We read with interest the Mir et al article on the effects of CD30 activation on anaplastic large-cell lymphoma and Hodgkin disease cell lines.1 The authors demonstrated apoptotic responses and lack of an nuclear factor (NF)–κB response, which is presumed to be responsible for the apoptotic response, following CD30 activation of the anaplastic large-cell lymphoma cell lines.
We performed numerous experiments on the cell lines mentioned in the study. Activation of the CD30 signaling pathway by HeFi-1, a CD30 activating antibody,2 caused a significant decrease in thymidine uptake of nodal ALCL cell line Karpas 299.3 But we did not observe apoptosis of the Karpas 299 cell line following CD30 activation measured by double staining with propidium iodide and Annexin V (not shown).
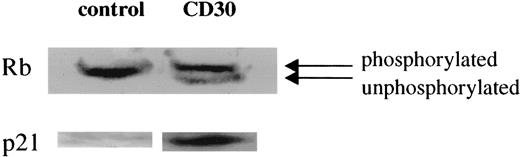
To determine the effects of CD30 activation on cell-cycle regulation, we investigated the status of retinoblastoma protein and p21 following CD30 activation by immunoblotting. Binding was detected using an ImmunStar chemiluminescence detection system (BioRad, Hercules, CA). There was an increase in the unphosphorylated retinoblastoma protein (Rb) in CD30-activated Karpas 299 cells at 40 hours after CD30 activation compared with untreated (control) Karpas 299 cells that had almost entirely phosphorylated Rb (Figure1). Likewise, at 40 hours there was no expression of p21 by the control cells while CD30 activated Karpas 299 cells had a high level of p21 expression (Figure 1).
Expression of Rb protein and p21 in Karpas 299 cells after CD30 activation.
After synchronization, cells were fed serum and allowed to grow. Forty hours after addition of serum, in the absence of HeFi-1 all the Rb protein expressed is in phosphorylated form (upper band), whereas the HeFi-1 treated cells demonstrate a significant amount of unphosphorylated Rb protein. p21 is not expressed in the control cells, whereas the CD30-activated cells have high p21 expression at 40 hours after CD30 activation.
Expression of Rb protein and p21 in Karpas 299 cells after CD30 activation.
After synchronization, cells were fed serum and allowed to grow. Forty hours after addition of serum, in the absence of HeFi-1 all the Rb protein expressed is in phosphorylated form (upper band), whereas the HeFi-1 treated cells demonstrate a significant amount of unphosphorylated Rb protein. p21 is not expressed in the control cells, whereas the CD30-activated cells have high p21 expression at 40 hours after CD30 activation.
We also investigated gene expression patterns of the Karpas 299 cells before and after CD30 activation by use of the Atlas human complementary DNA (cDNA) array for apoptosis (Clontech, Palo Alto, CA), which contains 205 genes associated with apoptosis and cell-cycle regulation. We found that upon CD30 activation most genes involved in cell-cycle progression showed decreased expression. Most significantly, proliferating cell nuclear antigen (PCNA), cyclins A, B, and H were down-regulated, as were the genes for caspase 1, 2, 3, and 4 (not shown). Expression of the gene for antiapoptotic protein IAP-1 was increased.
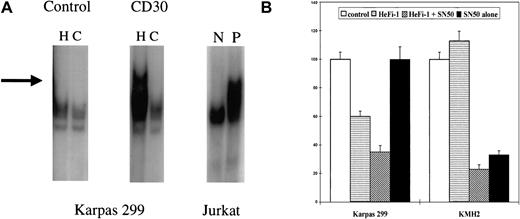
To understand the role of NF-κB in the growth inhibitory action of CD30, NF-κB activity was determined. Nuclear extracts from cell lines before and after stimulation with HeFi-1 for 1 hour were obtained. The extracts were incubated with 32P end-labeled NF-κB binding site oligos (5′-AGCTTGGGGTATTTCCAGCCG-3′) and excess cold oligos and run on a nondenaturing 6% PAGE (Gelshift kit, Geneka, Montreal, QC, Canada). There was a high constitutive activity in KMH2 while Karpas 299 had no activity. After incubation with HeFi-1 for 1 hour, KMH2 had no change in activity (not shown), but Karpas 299 cell line showed modest NF-κB activation (Figure2A). To further investigate the role of NF-κB on the CD30 action, we utilized a selective NF-κB inhibitor, SN50, which is a synthetic cell permeable peptide and inhibits NF-κB by binding to the nuclear localization sequence of the p50 subunit of NF-κB.4 The KMH2 cell line demonstrated a significant decrease in 3H-thymidine uptake within 24 hours with 50 μg/mL of NF-κB inhibitor SN50. Incubation with HeFi-1 combined with SN50 had no significant further effect on the KMH2 response. Karpas 299 was not growth-inhibited by SN50 alone, while the combined treatment with HeFi-1 and SN50 enhanced the inhibitory effect of HeFi-1 (Figure2B). Our results differ from the data presented by Mir et al in 2 respects. We could not demonstrate apoptosis in response to CD30 activation on Karpas 299 cells, while we did observe NF-κB activation after CD30 activation. Additionally, we have evidence of a cell-cycle–inhibitory effect of CD30 activation on the Karpas 299 cell line. The discrepancies between our results and theirs could be due to utilization of different CD30 activating antibodies or differences in the biologic properties of Karpas 299 cell lines, which could have been altered during culture. We have published our findings on cutaneous anaplastic large-cell lymphoma cell lines, which show either no response or a proliferative response to CD30 activation.5The cutaneous anaplastic large-cell lymphoma cell lines are also responsive to NF-κB and mitogen-activated protein kinase (MAPK) inhibitors when used in combination with CD30-activating antibodies. We conclude that NF-κB is a key element in regulating CD30 responses and that NF-κB inhibitors could be used alone or in combination with CD30 agonistic antibodies in the treatment of Hodgkin disease and anaplastic large-cell lymphomas. But the matter is not as straightforward as claimed in Mir et al1 since CD30 activation may also have cell-cycle–regulatory effects, as well as proapoptotic effects. Cell-cycle–inhibitory effects of CD30 activation require further investigation.
Effects of CD30 activation on NF-κB activity.
(A) NF-κB activity determined by the gel-shift assays. Karpas 299 cell line was incubated with HeFi-1 or isotype-specific control IgG for 1 hour. NF-κB activity was determined by mobility shift observed in the gel electrophoresis of nuclear extracts. The left lanes in individual figures are loaded with radioactive labelled oligoprobes representing NF-κB binding sites and nuclear extracts (H). The right lanes are loaded with radioactive probes plus excess amounts of (× 200) unlabeled probes. These lanes represent the negative controls for the assays and characterize which bands are specific for NF-κB binding activity (C). In the Karpas 299 cell line, there was no NF-κB activity after control IgG incubation; CD30 activation caused activation of NF-κB. Jurkat cell line nuclear extracts were used as controls. N is the negative control, and P is the positive control. (B) Effects of the NF-κB inhibitor SN50 on systemic ALCL and Hodgkin lymphoma cell lines. Proliferation assays were performed in the presence of HeFi-1 and the NF-κB inhibitor SN50 on the Karpas 299 and KMH2 cell lines. SN50 alone caused inhibition of the KMH2 cell line, whereas it had no effect on the Karpas 299 cell line. Combined treatment with HeFi-1 and SN50 enhanced the inhibitory effects of HeFi-1 on the Karpas 299 cell line but had no significant additional effect on the KMH2 cell line. The results are presented as percentages of 3H-thymidine incorporation values compared to controls (IgG incubated). Each of 3 experiments was done in triplicate. A representative experiment is shown.
Effects of CD30 activation on NF-κB activity.
(A) NF-κB activity determined by the gel-shift assays. Karpas 299 cell line was incubated with HeFi-1 or isotype-specific control IgG for 1 hour. NF-κB activity was determined by mobility shift observed in the gel electrophoresis of nuclear extracts. The left lanes in individual figures are loaded with radioactive labelled oligoprobes representing NF-κB binding sites and nuclear extracts (H). The right lanes are loaded with radioactive probes plus excess amounts of (× 200) unlabeled probes. These lanes represent the negative controls for the assays and characterize which bands are specific for NF-κB binding activity (C). In the Karpas 299 cell line, there was no NF-κB activity after control IgG incubation; CD30 activation caused activation of NF-κB. Jurkat cell line nuclear extracts were used as controls. N is the negative control, and P is the positive control. (B) Effects of the NF-κB inhibitor SN50 on systemic ALCL and Hodgkin lymphoma cell lines. Proliferation assays were performed in the presence of HeFi-1 and the NF-κB inhibitor SN50 on the Karpas 299 and KMH2 cell lines. SN50 alone caused inhibition of the KMH2 cell line, whereas it had no effect on the Karpas 299 cell line. Combined treatment with HeFi-1 and SN50 enhanced the inhibitory effects of HeFi-1 on the Karpas 299 cell line but had no significant additional effect on the KMH2 cell line. The results are presented as percentages of 3H-thymidine incorporation values compared to controls (IgG incubated). Each of 3 experiments was done in triplicate. A representative experiment is shown.
Strength of CD30 signal determines sensitivity to apoptosis
Levi et al have reported that treatment of the anaplastic large-cell lymphoma line Karpas 299 with the CD30-specific monoclonal antibody HeFi-1 does not induce apoptotic cell death.1-1,1-2In contrast, under the conditions described in our paper,1-3we readily observe apoptotic cell death in response to CD30 activation not only in the Karpas 299 line but also in a range of other CD30-positive cell lines of anaplastic large-cell lymphoma origin. In fact, our original decision to focus on the 2 CD30-sensitive cell lines Karpas 299 and Michel for the study described in our paper was based on 2 previous publications that demonstrated these lines' susceptibility to cell killing by the CD30-agonistic antibodies M44 and M67.1-4,1-5 Therefore, for our studies we used both M44 and M67 and found very similar proapoptotic effects with these 2 antibodies. The experiments reported by Levi et al differ substantially to those described in our paper. For example, the nuclear factor (NF)–κB status was evaluated by gel retardation one hour following activation, whereas in our paper we used a reporter assay to integrate κB-directed reporter gene activity over a 36-hour period. We feel it is important to note that our original model of CD30 regulation proposes that NF-κB induction occurs almost immediately after CD30 activation in a TRAF2-dependent fashion but that upon prolonged stimulation TRAF2 is degraded and NF-κB induction is impaired.1-6 Therefore, the gel retardation experiments performed by Levi et al and the reporter-gene analysis presented in our paper are consistent with our model.
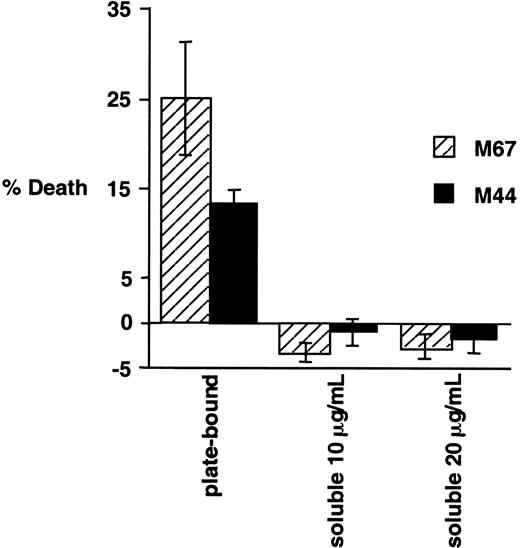
We have extensively compared the experimental procedures in our paper to those described in the above letter and in previous papers by Levi and colleagues. Possibly the most significant difference is the apparent use by Levi et al of antibody in its soluble form, whereas our experiments were performed using immobilized antibody, as detailed in “Materials and methods.” To examine this issue, we have compared the cytotoxic effects on Karpas 299 cells of the addition of antibodies to CD30 in soluble or plate-bound form. Karpas 299 cells were added to wells containing immobilized CD30-agonistic antibodies M67 and M44 or an isotype control antibody, exactly as described in our original paper. In parallel, these antibodies were provided in their soluble form, as used previously by Levi and colleagues, to Karpas 299 cells at 2 different concentrations. Consistent with our earlier report, incubation of Karpas 299 cells with immobilized antibodies to CD30 was found to potently induce cell death, whereas in the same experiment the addition of these antibodies in their soluble form did not induce cell death and actually slightly enhanced viability (Figure1-1). Also noteworthy is the fact that, in the earlier report by Gruss et al1-4 in which the cytotoxic effects of M44 and M67 were originally described, plate-bound antibodies were used. In preliminary experiments using plate-bound HeFi-1, we have also observed a proapoptotic effect (data not shown).
Effects of plate-bound and soluble CD30-agonistic antibodies on Karpas 299 cells.
Karpas 299 cells were treated for 20 hours either with plate-bound CD30 antibodies M44 and M67 (provided by Immunex Corporation), as indicated, exactly as described previously,1-3 or with the soluble antibodies at the indicated concentrations. Cell viabilities were evaluated by propidium iodide exclusion and flow cytometry, and normalized to the IgG1 isotype control as described previously.1-3 The experiment was performed in triplicate, and standard deviations are shown.
Effects of plate-bound and soluble CD30-agonistic antibodies on Karpas 299 cells.
Karpas 299 cells were treated for 20 hours either with plate-bound CD30 antibodies M44 and M67 (provided by Immunex Corporation), as indicated, exactly as described previously,1-3 or with the soluble antibodies at the indicated concentrations. Cell viabilities were evaluated by propidium iodide exclusion and flow cytometry, and normalized to the IgG1 isotype control as described previously.1-3 The experiment was performed in triplicate, and standard deviations are shown.
In summary, we suggest that apparently different experimental methods are the most likely explanation for the discrepancies between our report and that of Levi et al, in particular the use of immobilized antibody versus plate-bound antibody. The different effects of soluble and immobilized antibody are suggestive of an intriguing physiologic mechanism by which low levels of CD30 activation may induce cell-cycle arrest, activation of NF-κB, and the concomitant induction of antiapoptotic genes, whereas a stronger CD30-activation signal may result in a transient and more limited activation of NF-κB and ultimately cell death. The strength of the CD30 signal may be determined by numerous factors, including the density of CD30 receptors on the cell and the form of ligand (ie, membrane-bound or soluble). The threshold sensitivity of a given cell may also be determined by additional factors, such as the stability of intracellular signaling intermediates, particularly TRAF2. Thus, the apparent discrepancies between our data and those of Levi et al may reflect a novel physiologic function of CD30.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal