Interleukin-7 (IL-7) is the major thymopoietic cytokine. Injections of IL-7 after murine bone marrow transplantation (BMT) correct defects in thymic differentiation, including thymic hypocellularity, abnormal differentiation of CD3− CD4−CD8− (triple-negative [TN]) thymocytes into CD4+ CD8+ (double-positive [DP]) cells, and antigen-specific mature T-lymphocyte proliferation. To determine whether IL-7 production is decreased in BMT recipients, BMT was performed with congenic murine donor-recipient strains and escalating doses of pre-BMT conditioning. Increasing doses of radiation resulted in decreased thymic cellularity and maturation from the TN to the DP stage. Quantitative reverse transcription–polymerase chain reaction analyses demonstrated that intrathymic production of IL-7 was significantly decreased in irradiated mice than in nonirradiated controls. Decline in IL-7 transcript levels was correlated with the dose of radiation administered. Analyses of the numbers of CD45− major histocompatibility complex class II+ thymic stromal cells suggested that the mechanism for the decreased IL-7 production was loss of IL-7–producing thymic stromal cells. Experiments indicated that pre-BMT conditioning with radiation led to decreased stromal production of IL-7 and consequent blocks in the maturation of thymocytes. They provided a mechanism for both the abnormal thymopoiesis observed after BMT and the previously observed beneficial effects of IL-7 administration in murine models. Impaired production of IL-7 by thymic stroma may be a general model for the clinically observed adverse effects of cytotoxic therapy on thymopoiesis.
Introduction
The immune deficiency observed after bone marrow transplantation (BMT) is a major cause of morbidity and mortality in patients who undergo transplantation and results in prolonged susceptibility to infection.1,2 Some of the immunologic defects observed after BMT have included abnormalities of thymopoiesis, activation of T lymphocytes, and antibody production.3-6The thymus has been demonstrated to be a target of graft-versus-host disease (GVHD), and GVHD is associated with decreased thymopoietic capacity after BMT, resulting in decreased thymic output.7,8 However, abnormal numbers of circulating T lymphocytes have been observed in patients without GVHD, suggesting that other mechanisms besides GVHD suppress the production of new T lymphocytes.5 Thymopoietic defects may be due to the effects of radiation or chemotherapy on the thymic microenvironment. In addition, these effects may be age related. Analyses of patients undergoing either high-dose chemotherapy or BMT have shown an age-related decline in the production of new T lymphocytes.9-11 Abnormal numbers of T lymphocytes are especially evident in adult recipients of T-cell–depleted, matched, unrelated donor transplants, suggesting that the combined effects of age, alloreactivity, and high-dose cytotoxic therapy result in clinically significant defects in thymopoiesis.11
We have been studying the thymopoietic defects in BMT using syngeneic or congenic mice as a model. In a previous study, the administration of interleukin-7 (IL-7) after BMT resulted in the normalization of thymic numbers, subpopulations, and T-lymphocyte proliferative responses to mitogens and antigens.12 The pattern of thymic subpopulations in the control animals that received BMT but not IL-7 suggested a block in thymic differentiation. Control BMT animals had increased frequency of immature triple-negative (TN) thymocytes and decreased frequency of double-positive (DP) and single-positive (SP) CD4+ and CD8+ thymocytes representing later stages of thymic differentiation. Defects in thymopoiesis were similar to those observed in X-linked severe combined immune deficiency (X-SCID), caused by inherited defects of the γc component of the IL-7 receptor (IL-7R).13,14 IL-7 is a stimulus for proliferation, survival, and differentiation of immature thymocytes.15-18 IL-7 is normally made by a subset of thymic epithelial cells that express major histocompatibility complex (MHC) class II.19,20 The similarities between the thymic defects observed after BMT and in X-SCID led us to examine whether IL-7 production is normal in BMT recipients. We have previously demonstrated increased circulating levels of IL-7 after BMT.21 However, these studies measured steady-state levels of IL-7 that were probably a function of IL-7 production by many stromal and peripheral dendritic cell sources and of consumption by IL-7R–bearing cells.22Thus, analysis of the intrathymic production of IL-7 is necessary to determine whether the loss of IL-7 production contributes to the thymopoietic defects seen after BMT. In the present paper, we used a murine BMT model to test the hypothesis that pre-BMT radiotherapy inhibits IL-7 production and consequently interferes with post-BMT thymopoiesis. These studies demonstrated that IL-7–producing stromal cells, IL-7 mRNA production, and post-BMT thymopoiesis are radiosensitive.
Materials and methods
Animals
Recipient 4- to 6-week-old C57BL/6J mice expressing Ly5.2 (CD45.2) and B6.SJL mice congenic for Ly5.1 (CD45.1) (Jackson Laboratory, Bar Harbor, ME) were rested for 2 weeks after receipt. Untransplanted normal control mice were littermates of the recipient mice and were the same ages at the time they were killed. The animals were maintained in laminar flow cages with acidified water and antibiotics. Mice were killed with CO2 narcosis. All work was performed in accordance with protocols approved by the Animal Care Committee of the Children's Hospital Los Angeles.
Bone marrow transplantation procedure
Recipient CD45.2+ mice were prepared for transplantation with radiation given in 2 divided doses (650 cGy on day −1 and either 0, 350, 550, or 750 cGy on day 0). The radiation source was a linear accelerator with blocks to administer the dose at 120 cGy/min. Marrow from the CD45.1+ donor mice was obtained by perfusion of the femurs after they were killed. Nucleated marrow cells (2 × 106) were given per recipient. Mature T lymphocytes were removed from the marrow before infusion, using rat anti–Thy-1.2 monoclonal antibody (Pharmingen, San Diego, CA) and immunomagnetic beads (Dynal, Great Neck, NY). Each recipient mouse received fewer than 2000 Thy-1+ cells, as determined by fluorescence-activated cell sorting (FACS) of the infused marrow (less than 0.1% contamination). In each experiment, 2 mice received radiation but no cells were infused; the death of these mice was used to verify that a marrow-ablative dose of radiation had been given.
Immunophenotyping
At the time they were killed, thymus and spleen cells were obtained after teasing, and total cell number was determined. Cells (1 × 105) were stained with optimal concentrations of fluorescein isothiocyanate– or phycoerythrin-labeled monoclonal antibodies. Cells were stained with antibodies directed against CD3, CD4, CD8, Thy 1.2, Thy 1.1, CD45R, or isotype control antibodies (Pharmingen). After staining, cells were washed twice in phosphate-buffered saline and analyzed on the FACSCalibur or FACSVantage flow cytometers (Becton Dickinson, San Jose, CA). Five to 10 thousand gated events were acquired, and the number of cells positive for each antibody was determined by subtraction against the isotype control. The number of CD3− CD4−CD8− TN, CD4− CD8− DN, CD4+CD8+ DP, CD4+ CD8−(CD4+ SP), and CD4−CD8+ SP (CD8+ SP) cells was determined after staining with anti-CD3, CD4, and CD8 antibodies as previously described.11 Data were analyzed with Cellquest software.
Quantitative reverse transcription–polymerase chain reaction analyses
Real-time reverse transcription–polymerase chain reaction (RT-PCR) was used to quantitatively measure IL-7 transcripts produced by the thymus. Thymic tissue was harvested from unirradiated control mice and mice after graded doses of radiation. RNA was extracted with RNA STAT-60 isolation reagent (Tel-test, Friendswood, TX). Reverse transcription was performed with AMV reverse transcriptase (Gibco/BRL, Gaithersburg, MD), using random hexamers as primers (Gibco/BRL). Real-time PCR with the Taqman PCR assay was performed with the ABI 7700 Sequence Detector (PerkinElmer Applied Biosytems, Foster City, CA). The IL-7 gene was amplified with the forward primer 5′-GGAATTCCTCCACTGATCCTTG-3′ (bp 578-599, exon 2) and the reverse primer 5′-TTCCTGTCATTTTGTCCAATTCA-3′ (bp 707-685, exon 3) using the probe FAM–5′-CTGCTGCCTGTCACATCATCTGAGTGC-3′–TAMRA (bp 602-628) cDNA. β-Actin was amplified using the primers 5′-CAACGAGCGGTTCCGATG-3′ (bp 833-850, exon 3) and 5′-ATGGATGCCACAGGATTCCAT-3′ (bp 905-885, exon 4) using the probe FAM–5′ AGGCTCTTTTCCAGCCTTCCTTCTTGG-3′–TAMRA (bp 856-882). PCR amplification parameters were 95°C for 15 seconds and 60°C for 60 seconds. Amplification of an IL-7 cDNA clone over a range from 1.5 × 101 to 2.9 × 107 copies per reaction was used as a standard. Values obtained from the linear range of the PCR reaction of each experimental sample were first standardized to the β-actin signal to normalize loading and then compared to the signals from the cloned IL-7 cDNA to determine copy numbers of IL-7 per microgram RNA.
Isolation of CD45− MHC class II+ thymic stromal cells
The intact thymus was suspended in cold RPMI 1640 medium with 50 μg/mL DNAse I (Sigma, St Louis, MO) in a 35-mm tissue culture dish and cut into small fragments. The fragments were then gently stirred in RPMI 1640 and 12.5% fetal bovine serum with collagenase 500 μg/mL (Sigma) for 20 to 30 minutes at 37°C. After washing, the cells were then incubated overnight at 37°C and 5% CO2 in 6-well plates containing RPMI 1640 with 12.5% fetal bovine serum, 2 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. Nonadherent cells were removed by gentle washing. Adherent cells were detached with Cell Dissociation Buffer (Gibco/BRL). To quantify the numbers of CD45− MHC class II+ cells, the cells were stained with fluorescein anti–murine CD45 and PE-labeled anti–I-Ab (Aαb) antibodies (Pharmingen). The number of CD45− MHC class II+ cells was determined with the FACSCalibur flow cytometer and Cellquest software using previously defined parameters.19 20
Results
Inverse relationship between radiation dose before and thymocyte numbers after bone marrow transplantation
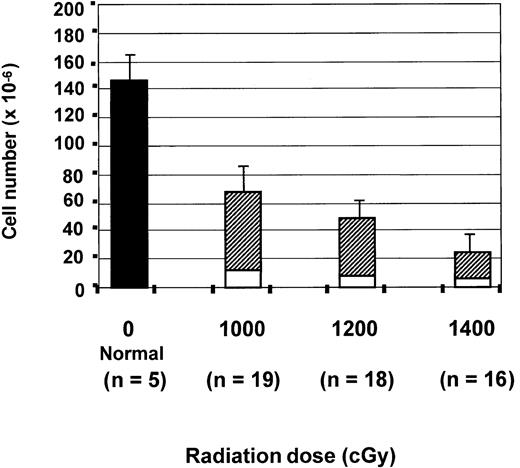
To test the effects of radiation on thymic reconstitution, mice were irradiated with total doses of either 1000, 1200, or 1400 cGy. The mean absolute number of thymocytes in normal mice was 142 × 106 ± 21 × 106 and was significantly greater than that seen in all transplantation groups on day 28 after BMT (P < .000 001). As shown in Figure1, the total number of thymocytes on day 28 after BMT was inversely related to the pre-BMT dose of radiation. After 1000 cGy preparation, there were 68 × 106 ± 14 × 106 thymocytes on day 28, which was significantly greater than the thymocyte numbers after 1200 or 1400 cGy. There were 51 × 106 ± 17 × 106 thymocytes seen after 1200 cGy, which was significantly greater than the 26 × 106 ± 13 × 106 cells seen after 1400 cGy.
Inverse relationship between pre-BMT radiation dose and thymic recovery on day 28.
The absolute number of thymocytes on day 28 in the normal C57/B6 controls (▪) and in the 3 groups of transplanted mice that received either 1000, 1200, or 1400 cGy. For the transplanted mice, the numbers of host (CD45.2+, ■) and donor-derived (CD45.1+, ▨) thymocytes are shown. All values are expressed as the mean cell number × 10−6 ± SD. Statistical differences were analyzed by 2-tailed t test with unequal distributions. For total cell numbers,P < .000 001 (normal vs BMT), P < .001 (1000 vs 1200 or 1400 cGy), P < .0001 (1200 vs 1400 cGy). For donor-derived cell numbers, P < .01 (1000 vs 1200 or 1400 cGy) and P < .0001 (1200 vs 1400 cGy). For recipient-derived cell numbers, P < .01 (1000 vs 1200 or 1400 cGy, P > .05 (1200 vs 1400 cGy).
Inverse relationship between pre-BMT radiation dose and thymic recovery on day 28.
The absolute number of thymocytes on day 28 in the normal C57/B6 controls (▪) and in the 3 groups of transplanted mice that received either 1000, 1200, or 1400 cGy. For the transplanted mice, the numbers of host (CD45.2+, ■) and donor-derived (CD45.1+, ▨) thymocytes are shown. All values are expressed as the mean cell number × 10−6 ± SD. Statistical differences were analyzed by 2-tailed t test with unequal distributions. For total cell numbers,P < .000 001 (normal vs BMT), P < .001 (1000 vs 1200 or 1400 cGy), P < .0001 (1200 vs 1400 cGy). For donor-derived cell numbers, P < .01 (1000 vs 1200 or 1400 cGy) and P < .0001 (1200 vs 1400 cGy). For recipient-derived cell numbers, P < .01 (1000 vs 1200 or 1400 cGy, P > .05 (1200 vs 1400 cGy).
Because the thymus on day 28 contains donor progeny and radioresistant host cells, we separately analyzed the donor- and host-derived thymocytes. C57BL6/J recipients expressed CD45.2, allowing host thymocytes to be distinguished from B6.SJL donor cells, which expressed CD45.1. There were 56 × 106 ± 13 × 106 donor-derived thymocytes after 1000 cGy, 43 × 106 ± 18 × 106 after 1200 cGy, and 19 × 106 ± 12 × 106 after 1400 cGy. Declines in the number of donor-derived cells in the BMT recipients prepared with 1000 cGy versus all other groups and with 1200 versus 1400 cGy were statistically significant (Figure 1).
There was also a 2-fold decrease in the number of residual host thymocytes as the radiation dose was increased from 1000 cGy (12 × 106 ± 5 × 106) to 1400 cGy (6.25 × 106 ± 3.7 × 106) (Figure 1). Results indicate that increased radiation doses increase the frequency of donor-derived thymocytes after BMT by elimination of residual host thymocytes but decrease the absolute number of donor-derived thymocytes, suggesting an impaired capacity of the thymus to support thymopoiesis.
Thymic maturation block after high-dose radiation
Previous analyses had demonstrated that IL-7 administration after BMT relieves a block in differentiation between the immature TN and later DP and SP stages of thymic differentiation.12 We determined whether the degree of maturational block was affected by the radiation dose. Normally TN thymocytes represent 2% to 4% of the total, whereas DP thymocytes are 80% to 92%, SP CD4 thymocytes 3% to 9%, and SP CD8 thymocytes 1% to 7%. As the dose of pre-BMT radiation was increased, the proportion of TN thymocytes increased and DP thymocytes decreased (Figures 2,3). Costaining with CD45.1 and Thy1 antibodies established that the observed increase in TN cells resulted from the increased frequency of donor-derived thymocytes, not recipient (CD45.1−) TN cells or non-T (Thy1−) lineage cells. The increased frequency of TN cells and the decreased frequency of DP cells are consistent with a defect in the microenvironment that affects maturation of donor TN cells. Overall reduced cellularity of the thymus after higher doses of radiation is also consistent with such a block in differentiation. Results were similar to those we previously described in animals that received BMT but not exogenous IL-7.12
FACS analyses of donor-derived CD3−CD4− CD8− (TN) thymocytes.
Thymocytes from representative normal and BMT recipients on day 28 were stained with anti-CD3–fluorescein isothiocyanate, a cocktail of anti-CD4–phycoerythrin and anti-CD8–phycoerythrin, and anti-Thy1–APC antibodies to determine the frequency of TN thymocytes. Analyses of the Thy1-positive cells are shown, with the percentages of TN thymocytes indicated for each mouse.
FACS analyses of donor-derived CD3−CD4− CD8− (TN) thymocytes.
Thymocytes from representative normal and BMT recipients on day 28 were stained with anti-CD3–fluorescein isothiocyanate, a cocktail of anti-CD4–phycoerythrin and anti-CD8–phycoerythrin, and anti-Thy1–APC antibodies to determine the frequency of TN thymocytes. Analyses of the Thy1-positive cells are shown, with the percentages of TN thymocytes indicated for each mouse.
Relationship between pre-BMT radiation dose and donor-derived thymic subpopulations on day 28.
Mean percentages of TN (■), DP (▪), SP CD4 (▨), and SP CD8 (░) thymocytes from the control group and the 3 groups of mice transplanted after either 1000, 1200, or 1400 cGy are shown. Each histogram represents mean percentage ± SD. Differences between groups were analyzed by 2-tailed t test with unequal distributions. For TN cells, P < .02 (normal vs 1400 cGy),P < .01 (1000 cGy vs 1400 cGy), and P > .05 (all other comparisons). For DP cells, P < .02 (normal vs 1400 cGy), P < .01 (1000 cGy vs 1400 cGy),P < .01 (1200 vs 1400 cGy), and P > .05 (all other comparisons).
Relationship between pre-BMT radiation dose and donor-derived thymic subpopulations on day 28.
Mean percentages of TN (■), DP (▪), SP CD4 (▨), and SP CD8 (░) thymocytes from the control group and the 3 groups of mice transplanted after either 1000, 1200, or 1400 cGy are shown. Each histogram represents mean percentage ± SD. Differences between groups were analyzed by 2-tailed t test with unequal distributions. For TN cells, P < .02 (normal vs 1400 cGy),P < .01 (1000 cGy vs 1400 cGy), and P > .05 (all other comparisons). For DP cells, P < .02 (normal vs 1400 cGy), P < .01 (1000 cGy vs 1400 cGy),P < .01 (1200 vs 1400 cGy), and P > .05 (all other comparisons).
Radiosensitivity of IL-7 transcript levels
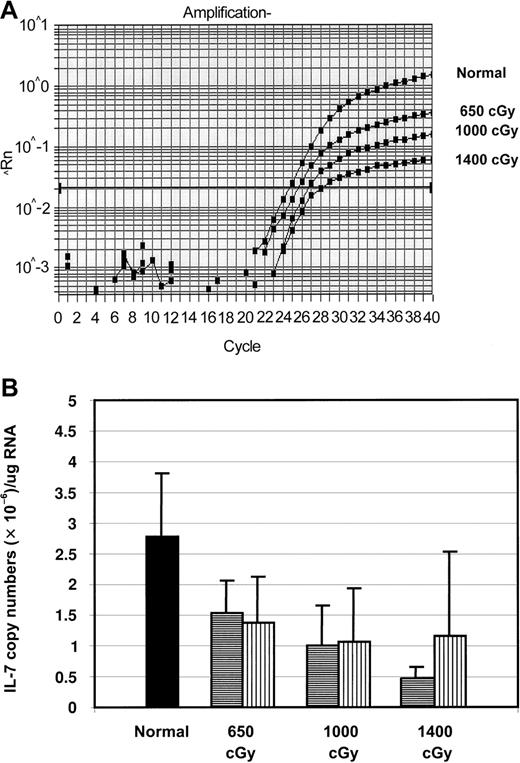
The increase in thymic immaturity observed with higher radiation doses was consistent with a block in differentiation between the TN and the DP stages. Because IL-7 is critical for the maturation of TN cells, we investigated the effects of increased radiation doses on IL-7 production. Real-time RT-PCR was used to quantitatively measure the levels of IL-7 mRNA. Levels of IL-7 produced 5 and 28 days after radiation were determined by extraction of total thymic RNA immediately after they were killed, then reverse transcription and amplification. In initial experiments, animals received radiation without subsequent BMT. Thymic levels of IL-7 mRNA were significantly decreased in irradiated mice compared to normal mice (Figure4A-B). On day 5 after irradiation, the mean level of IL-7 transcripts was 55% ± 19% of normal after 650 cGy, 36% ± 24% after 1000 cGy, and 17% ± 7% after 1400 cGy. Thus, radiation caused a rapid decrease in intrathymic IL-7 mRNA levels in a dose-dependent manner.
Levels of IL-7 transcripts in the thymus after BMT.
(A) Real-time RT-PCR plots of amplification of IL-7 from the thymuses of representative mice obtained 5 days after 650 cGy, 1000 cGy, and 1400 cGy and from a normal unirradiated mouse. (B) Copies of IL-7 transcripts per thymus in mice 5 and 28 days after BMT, expressed as copy numbers per thymus after 650, 1000, or 1400 cGy (n = 5 for all groups; P < .05 for all irradiated groups on day 5 vs normal; P > .05 on day 28). ▪ indicates day 0; ▤, day 5; ▥, day 28.
Levels of IL-7 transcripts in the thymus after BMT.
(A) Real-time RT-PCR plots of amplification of IL-7 from the thymuses of representative mice obtained 5 days after 650 cGy, 1000 cGy, and 1400 cGy and from a normal unirradiated mouse. (B) Copies of IL-7 transcripts per thymus in mice 5 and 28 days after BMT, expressed as copy numbers per thymus after 650, 1000, or 1400 cGy (n = 5 for all groups; P < .05 for all irradiated groups on day 5 vs normal; P > .05 on day 28). ▪ indicates day 0; ▤, day 5; ▥, day 28.
Because developing thymocytes have been shown to influence the thymic stroma, levels of IL-7 mRNA were then compared between mice that received radiation only (no BMT) and mice that also underwent transplantation. For the first 5 days after radiation treatment, there was no difference in the levels of IL-7 mRNA in irradiated mice not receiving transplants, and BMT mice. Because of hematopoietic toxicity, it was only possible to analyze later time points for IL-7 expression in the BMT mice. Defects in IL-7 production persisted for at least 1 month after BMT. On day 28 after BMT, all the BMT groups had equivalent levels of IL-7 transcripts that were less than half of normal but were not statistically significant (Figure 4B).
Loss of CD45− MHC class II+ thymic stromal cells
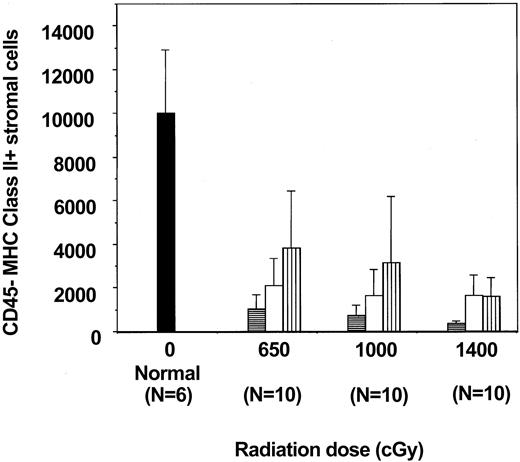
In the mouse, thymic IL-7 production resides in a subset of adherent cells that do not express CD45 but do express MHC class II antigens.19 20 To determine whether IL-7–producing cells were actually killed by the pre-BMT irradiation, we analyzed the number of CD45− MHC class II+ adherent cells in the thymus after different doses of irradiation and at different time points after BMT. The absolute number of CD45− MHC class II+ cells on day 5 after BMT was significantly lower than normal. Numbers of CD45− MHC class II+ cells increased by days 15 and 28 but were still significantly less than normal in all transplantation groups (Figure5). It was also evident that regeneration of the CD45− MHC class II+ stromal cells was greater after 650 cGy than after 1000 or 1400 cGy, but no statistically significant difference between 1000 and 1400 cGy was found. Results indicate that pre-BMT radiation decreases the number of CD45− MHC class II+ stromal cells in a dose-dependent manner and that recovery after BMT is also dose dependent.
Effects of radiation on thymic CD45− MHC class II+ adherent cells.
Recovery of CD45− MHC class II+ adherent cells from normal mice and groups of mice transplanted after either 650, 1000, or 1400 cGy and analyzed on day 5, 15, or 28 after BMT (n = 10 for each group and time point). Cells were collected from the thymus and plated overnight, and adherent cells were then analyzed. All values are the mean number of cells ± SD. Differences between groups were analyzed by 2-tailed t test with unequal distributions. On day 5 (▤), P < .001 (normal vs all BMT groups); on day 15 (■), (P < .001 normal vs BMT); and on day 28 (▥) (P < .01, normal vs BMT). On day 28,P < .04 (650 vs 1000 cGy), and P < .01 (650 vs 1400 cGy). ▪ indicates day 0.
Effects of radiation on thymic CD45− MHC class II+ adherent cells.
Recovery of CD45− MHC class II+ adherent cells from normal mice and groups of mice transplanted after either 650, 1000, or 1400 cGy and analyzed on day 5, 15, or 28 after BMT (n = 10 for each group and time point). Cells were collected from the thymus and plated overnight, and adherent cells were then analyzed. All values are the mean number of cells ± SD. Differences between groups were analyzed by 2-tailed t test with unequal distributions. On day 5 (▤), P < .001 (normal vs all BMT groups); on day 15 (■), (P < .001 normal vs BMT); and on day 28 (▥) (P < .01, normal vs BMT). On day 28,P < .04 (650 vs 1000 cGy), and P < .01 (650 vs 1400 cGy). ▪ indicates day 0.
Discussion
Reconstitution of immunity after BMT ultimately depends on the production of new lymphocytes from donor-derived hematopoietic stem cells. The difference in infectious complications and time to immune reconstitution in patients receiving unmanipulated marrow and T-cell–depleted marrow suggests that there is a transient role for adoptive transfer of mature T lymphocytes from the donor as a source of immune function early after BMT.23 Eventually, new T lymphocytes are produced from donor-derived hematopoietic stem cells and prothymocytes that mature in the host thymus. Thus, immune recovery after BMT is dependent on the maturational capacity of donor cells (transplanted hematopoietic stem cells) and host cells (thymic microenvironment).
In the present study, we have demonstrated that the dose of pre-BMT radiation has profound quantitative and qualitative effects on post-BMT thymopoiesis. Increasing doses of radiation decreased the capacity of the thymus to regenerate. There was an inverse relationship between the pre-BMT dose of radiation and the cellularity of the thymus. Decreased thymic cellularity was mainly attributed to decreased numbers of donor-derived thymocytes, not to increased destruction of recipient thymocytes. Furthermore, higher doses of radiation led to decreased maturation of the donor thymocytes, as evidenced by an increased proportion of immature TN cells and a decreased proportion of DP cells. In addition, the capacity of the thymic stroma to produce IL-7 was inversely related to the radiation dose given, providing at least one mechanism for the impaired thymopoiesis was observed. By day 28 after BMT, IL-7 transcript levels and numbers of IL-7–producing stromal cells were still abnormally low. We demonstrated that one mechanism of decreased thymic IL-7 production is the destruction of the stromal population that produces IL-7.
IL-7 is secreted by stromal cells from fetal liver, thymus, and bone marrow. In the murine thymus, IL-7 is produced by a subset of CD45− MHC class II+ epithelial cells distributed in both the cortex and the medulla.19,20 In murine thymic reaggregation assays, CD45− MHC class II+ stromal cells allow immature hematopoietic progenitors to undergo T-lymphoid differentiation.20 Specifically, CD45− MHC class II+ stromal cells permit the maturation of fetal liver–derived progenitors with germline T-cell receptor β (TCR-β) and TCR-α loci into cells with appropriate TCR-β and TCR-α rearrangements. IL-7 production by the CD45− MHC class II+ stromal cells is critical in these reaggregation assays because thymic differentiation is not observed when MHC class II+ stromal cells from IL-7 knock-out mice are used. In our analyses of the CD45− MHC II+ adherent cells after BMT, we found at least a 1-log decrease in this cell population, even at the lowest dose (650 cGy) of radiation used. Furthermore, higher doses resulted in greater losses of the CD45− MHC class II+ adherent cells, suggesting that radiation killed these cells in a dose-dependent manner.
Besides intrathymic secretion of IL-7, other functions of stromal cells—eg, expression of c-kit ligand or integrins—are important for thymopoiesis.24-26 However, the defects seen in BMT mice after radiation are most consistent with a loss of IL-7–mediated signaling.20 IL-7 has been shown in various genetic models to be essential for the survival, proliferation, and differentiation of immature thymocytes.14-18,27-35The relative increase in the proportion of TN thymocytes and the decrease in DP thymocytes in the irradiated mice are similar to those seen in X-SCID dogs that lack the γc subunit of the IL-7 receptor.14 It is likely that damage to the thymic microenvironment by pre-BMT conditioning led to decreased maturation of TN to DP thymocytes. In previous experiments, we have shown that the administration of recombinant IL-7 improves the thymopoietic defects seen after BMT, indicating that the loss of IL-7 production alone is sufficient to explain much of the impaired thymopoiesis we observed.12 Furthermore, cotransplantation of bone marrow stromal cells transduced with the IL-7 gene can also largely correct the thymopoietic defects (E. Bolotin et al, manuscript submitted, 2001).
RT-PCR data indicate that transcript levels for IL-7 decreased within 5 days of radiation treatment. The decrease in IL-7 production is likely to be greater than that observed by RT-PCR of whole thymic RNA. Using total thymic RNA as template was necessitated by the observation that IL-7 production by dissociated stroma is quickly down-regulated, making it impossible to assess IL-7 transcripts directly after sorting of CD45− MHC class II+ cells (V. Dunn, Y.-H. Hong, K.W., unpublished observation, January 2001). Therefore, we chose to measure IL-7 production from freshly isolated thymus without manipulation because this would introduce the least amount of artifact from changes in IL-7 gene expression during cell isolation. In the normal thymus, more than 99% of RNA is derived from thymocytes rather than from IL-7–producing stromal cells. In contrast, irradiated animals lost almost all thymocytes, leading to a proportionate increase in the thymic stromal elements. Because the IL-7 RT-PCR assays used total thymic RNA as the template, it is likely that the loss of IL-7 production by thymic stromal cells was significantly greater than what we were able to analyze from total thymic RNA.
Besides direct killing of thymic stromal cells, other mechanisms for the loss of IL-7 production have been described. Counter-regulatory cytokines, notably TGF-β, have been shown to decrease IL-7 mRNA production levels by marrow stroma.36 It is possible that increased TGF-β production in response to radiation also caused the down-regulation of IL-7 production. Other studies have indicated that decreased intrathymic IL-7 production underlies the abnormal thymopoietic capacity observed during aging and that IL-7 administration can correct thymic defects.37,38 The mechanism for the loss of intrathymic IL-7 production during aging is unknown. Our results differ from those of a recent study of decreased IL-7 production by bone marrow stromal cells in aged mice that demonstrated a posttranscriptional mechanism rather than destruction of the IL-7–producing subset.39 It is possible that the mechanisms for the effects of aging and radiation on IL-7 production may be different.
Loss of the IL-7–producing thymic stromal cells may be a common mechanism that underlies many pathogenic processes, leading to thymic insufficiency, such as radiation therapy, high-dose chemotherapy, GVHD, aging, and human immunodeficiency virus infection. Several clinical results have suggested that thymopoietic capacity is damaged by chemotherapy, radiotherapy, and GVHD. Adolescent and adult recipients of high, but nonmyeloablative doses of chemotherapy do not regenerate naive CD4+ T lymphocytes as well as young children do.9 Similarly, adults who undergo BMT have impaired production of naive CD4+ T cells.8,10,11 The nature of the thymopoietic defect is difficult to ascertain in the clinical setting because thymic biopsy samples are not taken after chemotherapy or radiotherapy. The present experiments demonstrating decreased IL-7 transcripts and abnormal thymic maturation provide a model for how patients may develop treatment-related thymic insufficiency. Analyses of the post-BMT thymus indicate that there is a capacity for regeneration of the CD45− MHC class II+ stromal cells after BMT. Further studies to determine the ontogeny of the regenerated CD45− MHC class II+ stromal cells may allow the development of strategies either to protect these cells from radiation or to stimulate their recovery with epithelial cell–specific cytokines such as keratinocyte growth factor.40 Reconstitution of thymopoiesis in patients may require direct efforts to restore the function of the thymic epithelial cells.
We thank Sally Worttman and Renee Workman of the Animal Care Facility at Children's Hospital Los Angeles, without whose assistance the experiments with high-dose radiation would not have been possible. We thank Jan Nolta, Mo Dao, Ellen Bolotin, and Robert Lavey for their helpful advice.
Supported by grants from the National Institutes of Health (1R01 HL54729, 1R21 HD/AI37598, 1P50 HL-54850), the T.J. Martell Foundation, and the American Medical Foundation for AIDS Research (02594-23-RGI).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kenneth Weinberg, Division of Research Immunology and Bone Marrow Transplantation, MS#62, Children's Hospital Los Angeles, 4650 Sunset Blvd, Los Angeles, CA 90027; e-mail:kweinberg@chla.usc.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal