobilized anti–Thy-1 inhibited the proliferation of both 2.10 and CTLL-2 cells in response to IL-2 over a wide range of concentrations. Analysis of the growth-inhibitory effects of anti–Thy-1 was extended to primary T cells, which require stimulation through TCR to induce expression of the high-affinity IL-2R and acquire responsiveness to IL-2. Thus, unstimulated CD8+lymph node T cells did not proliferate in response to exogenous IL-2 (Figure 1C). Primary CD8+ T cells were stimulated with anti-TCR, harvested, and restimulated with exogenous IL-2. As seen in Figure 1C, exogenous IL-2 induced the proliferation of activated primary T cells, which were inhibited by anti–Thy-1.
Anti–Thy-1 inhibits IL-2–induced T-cell proliferation.
The 2.10 T-cell clone (A) or the CTLL-2 T-cell line (B) were cultured in wells precoated with mAbs specific for Thy-1 (○) or an mAb isotype control (●) and the indicated concentration of IL-2. (C) Resting or TcR-stimulated CD8+ lymph node T cells were cultured in wells precoated with anti–Thy-1 or an isotype control in the presence of 15 U/mL IL-2. Uptake of 3H-thymidine was assessed after 20 hours of culture.
Anti–Thy-1 inhibits IL-2–induced T-cell proliferation.
The 2.10 T-cell clone (A) or the CTLL-2 T-cell line (B) were cultured in wells precoated with mAbs specific for Thy-1 (○) or an mAb isotype control (●) and the indicated concentration of IL-2. (C) Resting or TcR-stimulated CD8+ lymph node T cells were cultured in wells precoated with anti–Thy-1 or an isotype control in the presence of 15 U/mL IL-2. Uptake of 3H-thymidine was assessed after 20 hours of culture.
Anti-TCR–induced proliferation, but not IL-2 production, was inhibited by mAb specific for multiple GPI-AP.18 Therefore, we determined the effect of GPI-AP in addition to Thy-1 on T-cell proliferation induced by exogenous IL-2. Stable GPI+ and GPI− clonal variants of 2.10 were established by FACS sorting of cells based on the expression of Thy-1. GPI−2.10 were shown to lack expression of all GPI-AP because of a deficiency in PIG-P, a protein required for GPI anchor biosynthesis.25 The GPI-AP Thy-1, Ly6A/E, and CD48 are expressed on GPI+ but not on GPI− 2.10 T cells, as determined by flow cytometry (Figure2A-B). IL-2–induced proliferation of GPI+ 2.10 (Figure 2C) and CTLL-2 cells (data not shown) is inhibited by several mAbs specific for Thy-1 and by mAb specific for Ly6A/E and CD48. Proliferation of the GPI− clonal variant is unaffected by these mAbs, as predicted for cells that lack expression of these proteins, confirming that inhibition of proliferation is not caused by nonspecific toxic effects of the mAb preparations. In addition, immobilized mAbs specific for GPI-AP do not inhibit TCR-induced signaling, as assessed by the production of IL-2,18 demonstrating that not all signaling pathways are inhibited in these circumstances. IL-2–induced proliferation is not affected by isotype controls or by mAbs specific for the transmembrane proteins CD45 and CD5 (Figure 2C). In addition, IL-2–induced proliferation of GPI+, but not GPI−, 2.10 was inhibited by mAbs specific for Qa-2, CD55, and CD73, albeit to a lower extent, which in turn correlated with a decreased level of surface expression of these GPI-APs as detected by flow cytometry (data not shown). Thus, immobilized mAbs specific for all GPI-APs tested inhibit T-cell proliferation in response to exogenous IL-2.
IL-2–induced proliferation is inhibited by mAbs specific for the GPI-anchored proteins Thy-1, Ly-6A/E, and CD48.
The expression of Thy-1, Ly6A/E, and CD48 on GPI+ (A) and GPI− (B) variants of the 2.10 T-cell clone was analyzed by flow cytometry. The first histogram represents staining with secondary antibodies alone. (C) GPI+ and GPI− 2.10 T cells were cultured in wells precoated with the indicated mAbs and 15 U/mL IL-2. Uptake of 3H-thymidine is represented as the percentage of the proliferative response to IL-2 in the absence of added mAbs.
IL-2–induced proliferation is inhibited by mAbs specific for the GPI-anchored proteins Thy-1, Ly-6A/E, and CD48.
The expression of Thy-1, Ly6A/E, and CD48 on GPI+ (A) and GPI− (B) variants of the 2.10 T-cell clone was analyzed by flow cytometry. The first histogram represents staining with secondary antibodies alone. (C) GPI+ and GPI− 2.10 T cells were cultured in wells precoated with the indicated mAbs and 15 U/mL IL-2. Uptake of 3H-thymidine is represented as the percentage of the proliferative response to IL-2 in the absence of added mAbs.
Immobilized mAbs specific for GPI-AP result in the dissociation of IL-2–mediated survival and proliferation
The 2.10 T-cell clone is IL-2–dependent and undergoes apoptotic cell death on cytokine withdrawal. Although IL-2–mediated proliferation was inhibited by immobilized mAbs specific for Thy-1, IL-2 still supports cell survival in these circumstances. Figure3A demonstrates that most cells undergo apoptosis by 24 hours after the withdrawal of IL-2. In the presence of IL-2, cells cultured with anti–Thy-1 or an isotype control mAb remain viable, despite the growth inhibition mediated by anti–Thy-1 in cultures set up in parallel (Figure 3B). Similar results are observed using mAbs specific for CD48 or Ly6A/E and using CTLL-2 cells (data not shown). The increase in the number of control cells undergoing apoptosis at 48 hours is consistent with IL-2 use and catabolism by the proliferating cells. In the presence of immobilized anti–Thy-1, IL-2 is not used to aid proliferation, and it continues to support cell survival (Figure 3A).
Anti–Thy-1 inhibits IL-2–induced proliferation but not cell survival.
(A) GPI+ 2.10 T cells were cultured in the presence of immobilized anti–Thy-1 or an mAb isotype control, in the presence or absence of 15 U/mL IL-2. After 20 hours, the viability of cells in each condition was assessed by flow cytometry. (B) 3H-thymidine uptake in response to 15 U/mL IL-2 was assessed in cultures set up in parallel to those in panel A.
Anti–Thy-1 inhibits IL-2–induced proliferation but not cell survival.
(A) GPI+ 2.10 T cells were cultured in the presence of immobilized anti–Thy-1 or an mAb isotype control, in the presence or absence of 15 U/mL IL-2. After 20 hours, the viability of cells in each condition was assessed by flow cytometry. (B) 3H-thymidine uptake in response to 15 U/mL IL-2 was assessed in cultures set up in parallel to those in panel A.
The ability of IL-2 to support survival but not proliferation in the presence of immobilized mAbs specific for GPI-AP may be a result of residual signaling through IL-2R. Alternatively, survival may result from distinct signals induced through IL-2R that are differentially affected by GPI-AP. The activation of PI3K and its downstream effector protein kinase B have been implicated in IL-2–mediated survival.10,11 However, in the presence of immobilized anti–Thy-1, IL-2–induced PI3K activation, as determined by protein kinase B phosphorylation levels, was decreased relative to controls (data not shown). This result, in addition to our previous finding that immobilized anti–Thy-1 inhibits IL-2–induced IL-2R heterotrimerization,18 is consistent with the inhibition of all signaling pathways induced through IL-2R, and it suggests that viability may be mediated by residual signaling.
Another component of lipid rafts, the GM1 ganglioside, inhibits IL-2–induced proliferation
The ability of all GPI-APs tested to inhibit IL-2R–induced proliferation, despite their unrelated protein moieties, suggests that a characteristic imparted by the GPI anchor is critical for this inhibition. GPI anchoring results in the localization of proteins to lipid rafts, and we hypothesized that this localization of GPI-AP underlay their inhibitory capacity. Therefore, we determined the effect of immobilizing another component of lipid rafts, the GM1 ganglioside, on IL-2–induced proliferation.
To establish that plasma membrane compartmentalization occurs in cells expressing or lacking GPI-AP, lipid rafts were isolated from GPI+ and GPI− 2.10 lysed in buffer containing Brij58, a weak nonionic detergent that preserves lipid rafts. Fractions were collected after discontinuous sucrose density gradient ultracentrifugation, and the localization of proteins and glycolipids was assessed by immunoblotting. In both GPI+ and GPI− clonal variants, the transmembrane tyrosine phosphatase CD45 is found almost exclusively in soluble membranes whereas the GM1 ganglioside, detected using the β subunit of cholera toxin (CT), is found almost exclusively in lipid rafts (Figure4A-B). In addition, most Fyn and Thy-1 are found within lipid rafts. As predicted, Thy-1 cannot be detected in immunoblots of fractions isolated from GPI− cells (Figure4B).
Proliferative responses of T cells to TCR and IL-2 are inhibited by the lipid raft component GM1.
(A-B) Plasma membrane compartmentalization into lipid rafts in the presence or absence of GPI-anchored proteins. Lysates of GPI+ (A) or GPI− (B) 2.10 T cells were subjected to discontinuous sucrose density gradient ultracentrifugation. Fractions (Fr) were collected from the top of the gradient, and the distribution of CD45, Fyn, Thy-1, and GM1 was analyzed by immunoblotting. Fractions corresponding to lipid rafts and soluble membranes are indicated. (C-D) Immobilized CT inhibits anti-TCR and IL-2–induced proliferation. GPI+ and GPI−2.10 T cells were cultured in wells precoated with CT (▪), anti–Thy-1 (■), or an isotype control (░) mAb and coimmobilized anti-TcR-Cβ (C) or in the presence of 15 U/mL IL-2 (D).3H-thymidine uptake was assessed after 20 hours of culture.
Proliferative responses of T cells to TCR and IL-2 are inhibited by the lipid raft component GM1.
(A-B) Plasma membrane compartmentalization into lipid rafts in the presence or absence of GPI-anchored proteins. Lysates of GPI+ (A) or GPI− (B) 2.10 T cells were subjected to discontinuous sucrose density gradient ultracentrifugation. Fractions (Fr) were collected from the top of the gradient, and the distribution of CD45, Fyn, Thy-1, and GM1 was analyzed by immunoblotting. Fractions corresponding to lipid rafts and soluble membranes are indicated. (C-D) Immobilized CT inhibits anti-TCR and IL-2–induced proliferation. GPI+ and GPI−2.10 T cells were cultured in wells precoated with CT (▪), anti–Thy-1 (■), or an isotype control (░) mAb and coimmobilized anti-TcR-Cβ (C) or in the presence of 15 U/mL IL-2 (D).3H-thymidine uptake was assessed after 20 hours of culture.
Immobilized CT inhibited the proliferation of both GPI+ and GPI− cells in response to IL-2 produced endogenously on stimulation with anti-TCR (Figure 4C) or provided exogenously (Figure4D). Immobilized mAbs specific for Thy-1 inhibited anti-TCR and IL-2–induced proliferation of GPI+ but not GPI− cells. The effects of immobilizing GM1 using CT appeared identical to those of immobilizing GPI-AP using mAbs because IL-2–induced proliferation but not cell survival or TCR-induced production of IL-2 was inhibited (data not shown). These results are consistent with the ability of components of lipid rafts to modify IL-2R signaling in T cells.
IL-2Rα is enriched in lipid rafts, but IL-2R signaling occurs in soluble membranes
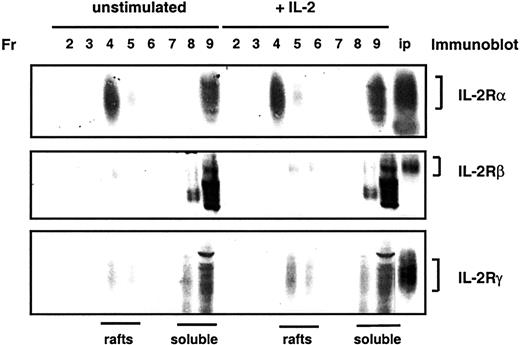
The ability of components of lipid rafts to inhibit IL-2–induced proliferation suggests that lipid rafts may play a role in the regulation of IL-2R signaling. As a first step in assessing the role of lipid rafts in IL-2R signaling, we determined the localization of IL-2R chains. CTLL-2 cells are maintained in IL-2; therefore, to minimize potential effects of IL-2 on the distribution of its receptor chains, cells were cultured for 16 hours in low concentrations of IL-2. In these circumstances, IL-2R signaling, assessed by the tyrosine phosphorylation of JAK1 and JAK3, was not detectable. CTLL-2 cells were treated no further (unstimulated) or were stimulated for 10 minutes with 400 U/mL IL-2 before lysis, and membranes were fractionated by sucrose density gradient ultracentrifugation. Immunoblot analysis of fractions from these gradients revealed that a large proportion (58%) of IL-2Rα was localized in lipid rafts (Figure 5). In contrast, most IL-2Rβ and IL-2Rγ were detected in soluble membranes. No significant differences in the localization of IL-2Rα, IL-2Rβ, or IL-2Rγ were detected on stimulation of cells with IL-2.
IL-2Rα, but not IL-2Rβ or IL-2Rγ, is enriched in lipid rafts.
CTLL-2 cells were left untreated or were stimulated for 10 minutes with IL-2 before lysis. Lysates were subjected to discontinuous sucrose density gradient ultracentrifugation, and fractions (Fr) were collected and separated by SDS-PAGE. Localization of IL-2α, IL-2Rβ, and IL-2Rγ chains was analyzed by immunoblotting. The final lane in each blot consists of immunoprecipitations of IL-2Rα, IL-2Rβ, or IL-2Rγ chains as positive controls for immunoblotting.
IL-2Rα, but not IL-2Rβ or IL-2Rγ, is enriched in lipid rafts.
CTLL-2 cells were left untreated or were stimulated for 10 minutes with IL-2 before lysis. Lysates were subjected to discontinuous sucrose density gradient ultracentrifugation, and fractions (Fr) were collected and separated by SDS-PAGE. Localization of IL-2α, IL-2Rβ, and IL-2Rγ chains was analyzed by immunoblotting. The final lane in each blot consists of immunoprecipitations of IL-2Rα, IL-2Rβ, or IL-2Rγ chains as positive controls for immunoblotting.
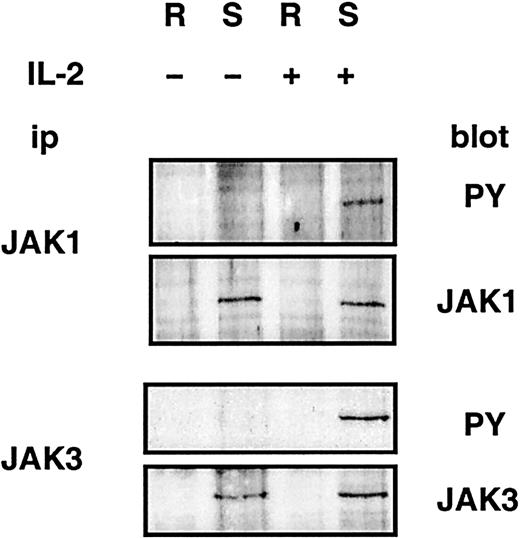
JAK1 and JAK3 kinases are constitutively associated with the IL-2Rβ and IL-2Rγ chains, respectively,8 9 and their activation after stimulation by IL-2 is critical for signaling through the IL-2R. Therefore, we assessed the localization of JAK1 and JAK3. The kinases were immunoprecipitated from pooled fractions of sucrose density gradients derived from CTLL-2 cells corresponding to lipid rafts and soluble membranes, as determined by blotting for GM1. Figure 6demonstrates that JAK1 and JAK3 are found in soluble membranes. In addition, JAK1 and JAK3 molecules involved in IL-2R signaling, assessed by IL-2–induced tyrosine phosphorylation, were found in soluble membranes. JAK1 and JAK3, and IL-2Rβ and IL-2Rγ, were not detected in lipid rafts at multiple additional time points after stimulation with IL-2 (1, 3, 9, or 27 minutes; data not shown). Similar results were observed using 2.10 (data not shown).
JAK1 and JAK3 are localized in detergent-soluble membranes.
CTLL-2 cells were left untreated or were stimulated for 10 minutes with IL-2 before lysis and sucrose density gradient ultracentrifugation. JAK1 and JAK3 were immunoprecipitated from pooled fractions of sucrose gradients corresponding to lipid rafts (R) and soluble membranes (S). Immunoprecipitations were split in 2 and resolved by SDS-PAGE, and immunoblotting was performed using phosphotyrosine (PY)–, JAK1-, or JAK3-specific antibodies.
JAK1 and JAK3 are localized in detergent-soluble membranes.
CTLL-2 cells were left untreated or were stimulated for 10 minutes with IL-2 before lysis and sucrose density gradient ultracentrifugation. JAK1 and JAK3 were immunoprecipitated from pooled fractions of sucrose gradients corresponding to lipid rafts (R) and soluble membranes (S). Immunoprecipitations were split in 2 and resolved by SDS-PAGE, and immunoblotting was performed using phosphotyrosine (PY)–, JAK1-, or JAK3-specific antibodies.
IL-2 binding to receptor chains can be assessed using labeled cytokine. CTLL-2 cells were incubated with 125I-labeled IL-2, which was subsequently chemically cross-linked to bound receptor chains. Fractions from sucrose density gradients corresponding to lipid rafts and soluble membranes were determined by blotting for GM1 (Figure 7A). Proteins in these fractions were separated by SDS-PAGE, and proteins cross-linked to 125I-labeled IL-2 were visualized by autoradiography. 125I-labeled IL-2 was cross-linked to IL-2Rα, IL-2Rβ, and IL-2Rγ chains in soluble membranes but not in lipid rafts (Figure 7B). This result is consistent with the presence of the signaling complex, composed of IL-2 and IL-2Rα, IL-2Rβ, and IL-2Rγ in soluble membranes exclusively.
The heterotrimeric receptor complex composed of IL-2 bound to IL-2Rα, IL-2Rβ, and IL-2Rγ is detected in detergent-soluble membranes.
(A) CTLL-2 cells were incubated with 125I-labeled IL-2, and bound IL-2 was cross-linked to cell-surface proteins. After lysis, sucrose density gradient ultracentrifugation was performed. Fractionation (Fr) into lipid rafts and soluble membranes was determined by immunoblotting for GM1. (B) Proteins in fractions corresponding to lipid rafts and soluble membranes were resolved by SDS-PAGE, and proteins cross-linked to 125I-labeled–IL-2 were visualized by autoradiography. Bands corresponding to125I–IL-2 cross-linked to IL-2Rα, IL-2Rβ, and IL-2Rγ are indicated.
The heterotrimeric receptor complex composed of IL-2 bound to IL-2Rα, IL-2Rβ, and IL-2Rγ is detected in detergent-soluble membranes.
(A) CTLL-2 cells were incubated with 125I-labeled IL-2, and bound IL-2 was cross-linked to cell-surface proteins. After lysis, sucrose density gradient ultracentrifugation was performed. Fractionation (Fr) into lipid rafts and soluble membranes was determined by immunoblotting for GM1. (B) Proteins in fractions corresponding to lipid rafts and soluble membranes were resolved by SDS-PAGE, and proteins cross-linked to 125I-labeled–IL-2 were visualized by autoradiography. Bands corresponding to125I–IL-2 cross-linked to IL-2Rα, IL-2Rβ, and IL-2Rγ are indicated.
Additional support for IL-2R signaling occurring within soluble membranes was derived by assessing the effect of disrupting the integrity of lipid rafts. This was achieved using methyl-β-cyclodextrin (MCD), which results in the extraction of cholesterol by forming inclusion complexes within a hydrophobic cyclodextrin cavity.24 MCD did not inhibit IL-2–induced tyrosine phosphorylation of JAK1 and JAK3 in CTLL-2 cells (Figure8A-B). As a positive control for the disruption of lipid rafts by MCD, we assessed the effects on a TCR-induced signaling pathway known to be dependent on lipid rafts.26 Although IL-2–induced tyrosine phosphorylation of JAK1 in 2.10 was unaffected by MCD (Figure 8C), TCR-induced tyrosine phosphorylation of PLCγ1 was virtually ablated (Figure 8D).
Disruption of lipid raft integrity does not affect IL-2–induced tyrosine phosphorylation of JAK1 and JAK3.
(A-B) CTLL-2 cells were left untreated or were incubated with 10 mM MCD before stimulation with IL-2. JAK1 and JAK3 were immunoprecipitated from postnuclear lysates. Immunoprecipitations were split in 2 and resolved by SDS-PAGE, and immunoblotting was performed using phosphotyrosine (PY)–, JAK1-, or JAK3-specific antibodies. (C-D) 2.10 cells were left untreated or were incubated with 10 mM MCD. (C) Cells were stimulated with IL-2, and JAK1 immunoprecipitated from postnuclear lysates was immunoblotted using anti-PY or anti-JAK1. (D) Cells were stimulated with anti-TCR, and PLCγ1 immunoprecipitated from postnuclear lysates was immunoblotted using anti-PY or anti-PLCγ1.
Disruption of lipid raft integrity does not affect IL-2–induced tyrosine phosphorylation of JAK1 and JAK3.
(A-B) CTLL-2 cells were left untreated or were incubated with 10 mM MCD before stimulation with IL-2. JAK1 and JAK3 were immunoprecipitated from postnuclear lysates. Immunoprecipitations were split in 2 and resolved by SDS-PAGE, and immunoblotting was performed using phosphotyrosine (PY)–, JAK1-, or JAK3-specific antibodies. (C-D) 2.10 cells were left untreated or were incubated with 10 mM MCD. (C) Cells were stimulated with IL-2, and JAK1 immunoprecipitated from postnuclear lysates was immunoblotted using anti-PY or anti-JAK1. (D) Cells were stimulated with anti-TCR, and PLCγ1 immunoprecipitated from postnuclear lysates was immunoblotted using anti-PY or anti-PLCγ1.
Thus, although IL-2Rα was enriched in lipid rafts, the signaling components of the IL-2R, IL-2Rβ, and IL-2Rγ chains, and of JAK1 and JAK3 phosphorylated in response to IL-2, were not found in lipid rafts. The active heterotrimeric receptor complex composed of IL-2 bound to IL-2Rα, IL-2Rβ, and IL-2Rγ was not detected in lipid rafts. In addition, IL-2R–induced signaling was not affected by the disruption of lipid rafts. Taken together, these results support the conclusion that IL-2R signaling occurs in soluble membranes.
Immobilized anti–Thy-1 results in an increased proportion of IL-2Rα in lipid rafts
The ability of lipid raft components to block IL-2R signaling suggests that rafts can regulate signaling through IL-2R, despite evidence that signaling occurs in soluble membranes. Lipid rafts may regulate IL-2R signaling by segregating elements of the receptor complex in the plasma membrane. IL-2 may result in the dissociation of IL-2Rα from lipid rafts and its interaction with IL-2Rβ and IL-2Rγ in soluble membranes to initiate signaling. The inhibition of IL-2R signaling, observed on immobilization of GPI-AP or GM1 in lipid rafts, may be owing to impairment of the mobility of IL-2Rα, thus preventing its dissociation from rafts. IL-2Rα may be in a dynamic equilibrium between lipid rafts and soluble membranes, and immobilization of lipid rafts components may shift this equilibrium by trapping IL-2Rα chains in lipid rafts. In either case, the prediction would follow that a greater proportion of IL-2Rα would be present in rafts in these circumstances. We therefore assessed whether immobilization of components of lipid rafts affected the distribution of IL-2Rα.
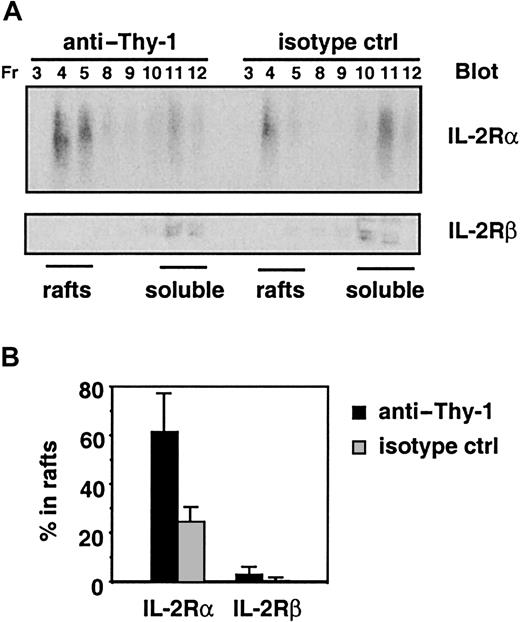
Figure 9 demonstrates that the proportion of IL-2Rα localized in lipid rafts was higher in cells that had been cultured in the presence of immobilized anti–Thy-1, relative to an isotype-matched mAb (61.4% ± 15.8% vs 24.6% ± 6.0%). In contrast, IL-2Rβ was not enriched in lipid rafts in the presence or absence of anti–Thy-1, and the proportion of IL-2Rβ in rafts was less than 5% in all experiments. Thus, in the presence of immobilized anti–Thy-1, IL-2Rα was further enriched in lipid rafts and is segregated from IL-2Rβ and IL-2Rγ localized in soluble membranes. This demonstration is consistent with our earlier report that IL-2–induced receptor heterotrimerization was inhibited by anti–Thy-1.18
The proportion of IL-2Rα in lipid rafts is increased in cells cultured in the presence of immobilized anti–Thy-1.
(A) CTLL-2 cells were cultured in the presence of immobilized anti–Thy-1 or an isotype-matched control (ctrl) mAb before lysis and sucrose density gradient ultracentrifugation. The localization of IL-2Rα and IL-2Rβ chains was assessed by immunoblotting the fractions of the sucrose gradients corresponding to lipid rafts and soluble membranes. (B) The proportion of IL-2Rα and IL-2Rβ in lipid rafts and soluble membranes was quantified by scanning densitometry. Data shown are represented as the percentages of the total IL-2Rα or IL-2Rβ localized in lipid rafts and are the averages from 3 independent experiments.
The proportion of IL-2Rα in lipid rafts is increased in cells cultured in the presence of immobilized anti–Thy-1.
(A) CTLL-2 cells were cultured in the presence of immobilized anti–Thy-1 or an isotype-matched control (ctrl) mAb before lysis and sucrose density gradient ultracentrifugation. The localization of IL-2Rα and IL-2Rβ chains was assessed by immunoblotting the fractions of the sucrose gradients corresponding to lipid rafts and soluble membranes. (B) The proportion of IL-2Rα and IL-2Rβ in lipid rafts and soluble membranes was quantified by scanning densitometry. Data shown are represented as the percentages of the total IL-2Rα or IL-2Rβ localized in lipid rafts and are the averages from 3 independent experiments.
Discussion
These results demonstrate that immobilized mAbs specific for GPI-AP inhibit IL-2–induced proliferation of primary T cells and in 2 T-cell lines, GPI+ 2.10 and CTLL-2 cells. Multiple GPI-APs, including Thy-1, Ly6A/E, and CD48, can mediate this effect, suggesting that the ability to affect IL-2R signaling reflects a common feature of these proteins. An important characteristic imparted by the GPI anchor is localization to lipid rafts. Lipid rafts are detergent-insoluble microdomains in the plasma membrane that are enriched in cholesterol and glycosphingolipids, including the ganglioside GM1.3Consistent with the notion that their localization to lipid rafts underlies the capacity of GPI-AP to modify IL-2R signaling, immobilization of GM1 using the β subunit of CT also inhibits IL-2–induced proliferation.
The ability of multiple components of lipid rafts to modify cellular responsiveness to IL-2 suggests that lipid rafts can regulate IL-2R signaling, notwithstanding the fact that IL-2R signaling does not appear to occur in these microdomains. Although IL-2Rα was enriched in lipid rafts isolated by sucrose density gradient ultracentrifugation, the IL-2Rβ and IL-2Rγ chains responsible for signal transduction were found in detergent-soluble membranes before and after stimulation of cells with IL-2. In addition, the Janus family kinases phosphorylated in response to IL-2, JAK1, and JAK3 were not detected in lipid rafts. Disruption of lipid rafts using MCD did not perturb IL-2R–induced signaling. Finally, in cells proliferating in response to IL-2, the active heterotrimeric receptor complex composed of IL-2Rα, IL-2Rβ, and IL-2Rγ bound to125I-labeled IL-2 was detected only in soluble membranes.
The function of lipid rafts in the coordination of signaling may be 2-fold. Several membrane receptors are inducibly recruited to or stabilized within these domains, including TCR, BCR, and FcεRI, and the subsequent activation of critical signaling molecules enriched in rafts may facilitate signaling.2 However, lipid rafts may also function to segregate signaling molecules in resting cells, maintaining low levels of signaling in the absence of stimulation. Thus, though the disruption of lipid rafts resulted in decreased TCR/CD3-induced signaling,26 increased basal levels of tyrosine phosphorylation were observed on treatment with MCD.27 In addition, MCD resulted in activation of the Ras-ERK pathway.27,28 Furthermore, the localization of Src PTKS in rafts may segregate these kinases from their substrates. Palmitoylation of Fyn, which results in its localization to lipid rafts, prevented its ability to phosphorylate a nonlipid raft resident chimeric Igα molecule.29 Src, which is not palmitoylated, and a nonpalmitoylated mutant of Fyn were able to phosphorylate Igα, suggesting that membrane compartmentalization regulates protein–protein interactions.
Thus, lipid rafts may be involved in the spatial regulation of intermolecular associations in the plasma membrane. In the context of TCR or BCR signaling, rafts result in a segregation of enzymes and substrates, and signaling is initiated on the regulated association of receptors with lipid rafts.2 In the context of IL-2R signaling, lipid rafts may mediate the segregation of receptor chains. Lipid raft components can affect the initiation of signaling in both receptor systems by interfering with the regulated assembly of molecules. The first indications that lipid rafts played a role in TCR/CD3-induced signaling were the demonstrations that mAbs specific for GPI-AP could inhibit or potentiate signaling through the antigen receptor [reviewed in 30], possibly by modifying the association of TCR/CD3 with lipid rafts. The demonstration herein that components of rafts modify IL-2 responsiveness similarly implicates lipid rafts in the regulation of IL-2R signaling. Lipid rafts may also be involved in regulating signaling through multiple cytokine receptors, as suggested by our preliminary evidence that the immobilization of GPI-AP inhibits the responsiveness of T cells to IL-4 and IL-15, of B cells to IL-7, and of mast cells to IL-3 (M.D.M. and M.J., unpublished observations, 2000).
Fluorescence resonance energy transfer analysis has revealed that IL-2Rα, IL-2Rβ, and IL-2Rγ chains are loosely associated in resting T-cell lymphoma lines.12 On IL-2 binding, the chains are brought closer, consistent with strengthened interactions or movement of the chains in the plasma membrane. IL-2Rα may be localized at the periphery of lipid rafts, where it can maintain a loose association with IL-2Rβ and IL-2Rγ. Binding of IL-2 and the subsequent strengthening of the interactions among IL-2Rα, IL-2Rβ, and IL-2Rγ may result in the dissociation of IL-2Rα from lipid rafts and the initiation of signaling in soluble membranes. Alternatively, signaling may be mediated only by IL-2Rα in soluble membranes. We propose that the inhibition of IL-2–induced proliferation observed on immobilization of GPI-AP or GM1 reflects effects on the mobility of IL-2Rα. These inhibitory effects may involve steric hindrance of the association of IL-2Rα with IL-2Rβ and IL-2Rγ, blocking a ligand-induced dissociation of IL-2Rα from lipid rafts or through a shift in the equilibrium such that IL-2Rα becomes trapped in lipid rafts. In support of its altered mobility, the proportion of IL-2Rα in lipid rafts is 2.5-fold higher in cells cultured in the presence of immobilized anti–Thy-1 than in controls.
No significant differences in the membrane distribution of IL-2Rα were detected on the stimulation of cells with IL-2 for 1 to 27 minutes (Figure 7 and data not shown). Because the levels of IL-2Rα chain expression on cell surfaces exceed those of IL-2Rβ and IL-2Rγ chains,31 only a fraction of available IL-2Rα will dissociate from lipid rafts and participate in signaling in detergent-soluble membranes. In these acute experiments, the change in the distribution of IL-2Rα is not detectable using Western blot analysis. In contrast, the difference in the proportion of IL-2Rα in lipid rafts in Figure 7 and the isotype control in Figure 9 may reflect ligand-induced changes in the localization of IL-2Rα with prolonged exposure to IL-2. In Figure 7, lipid rafts were isolated from “starved” CTLL-2 cells, cultured for 16 hours in minimal concentrations of IL-2 compatible with sustaining survival. In these circumstances, the proportion of IL-2Rα in lipid rafts varied between 37% and 70% in 8 experiments. Results presented in Figure 9 are derived from rafts isolated from CTLL-2 cells in log phase response to IL-2, circumstances in which 24.6% ± 6.0% of IL-2Rα was localized in rafts. This difference might have resulted from a shift in the equilibrium of IL-2Rα between rafts and soluble membranes as a consequence of prolonged stimulation with IL-2 and the resultant dissociation of IL-2Rα from rafts. Further, the IL-2R complex is internalized on IL-2 binding. IL-2Rα is subsequently recycled to the plasma membrane,32 and it is unknown whether recycling IL-2Rα molecules localize to lipid rafts or soluble membranes. Thus, though the dynamic equilibrium of IL-2Rα localization is altered in the presence of ligand, the time needed to observe a re-equilibration of IL-2Rα likely reflects constraints imposed by receptor physiology (re-use) and the relative abundance of IL-2R chains.
This report confirms the findings of 2 recent reports demonstrating the localization of IL-2Rα in lipid rafts,15,33 and it extends the analysis to IL-2Rβ, IL-2Rγ, and ligand binding. Moreover, these results provide the first demonstration of a functional role for lipid rafts in the regulation of IL-2R signaling. Field et al33 demonstrated that in transfected Chinese hamster ovary cells, IL-2Rα became associated with TX-100 insoluble domains on cross-linking. In contrast, we observed an IL-2–independent enrichment of IL-2Rα in lipid rafts. This apparent contradiction likely reflects the differing detergents used (0.5% Brij58 vs 0.05% TX-100) and the differing detergent-to-cell ratios, and it highlights a limitation imposed by the methodology used to examine lipid raft constituents. Specifically, weak associations of proteins with lipid rafts may be disrupted using detergents. Field et al33,34also observed that FcεRI aggregation was required for its association with TX-100–insoluble domains. However, recent data suggest that FcεRI localization in rafts is constitutive but weak and that it is strengthened and rendered detergent resistant on cross-linking. High-resolution immunogold labeling and electron microscopy revealed that in unstimulated cells, monomeric FcεRI is distributed in small clusters that also contain the Src family PTK Lyn and that likely represent lipid rafts.35 Similarly, the association of IL-2Rα with lipid rafts is likely constitutive but weak, and we have observed that though it is maintained in the presence of Brij58, 0.5% TX-100 disrupts the association of IL-2Rα, but not of Fyn and Thy-1, with lipid rafts (data not shown). Consistent with this interpretation, relative to other nonionic detergents, Brij58 has been shown to better preserve associations of proteins with lipid rafts.36Using immunogold labeling and electron microscopy, Vereb et al15 demonstrated that IL-2Rα exists in clusters in the presence or absence of IL-2. Clusters of IL-2Rα colocalized with clusters of CD48; moreover, the cluster size was modulated by MCD, suggesting they represented lipid rafts. The present study demonstrates that only IL-2Rα is enriched in lipid rafts isolated in the presence of Brij58. However, it remains possible that IL-2Rβ and IL-2Rγ associate with rafts even more weakly than IL-2Rα and that this association is sensitive to Brij58. Electron microscopic analyses of IL-2Rα, IL-2Rβ, and IL-2Rγ before and after stimulation with IL-2 should provide a definitive picture of the membrane compartmentalization of IL-2R.
A recent report detected interferon receptors JAK1 and JAK3 in caveolae isolated from mouse embryonic fibroblasts.37 Caveolae, flask-shaped membrane invaginations, are a subset of lipid rafts. Although these 2 plasma membrane domains share common features, including detergent insolubility, they can be separated experimentally and show differing protein composition and morphology.1 In addition, lipid rafts are present in cells, including lymphocytes, that do not express caveolin, a cholesterol-binding integral membrane protein essential for caveolae formation.1 The caveolar localization of JAKs may not be relevant to their subcellular localization in lymphocytes because many molecules localize to caveolae through a direct interaction with caveolin. Indeed, examination of the sequences of all JAK kinases reveals the presence of a caveolin binding motif, ΦXΦXXXXΦ, where Φ represents any of the aromatic amino acids tyrosine, phenylalanine, or tryptophan.38Furthermore, Takaoka et al37 isolated caveolae using a detergent-free method. Because differential results with regard to the localization of some proteins have been observed using detergent and nondetergent fractionation of membranes,39 40 further study is required to confirm the caveolar localization of JAKs in fibroblasts.
The results presented herein demonstrate that components of lipid rafts inhibit IL-2–induced proliferation in vitro; however, potential roles for GPI-AP and lipid raft–associated gangliosides in regulating IL-2R–mediated signaling in vivo remain to be determined. T-cell proliferative responses appear normal in the absence of GPI-AP in mice bearing T-cell–specific disruption of a gene critical for GPI anchor biosynthesis.41 In contrast, T cells from mice lacking all complex gangliosides, including GM1, because of targeted deletion of the gene encoding the GM2/GD2 synthase display decreased IL-2–induced signaling and proliferation.42 Whether the defects in IL-2R signaling in these cells relate to lipid rafts awaits further investigation.
We thank Drs B. Leung and S. Ilangumaran for helpful discussions and reading of the manuscript, and we thank Drs B. Drucker for kindly providing us with the 4G10 mAb, F. Fitch for the D7 hybridoma, T. Malek for the 5H4 and 3E12 mAbs, H. Reiser for the 5-8A10 hybridoma, D. Sachs for the Qa-2–specific hybridomas, L. Thompson for the TY/23 mAb, and A. Veillette for the Fyn-specific antiserum.
Supported by the Canadian Institutes for Health Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Michael Julius, Sunnybrook and Women's College, Health Sciences Centre, Room A3 33, 2075 Bayview Ave, Toronto, Ontario, Canada M4N 3M5; e-mail: michael.julius@utoronto.ca.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal