An unusual CD18 monoclonal antibody (mAb) MEM-148 binds, in contrast to standard CD18 mAbs, specifically to peripheral blood monocytes and neutrophils activated by various stimuli such as phorbol myristate acetate, opsonized zymosan, heat-aggregated immunoglobulin, and (after priming with lipopolysaccharide, tumor necrosis factor, or granulocyte-macrophage colony-stimulating factor) also by formyl-methionyl-leucyl-phenylalanine. In addition, in vivo activated neutrophils obtained from urine of patients following recent prostatectomy were also strongly positive for MEM-148. On the activated myeloid cells the mAb recognized a 65- to 70-kd protein identified immunochemically and by mass spectrometric peptide sequencing as a membrane-anchored fragment of CD18 (the common chain of leukocyte integrins) produced by proteolytic cleavage. The CD18 fragment originated mainly from integrin molecules stored intracellularly in resting cells, it was unassociated with CD11 chains, and its formation was inhibited by several types of protease inhibitors. Thus, the 65- to 70-kd CD18 fragment represents a novel abundant activation marker of myeloid cells of so far unknown function but possibly involved in conformational changes in leukocyte integrin molecules resulting in increased affinity to their ligands.
Introduction
Activation of myeloid cells by various physiologic and experimental stimuli is accompanied by multiple surface changes connected mainly to degranulation (externalization and thus enhanced surface expression of several membrane proteins stored in cytoplasmic granules), proteolytic shedding, and internalization of distinct sets of molecules. Thus, activated blood myeloid cells typically up-regulate surface expression of complement receptor type 3 (CR3; CD11b/CD18), alkaline phosphatase, or chemotactic receptors1 and down-modulate surface density of lipopolysaccharide (LPS) receptor CD14, adhesion receptors CD44 and CD62L, or antiadhesion sialoglycoprotein CD43.2
Leukocyte integrins are major adhesion molecules of white blood cells. They are noncovalent transmembrane heterodimers composed of the common β2 chain (CD18; 95 kd) and one of α chains, αL (CD11a; 180 kd), αM (CD11b; 170 kd), αX (CD11c; 150 kd), or αD (CD11d; 150 kd). Leukocyte (β2) integrins display a remarkable redundancy of ligand binding. The CD11a/CD18 heterodimer called leukocyte function-associated antigen-1 (LFA-1) is the major leukocyte adhesion receptor recognizing differentially expressed membrane ligands ICAM-1 (CD54), ICAM-2 (CD102), ICAM-3 (CD50), ICAM-4 (carrying Landsteiner-Wiener (LW) blood group antigens), and ICAM-5 (telencephalin). CD11b/CD18 and CD11c/CD18 heterodimers are usually called complement receptor type 3 (CR3) and type 4 (CR4), respectively, because they bind iC3b fragments of complement, but they also recognize ICAMs as well as a number of other ligands, such as extracellular matrix proteins. (Leukocyte integrins and their ligands are reviewed elsewhere.3-6) Leukocyte integrins can exist in still poorly characterized activated states based on increased affinity or avidity.7-13 The adhesiveness of integrin molecules is regulated from the cell interior (inside-out signaling) via interactions through their cytoplasmic tails involving allosteric and mobility changes. Ligand binding is influenced also extracellularly by coordination of distinct divalent cations and can result in triggering of intracellular signals (outside-in signaling).
In this report we describe a novel activation marker abundantly expressed on the surface of activated monocytes and neutrophils—a 65- to 70-kd proteolytic fragment of the common β chain of leukocyte (β2) integrins, CD18, as detected by a monoclonal antibody (mAb) recognizing a unique CD18 epitope.
Materials and methods
Reagents and antibodies
If not stated otherwise, all reagents were purchased from Sigma (St Louis, MO). CD18 mAbs MEM-48 (IgG1 [immunoglobulin G1]) and MEM-148 (IgG1) were obtained by standard techniques from mice immunized with peripheral blood mononuclear cells and their CD18 specificity was unambiguously determined in our laboratory.14,15 The CD18 specificity of MEM-148 was confirmed during the 6th Human Leukocyte Differentiation Antigen (HLDA) Workshop.16 The mAbs MEM-83 (IgG1; CD11a), MEM-170 (IgG1; CD11b), MEM-135 (IgG1; HLA class I), and B2M-02 (IgG1; β2-microglobulin) were also produced and characterized in our laboratory. The mAb LeuM5 (IgG2b; CD11c) was obtained within the 6th HLDA Workshop and 6.7 (IgG1; CD18) was kindly provided by Dr A. Bensussan (INSERM 448, Creteil, France).
Cells
Fresh buffy coats (a mixture of peripheral blood leukocytes [PBLs]) in the heparinized plasma and neutrophils isolated from urine of patients after prostatectomy were used. Monocytes and neutrophils were separated by Ficoll (Pharmacia, Uppsala, Sweden) gradient centrifugation and were further purified based on adhesion to polystyrene Petri dishes (monocytes) or osmotic lysis of erythrocytes (neutrophils); their purity was greater than 92% and 95%, respectively. Cells were treated for up to 2 hours at 37°C with various activating and priming agents: phorbol myristate acetate (PMA; 100 ng/mL), opsonized zymosan (OZ; 300 μg/mL; opsonized with 50% human serum for 30 minutes at 37°C), heat-aggregated immunoglobulin (HA-Ig; 100 μg/mL; prepared by heating 100 mg/mL human IgG for 2 hours at 63°C and purified by gel filtration on Superdex 200 HR 10/30 column; Pharmacia), LPS (100 ng/mL; Difco, Detroit, MI), tumor necrosis factor (TNF; 20 ng/mL; R & D Systems, Minneapolis, MN), granulocyte-macrophage colony-stimulating factor (GM-CSF; 100 ng/mL; Sandoz, Basel, Switzerland), or formyl-methionyl-leucyl-phenylalanine (fMLP; 1 μg/mL). Before treatment with 1 μg/mL fMLP the cells were primed with LPS, TNF, or GM-CSF for 30 minutes at 37°C. In some experiments cells were preincubated (10 minutes, 37°C) before the PMA treatment with protease inhibitors: diisopropyl fluorophosphate (DFP; 5 mM), 4-(2-aminoethyl)-benzenesulfonyl fluoride (AEBSF; 1 mM), 1,10-phenanthroline (Phen; 5 mM), iodoacetic acid (IAA; 2 mM), Nα-p-tosyl-l-lysine chloromethyl ketone (TLCK; 0.1 mM), N-tosyl-l-phenylalanine chloromethyl ketone (TPCK; 0.1 mM), aprotinin (10 μg/mL), phenylmethylsulfonylfluoride (PMSF; 0.8 mM), EDTA (5 mM), ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (5 mM; EGTA) or [(2S,3R)-3-amino-2-hydroxy-4-phenylbutanoyl]-l-leucine (bestatin; 25 μg/mL). Low pH–treated cells were prepared by incubation (2 minutes on ice) in isotonic citrate/phosphate buffer (pH 3.5) and immediate neutralization in Hanks balanced salt solution (HBSS).
Flow cytometry
Cells were stained with mAbs (20 μg/mL) for 30 minutes on ice and washed in HBSS containing 0.2% gelatin and 0.1% NaN3without Ca++ and Mg++ followed by fluorescein-labeled goat F(ab′)2 antimouse Ig (Jackson Immunoresearch, West Grove, PA). Propidium iodide (PI; 0.1 μg/mL) and LDS-751 (0.2 μg/mL; Exciton, Dayton, OH) were added prior to measurement on a FACSort flow cytometer (Becton Dickinson, Mountain View, CA) in a standard 3-color setup. At least 104 viable nucleated cells (PI−, LDS-751+) were collected for each sample. Leukocyte populations were resolved on the basis of their scatter properties.
Immunoprecipitation, sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and Western blotting
Cells were surface biotinylated using biotinamidocaproic acid 3-sulfo-N-hydoxysuccinimide ester and solubilized on ice in isotonic lysis buffer containing 1% Nonidet P40 (NP40) detergent and standard mixture of protease inhibitors; the supernatant was used directly for sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting, immunoprecipitation (solid-phase immunoisolation technique), or immunoaffinity chromatography. Immunoprecipitated biotinylated proteins were detected on the blots by streptavidin-peroxidase conjugate and luminographic visualization. The immunoaffinity columns were washed with 10 volumes of the lysis buffer, the adsorbed proteins were eluted with alkaline buffer (0.1 M glycine-NaOH, pH 11.5, containing 0.1% NP40), and separated by nonreducing SDS-PAGE. Details of biochemical methods were described previously.15 Densitometric evaluation of the zones on Western blots has been performed on LAS-1000 (Fuji Photo Film, Tokyo, Japan).
Native electrophoresis and 2-dimensional electrophoresis (native versus SDS-PAGE)
Native non–SDS-PAGE (blue native electrophoresis, BNE) was performed essentially as described elsewhere.17 Briefly, 5 × 106 cells were solubilized in 200 μL of the native lysis buffer (1% n-dodecyl β-d-maltoside, 5 mM iodoacetamide and 1 mM 4-[2-aminoethyl]benzenesulfonyl fluoride in 750 mM aminocaproic acid, 50 mM Bis-tris, pH 7.0) and centrifuged for 3 minutes at 20 000g; Coomassie brilliant blue G was mixed with the supernatant to yield the final concentration of 0.25%. The 5-μL samples were run on water-cooled gradient gels (6%-15%). A strip of the gel corresponding to single lane was equilibrated in the nonreducing sample buffer for SDS-PAGE and used for second-dimension separation by standard SDS-PAGE (7.5% gel). The separated proteins were then electroblotted onto polyvinylidene difluoride (PVDF) membrane and visualized by immunoperoxidase staining. The molecular weight standards used for the first-dimension separation (BNE) were monomers and oligomers (obtained by chemical cross-linking) of bovine serum albumin or mouse IgG1 mAb.
Mass spectrometric analysis
The Coomassie brilliant blue–stained protein band was cut from the gel and washed several times with 10 mM dithiotreitol (DTT) and 0.1 M 4-ethylmorpholine acetate (pH 8.1) in 50% acetonitrile. After complete destaining, the gel was washed with water, shrunk by dehydration with acetonitrile, and reswollen again in water. Next, the gel was partly dried using a SpeedVac concentrator (Savant Instruments, Holbrook, NY) and then reconstituted with cleavage buffer containing 0.01% 2-mercaptoethanol, 0.1 M 4-ethylmorpholine acetate, 10% acetonitrile, 1 mM CaCl2, and sequencing grade trypsin (Promega, Madison, WI; 50 ng/μL). Digestion was carried out overnight at 37°C; the resulting peptide mixture was extracted with 50% acetonitrile/1% trifluoroacetic acid and separated on the C18 reverse-phase column (0.3 × 150 mm; LC Packings, Amsterdam, The Netherlands) using gradient elution (5% acetonitrile/0.5% acetic acid to 95% acetonitrile/0.4% acetic acid). The column was linked to the nano-electrospray ionization (ESI) interface of the mass spectrometer. Positive full scan and collision-induced dissociation mass spectra were recorded on a LCQ DECA ion trap mass spectrometer (Thermoquest, San Jose, CA) equipped with nano-ESI ion source and interpreted manually. Spray voltage was held at 2.2 kV, tube lens voltage was −10 V. The heated capillary was kept at 150°C with a voltage of 32 V. Full scan spectra were acquired over m/z range 300 to 2000 d. Collision energy was kept at 42 units and the activation time was 30 ms. Collisions were done from the first intense ion in the each chromatographic peak; every 2 scans were accumulated.
Results
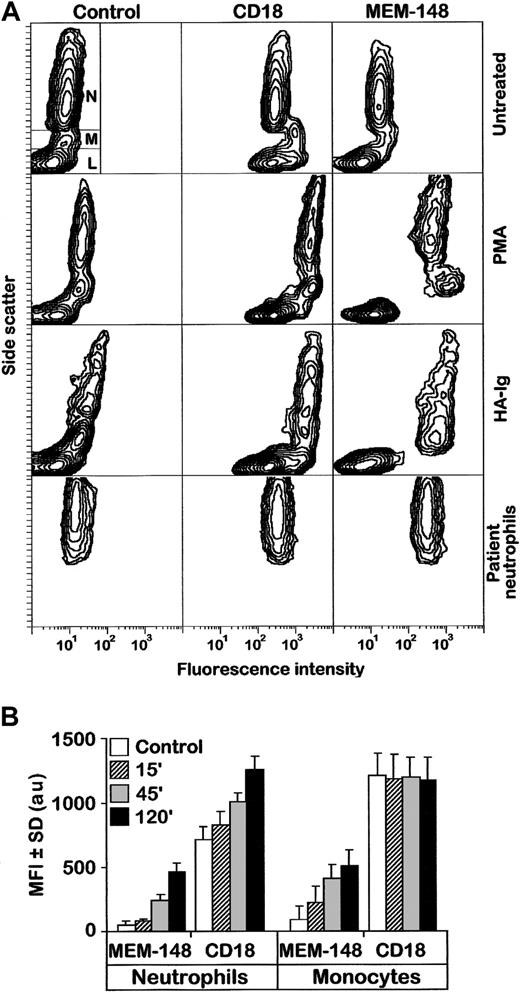
The MEM-148 mAb (either intact IgG or its Fab fragment) bound only very weakly to resting PBLs, but the respective antigen was up-regulated on monocytes and neutrophils activated by PMA, OZ, HA-Ig, and (after priming with either LPS, TNF, or GM-CSF) also by fMLP (Figure 1A and data not shown). The MEM-148 epitope became clearly detectable after 15 minutes of treatment and gradually increased during 2 hours (Figure 1B and data not shown). Its appearance was closely paralleled by microscopically observable firm aggregation of the myeloid cells or their adhesion to serum-coated plastic, which is well known to be elicited by all these treatments (not shown). Neutrophils obtained from urine of prostatectomy patients were strongly positive for MEM-148 without any treatment (Figure 1A); these cells were obviously naturally activated as a consequence of local inflammation. Moreover, all PBLs (including lymphocytes) became strongly positive for MEM-148 after a brief exposure to acidic solutions, MEM-148 stained 78- to 96-kd zones corresponding to various forms of CD18 on Western blots of nonreduced leukocyte detergent lysates, and strongly reacted with CD18-transfected COS-7 cells.15 Thus, MEM-148 obviously reacts with an epitope of CD18, which is inaccessible in CD11/CD18 heterodimers present on resting leukocytes but becomes exposed during dissociation of the heterodimers by low pH or SDS and also as a result of a conformational change caused by cell activation.
Activation of monocytes and neutrophils is accompanied by expression of a unique CD18 epitope recognized by mAb MEM-148.
(A) PBLs were left either untreated or treated with PMA or HA-Ig for 2 hours, stained by MEM-148, and analyzed by flow cytometry. As controls, an irrelevant IgG1 mAb (Control) and a standard CD18 mAb MEM-48 (CD18) were used. The results are expressed as standard side scatter (y-axis) versus logarithmic fluorescence intensity (x-axis) contour plots. The gates corresponding to neutrophils (N), monocytes (M), and lymphocytes (L) are shown in the first plot (top, left). The bottom panel shows staining of untreated neutrophils isolated from urine of a patient following prostatectomy. (B) Purified monocytes and neutrophils were activated with PMA and at different time points stained with MEM-148 or standard CD18 mAb (MEM-48). Results from 3 experiments are represented as mean fluorescence intensity (MFI) ± SD values (arbitrary units) calculated from geometric means of fluorescence intensity after subtraction of irrelevant isotype control.
Activation of monocytes and neutrophils is accompanied by expression of a unique CD18 epitope recognized by mAb MEM-148.
(A) PBLs were left either untreated or treated with PMA or HA-Ig for 2 hours, stained by MEM-148, and analyzed by flow cytometry. As controls, an irrelevant IgG1 mAb (Control) and a standard CD18 mAb MEM-48 (CD18) were used. The results are expressed as standard side scatter (y-axis) versus logarithmic fluorescence intensity (x-axis) contour plots. The gates corresponding to neutrophils (N), monocytes (M), and lymphocytes (L) are shown in the first plot (top, left). The bottom panel shows staining of untreated neutrophils isolated from urine of a patient following prostatectomy. (B) Purified monocytes and neutrophils were activated with PMA and at different time points stained with MEM-148 or standard CD18 mAb (MEM-48). Results from 3 experiments are represented as mean fluorescence intensity (MFI) ± SD values (arbitrary units) calculated from geometric means of fluorescence intensity after subtraction of irrelevant isotype control.
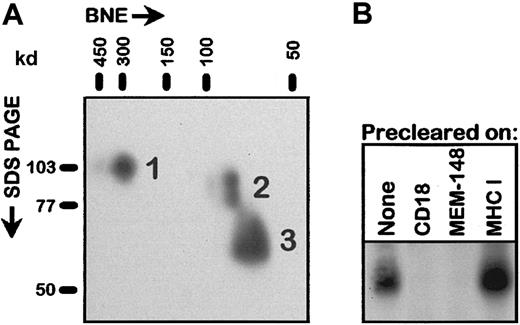
Surprisingly, MEM-148 specifically immunoprecipitated a protein of 65- to 70-kd from detergent lysates of activated, surface biotinylated PBLs. This protein was also immunoprecipitated (in addition to the CD11/CD18 heterodimers) by standard CD18 mAbs but not by mAbs to CD11a, CD11b, or CD11c (Figure 2A). This zone was more prominent in the immunoprecipitates obtained from the cells that were surface labeled after PMA activation rather than before the activation, indicating that most of the 65- to 70-kd protein (or its precursor) appeared on the cell surface only after the activation. The kinetics of appearance of this molecule following activation by PMA closely paralleled the increase of the MEM-148 positivity (Figures 1B and 2B). A protein of apparently identical size and recognized not only by MEM-148 but also by at least some other CD18 mAbs could be clearly detected (in addition to the intact CD18 molecular species) by Western blotting in the lysates of PBLs activated by various stimuli (Figure2C) and in the lysates of in vivo activated neutrophils isolated from urine of prostatectomized patients (not shown). These results indicated that the 65- to 70-kd protein is a truncated form of CD18, apparently unassociated with CD11 chains. Indeed, analysis by 2-dimensional electrophoresis (native BNE versus nonreducing SDS-PAGE) demonstrated that the 65- to 70-kd CD18 form was not associated with either CD11 chains or any other proteins (Figure 3A). To confirm that this protein is actually a form of CD18 and not a different, cross-reactive protein, detergent cell lysate of PMA-activated PBLs was first precleared on a standard anti-CD18 immunosorbent and the unbound fraction was subjected to SDS-PAGE and Western blotting using MEM-148. As shown in Figure 3B, the 65- to 70-kd protein was quantitatively adsorbed to the CD18 immunosorbent. Finally, the protein was immunopurified from the lysate of PMA-activated PBLs and analyzed by mass spectrometric peptide mapping and sequencing; 4 CD18-derived peptides were unambiguously identified by sequencing (Table 1). The results of this peptide mapping suggest that the fragment comprises the major C-terminal part of CD18 involving at least residues 345 to 733. However, it is not clear at this moment where exactly is the N-terminus of the fragment.
The activation epitope recognized by MEM-148 is present on a 65- to 70-kd protein.
(A) Immunoprecipitation from surface-biotinylated PBLs. The cells were biotinylated either before or immediately after 2-hour PMA treatment; control cells were biotinylated and left untreated. The cell lysates (1% NP40) were immunoprecipitated by means of mAbs to the indicated molecules (MEM-148; 6.7, CD18; MEM-83, CD11a; MEM-170, CD11b; LeuM5, CD11c; B2M-02, β2-microglobulin) and the immunoprecipitates subjected to SDS-PAGE followed by Western blotting using streptavidin-peroxidase to reveal biotinylated proteins. (B) Isolated neutrophils or monocytes were activated with PMA as in Figure1B or left untreated for 120 minutes (Control) and at the indicated time points (15-120 minutes) analyzed by SDS-PAGE and immunoblotting using MEM-148 for detection (top panels); the bottom panel shows quantitative densitometric evaluation (plotted for the respective lanes as ratio of the signal from the 65- to 70-kd zone [closed arrowhead] versus the upper 78- to 96-kd CD18 zone [open arrowhead]). (C) Western blotting analysis of PBLs after various activating treatments. PBLs were activated using the indicated stimuli for 2 hours (for the different activation protocols see “Materials and methods”). The blot was immunoperoxidase-stained with the standard CD18 mAb MEM-48; a very similar, albeit weaker, staining pattern was obtained also with MEM-148 (not shown). Open and closed arrowheads in all parts of the Figure 2 indicate the zones corresponding to the uncleaved CD18 species (78-96 kd) and the 65- to 70-kd fragment, respectively.
The activation epitope recognized by MEM-148 is present on a 65- to 70-kd protein.
(A) Immunoprecipitation from surface-biotinylated PBLs. The cells were biotinylated either before or immediately after 2-hour PMA treatment; control cells were biotinylated and left untreated. The cell lysates (1% NP40) were immunoprecipitated by means of mAbs to the indicated molecules (MEM-148; 6.7, CD18; MEM-83, CD11a; MEM-170, CD11b; LeuM5, CD11c; B2M-02, β2-microglobulin) and the immunoprecipitates subjected to SDS-PAGE followed by Western blotting using streptavidin-peroxidase to reveal biotinylated proteins. (B) Isolated neutrophils or monocytes were activated with PMA as in Figure1B or left untreated for 120 minutes (Control) and at the indicated time points (15-120 minutes) analyzed by SDS-PAGE and immunoblotting using MEM-148 for detection (top panels); the bottom panel shows quantitative densitometric evaluation (plotted for the respective lanes as ratio of the signal from the 65- to 70-kd zone [closed arrowhead] versus the upper 78- to 96-kd CD18 zone [open arrowhead]). (C) Western blotting analysis of PBLs after various activating treatments. PBLs were activated using the indicated stimuli for 2 hours (for the different activation protocols see “Materials and methods”). The blot was immunoperoxidase-stained with the standard CD18 mAb MEM-48; a very similar, albeit weaker, staining pattern was obtained also with MEM-148 (not shown). Open and closed arrowheads in all parts of the Figure 2 indicate the zones corresponding to the uncleaved CD18 species (78-96 kd) and the 65- to 70-kd fragment, respectively.
The 65- to 70-kd molecule is a free fragment of CD18 unassociated with CD11 chains.
(A) PMA-activated PBLs were lysed in 1% n-dodecyl β-d-maltoside and subjected to BNE followed by the second-dimension SDS-PAGE, electroblotting, and immunoperoxidase detection with a standard CD18 mAb MEM-48. The numbered spots correspond to (1) CD18 molecules present in the noncovalent integrin heterodimers, (2) free intracellular precursor forms of CD18 described earlier,15 and (3) free 65- to 70-kd CD18 fragment. It should be noted that under the conditions of BNE the noncovalent integrin heterodimeric complexes are preserved. Positions of molecular weight standards in both dimensions are indicated. (B) Detergent lysates of PMA-activated PBLs were passed through immunosorbents based on immobilized mAbs to the indicated antigens: CD18 (MEM-48), MEM-148, MHC class I (MEM-135; negative control), and the unbound fractions or whole cell lysate (None) were subjected to SDS-PAGE and Western blotting using MEM-148; only the relevant 50- to 80-kd region of the blot is shown.
The 65- to 70-kd molecule is a free fragment of CD18 unassociated with CD11 chains.
(A) PMA-activated PBLs were lysed in 1% n-dodecyl β-d-maltoside and subjected to BNE followed by the second-dimension SDS-PAGE, electroblotting, and immunoperoxidase detection with a standard CD18 mAb MEM-48. The numbered spots correspond to (1) CD18 molecules present in the noncovalent integrin heterodimers, (2) free intracellular precursor forms of CD18 described earlier,15 and (3) free 65- to 70-kd CD18 fragment. It should be noted that under the conditions of BNE the noncovalent integrin heterodimeric complexes are preserved. Positions of molecular weight standards in both dimensions are indicated. (B) Detergent lysates of PMA-activated PBLs were passed through immunosorbents based on immobilized mAbs to the indicated antigens: CD18 (MEM-48), MEM-148, MHC class I (MEM-135; negative control), and the unbound fractions or whole cell lysate (None) were subjected to SDS-PAGE and Western blotting using MEM-148; only the relevant 50- to 80-kd region of the blot is shown.
Peptides identified in the 65- to 70-kd fragment by mass spectrometric sequencing
| Position in CD18 polypeptide chain . | Sequence* . |
|---|---|
| 345-357 | VFLDHNALPDTLK |
| 612-619 | FEKGPFGK |
| 663-671 | YLIYVDESR |
| 723-733 | SQWNNDNPLFK |
| Position in CD18 polypeptide chain . | Sequence* . |
|---|---|
| 345-357 | VFLDHNALPDTLK |
| 612-619 | FEKGPFGK |
| 663-671 | YLIYVDESR |
| 723-733 | SQWNNDNPLFK |
Single-letter amino acid codes.
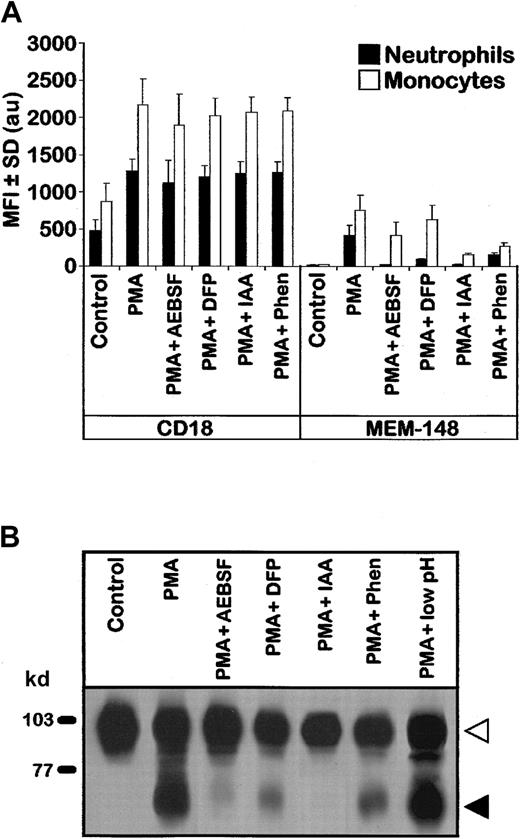
Expression of the MEM-148 epitope on the surface of activated myeloid cells, as well as the appearance of the 65- to 70-kd CD18 form, were markedly suppressed by several types of protease inhibitors (Figure4). It should be noted that under the conditions used, degranulation induced by the activation stimuli was essentially unaffected by the inhibitors as judged by typical increased expression of leukocyte integrins (Figure 4A). Interestingly, inhibitors of serine proteases (DFP, AEBSF), cysteine proteases (IAA), and metalloproteases (Phen) exhibited partial inhibitory effects (see “Discussion”). Notably, serine protease inhibitors DFP and AEBSF were only very slightly inhibitory on monocytes as compared to neutrophils. The 65- to 70-kd fragment of CD18 could not be removed from the cell surface by acid washing (Figure 4B, last lane) indicating that it possesses the transmembrane segment anchoring it firmly to the cell surface. This conclusion is strongly supported also by the fact that it contains a peptide (723-733) originating from the cytoplasmic domain (Table 1).
Expression of the activation-induced CD18 fragment is suppressed by protease inhibitors.
(A) PBLs (as shown in Figure 1) were first preincubated with the indicated protease inhibitors, then activated with PMA, stained with MEM-148 or standard CD18 mAb (MEM-48), and analyzed by flow cytometry. Results from 3 experiments are represented as MFI ± SD values (arbitrary units) calculated from geometric means of fluorescence intensity for gated neutrophils and monocytes after subtraction of irrelevant isotype control. Other protease inhibitors tested (TLCK, TPCK, aprotinin, PMSF, bestatin, EDTA, or EGTA) showed less than 10% inhibition as detected by flow cytometry; actually, further increase of the MEM-148 epitope was observed when the PMA treatment was performed in the presence of 5 mM EDTA or EGTA (not shown). (B) Detergent lysates of the cells treated with the indicated protease inhibitors and PMA (as in panel A) were analyzed by SDS-PAGE and immunoblotting (staining with CD18 mAb MEM-48). The last lane shows PBLs treated with PMA for 2 hours and then exposed for 2 minutes at 0°C to pH 3.5 to wash away any noncovalently associated and nonmembrane-anchored protein fragments. Control low pH sensitive protein β2-microglobulin was fully dissociated from the cell surface under these experimental conditions (not shown). Open and closed arrowheads indicate the zones corresponding to the uncleaved CD18 and the fragment, respectively.
Expression of the activation-induced CD18 fragment is suppressed by protease inhibitors.
(A) PBLs (as shown in Figure 1) were first preincubated with the indicated protease inhibitors, then activated with PMA, stained with MEM-148 or standard CD18 mAb (MEM-48), and analyzed by flow cytometry. Results from 3 experiments are represented as MFI ± SD values (arbitrary units) calculated from geometric means of fluorescence intensity for gated neutrophils and monocytes after subtraction of irrelevant isotype control. Other protease inhibitors tested (TLCK, TPCK, aprotinin, PMSF, bestatin, EDTA, or EGTA) showed less than 10% inhibition as detected by flow cytometry; actually, further increase of the MEM-148 epitope was observed when the PMA treatment was performed in the presence of 5 mM EDTA or EGTA (not shown). (B) Detergent lysates of the cells treated with the indicated protease inhibitors and PMA (as in panel A) were analyzed by SDS-PAGE and immunoblotting (staining with CD18 mAb MEM-48). The last lane shows PBLs treated with PMA for 2 hours and then exposed for 2 minutes at 0°C to pH 3.5 to wash away any noncovalently associated and nonmembrane-anchored protein fragments. Control low pH sensitive protein β2-microglobulin was fully dissociated from the cell surface under these experimental conditions (not shown). Open and closed arrowheads indicate the zones corresponding to the uncleaved CD18 and the fragment, respectively.
Taken together, our data show that activation of peripheral blood monocytes and neutrophils is accompanied by formation of large amounts of a 65- to 70-kd proteolytic transmembrane fragment of CD18 unassociated with other proteins.
Discussion
Adhesion of blood myeloid cells to endothelia of blood vessels in sites of inflammation and their subsequent transmigration into the tissue is accompanied by major reorganization of their surface—rapid up-regulation of numerous receptors and adhesion molecules and parallel down-regulation of other surface molecules. This process is based mainly on (1) externalization of molecules stored in the resting cells in the membranes of several types of cytoplasmic vesicles (granules), (2) endocytosis (internalization) of other cell surface molecules, and (3) proteolytic cleavage of specific surface proteins resulting in their removal (shedding) or structural and functional modifications.1,2 Extensive proteolytic activity of leukocytes is also directed toward endothelia and extracellular matrix in inflamed sites.18,19 The β2-integrins are crucial players in the later steps of leukocyte firm adhesion and spreading on the endothelia and also in the following transmigration process.3,5,20-22 These adhesion molecules are strongly up-regulated on the surface of activated leukocytes and converted into so far poorly characterized high-affinity molecular conformations23-25 or aggregated into high-avidity clusters.13
In this study, we show that CD18, a common chain of β2-integrins, is proteolytically cleaved on the surface of activated myeloid cells. The resulting free 65- to 70-kd fragment of CD18 is expressed apparently as a free molecule unassociated with CD11 chains or other molecules and represents a novel abundant activation marker of myeloid cells. This fragment is most likely produced by proteases released from secretory granules of the activated cells or by activated membrane-associated proteases1,2,18,19,26 and comes predominantly from integrin molecules stored intracellularly in resting cells (ie, from intracellular mature heterodimers or perhaps also from unassociated intracellular CD18 chains demonstrated recently by us15). Our somewhat surprising finding that inhibitors of several protease classes (serine-, cysteine-, and metalloproteases) block the production of this fragment can be plausibly explained by possible dependence of this process on a cascade of consecutively activated proteases analogous to similar previously described cases.18,27-31 Another possibility is that several close cleavage sites may exist in the CD18 molecule, which are used by different proteases. This would be in agreement with the observation that the 65- to 70-kd fragment migrates as a relatively broad zone on SDS-PAGE, indicating a size heterogeneity. Actually, members of 2 serine protease systems, namely uPAR (CD87)32 and leukocyte elastase,33 have been shown to interact with β2-integrins and modulate their adhesive capacity.33,34 In addition to the protease inhibition, the effect of cysteine protease inhibitor might reflect an alteration in extracellular cysteine-rich domain conformation to facilitate the protease access. It should be noted that proteolytic cleavage of CD18 occurs in the presence of serum, which is known to contain high concentrations of endogenous protease inhibitors.18
Transmembrane fragments of CD18 produced by the activation-induced proteolytic cleavage obviously loose their association with CD11 chains and expose the epitope recognized by mAb MEM-148, which is sterically blocked in the intact integrin heterodimers.15 It is not clear what happens to the respective CD11 chains during this process (we did not have suitable antibodies reactive with free CD11 chains or their fragments). One speculative but attractive possibility is that the proteolytic cleavage and concomitant dissociation of the major CD18 fragment may uncover ligand-binding sites in the α chains (CD11). Such free CD11 chains (perhaps still associated with a putative small CD18 N-terminal fragment containing I-like domain35) might represent an unconventional form of a “high-affinity conformation” of the β2-integrin molecules and thus contribute to the known activation-induced homotypic aggregation. This would be in agreement with the previously described relaxation of conformational constraints in both cytoplasmic36 and extracellular cysteine-rich domains37 of CD18 accompanying the transition into the activated state.
Actually, a proteolytic activation of platelet β3-integrins has been already described.38On the other hand, the observed cleavage of CD18 may simply be a first step of a degradative process down-regulating the amounts of functional cell surface β2-integrins in the later phases of the adhesion process as described before.39 However, that process appears to exhibit a different cleavage site specificity, kinetics, and sensitivity to protease inhibitors.39 It can be even speculated that the proteolytic fragments of CD18 might serve some unknown functions possibly quite unrelated to adhesion; for example, proteolytic fragments of other adhesion molecules, CD62E and CD106, act as chemotactic factors for endothelial cells and promote angiogenesis.40 Finally, it would be interesting to examine whether other activation-dependent anti-CD18 mAbs, such as KIM127,41 CBRM1/19,42 and 240Q43also recognize specifically the CD18 fragment described here; unfortunately these mAbs were not available to us in the present study.
We thank Dr A.Bensussan for providing us the 6.7 CD18 mAb.
Supported by grant 310/99/0349 from the Grant Agency of the Czech Republic and from the project Center of Molecular and Cellular Immunology LN00A026, Ministry of Education of the Czech Republic.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Dr Václav Hořejšı́, Institute of Molecular Genetics, Academy of Sciences of the Czech Republic, Vı́deňská 1083, 142 20, Prague 4, Czech Republic; e-mail: horejsi@biomed.cas.cz.

![Fig. 2. The activation epitope recognized by MEM-148 is present on a 65- to 70-kd protein. / (A) Immunoprecipitation from surface-biotinylated PBLs. The cells were biotinylated either before or immediately after 2-hour PMA treatment; control cells were biotinylated and left untreated. The cell lysates (1% NP40) were immunoprecipitated by means of mAbs to the indicated molecules (MEM-148; 6.7, CD18; MEM-83, CD11a; MEM-170, CD11b; LeuM5, CD11c; B2M-02, β2-microglobulin) and the immunoprecipitates subjected to SDS-PAGE followed by Western blotting using streptavidin-peroxidase to reveal biotinylated proteins. (B) Isolated neutrophils or monocytes were activated with PMA as in Figure1B or left untreated for 120 minutes (Control) and at the indicated time points (15-120 minutes) analyzed by SDS-PAGE and immunoblotting using MEM-148 for detection (top panels); the bottom panel shows quantitative densitometric evaluation (plotted for the respective lanes as ratio of the signal from the 65- to 70-kd zone [closed arrowhead] versus the upper 78- to 96-kd CD18 zone [open arrowhead]). (C) Western blotting analysis of PBLs after various activating treatments. PBLs were activated using the indicated stimuli for 2 hours (for the different activation protocols see “Materials and methods”). The blot was immunoperoxidase-stained with the standard CD18 mAb MEM-48; a very similar, albeit weaker, staining pattern was obtained also with MEM-148 (not shown). Open and closed arrowheads in all parts of the Figure 2 indicate the zones corresponding to the uncleaved CD18 species (78-96 kd) and the 65- to 70-kd fragment, respectively.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/5/10.1182_blood.v98.5.1561/5/m_h81711466002.jpeg?Expires=1769284554&Signature=dMqcOFz3jgtJ~Dp1~Q6E0sXFjoccWHEnc~EFe2-gshyquzrjFbY7yJmQ40UBQU4HkCGqQWc4He5YDoDOc-sDLt6lbnVehqRE37I--fD1rzeM5vOFKyYhTMRsAIeglIYjFTo9ulimpd9wAGnBIJZ0tbLptLPQwI2gccky5ZxC7bgw6xeyMyZbXPK-gEOXyV-con90bwSsY1SHqNR4m6Z7WVkfNrXlXNp0tJe3kX-ROPEfGQR8HGg2aPmCV4PX9moQmw3kjKdyHgO2Lu7qjj~u8miD9VPGfiQom7HnfJXakgL2dYvIQmvEMazEjQRiqqjebhpHoqaJvqLBDM1eHAJidQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal