Conditionally replicating viruses are promising agents for the treatment of malignancy. Here it is shown that the live attenuated Edmonston-B vaccine strain of measles virus (MV-Edm) replicates selectively in human myeloma cells and has potent antitumor activity. In vitro, replication of MV-Edm was restricted in phytohemagglutinin (PHA)-stimulated peripheral blood lymphocytes (PBLs) but proceeded efficiently in a panel of 6 myeloma cell lines—ARH-77, RPMI 8226, JJN-3, MM1, KAS-6/1, and KMS-11—and in primary myeloma cells isolated by CD138 sorting from the bone marrow aspirates of 6 patients. MV-Edm infection induced potent cytopathic effects in these myeloma cells, resulting in the formation of multinucleated syncytia that eventually became nonviable. In contrast, syncytial formation in PHA-stimulated PBLs was minimal after MV-Edm infection. In vivo, MV-Edm was antitumorigenic and inhibited the establishment of myeloma cells as xenografts in immunocompromised mice. When injected directly into ARH-77 myeloma xenografts in the mice, MV-Edm caused complete regression of these xenografts. MV-Edm administered intravenously into the tail veins of mice also showed significant antineoplastic activity against established RPMI 8226 and ARH-77 xenografts. In particular, the ARH-77 myeloma xenografts were exquisitely sensitive to MV-Edm therapy, and tumors in all mice regressed completely. In light of its selectivity for myeloma cells and its potent antineoplastic activity against myeloma xenografts in vivo, MV-Edm merits further development for the treatment of multiple myeloma.
Introduction
Attenuated viruses with tumor specificity have attracted considerable interest as novel anticancer agents, and clinical testing of several such agents is under way.1Tumor selectivity of these viruses has been attributed to various intracellular restrictions to their life cycles that are strongly inhibitory to virus propagation in nontransformed cells but that are overridden by cellular factors present in neoplastic cells.2-6
Measles is an acute viral disease caused by a negative-strand RNA virus of the family Paramyxoviridae, which remains responsible for approximately 1 million deaths each year.7 In the early stages of measles virus (MV) infection, the lymphoid organs and tissues are predominant sites for viral replication, leading to the formation of giant reticuloendothelial (Warthin-Finkeldey) cells in the tissues.7 Measles is prevented by the use of a live attenuated virus vaccine now routinely administered during childhood. The Edmonston-B vaccine strain of measles virus (MV-Edm) was attenuated by serial tissue culture passage of a clinical isolate.7Despite its profound attenuation as a human pathogen, MV-Edm replicates more efficiently than nonattenuated measles virus in many primate cell lines, inducing cell cell fusion and the formation of characteristic multinucleated syncytia.8
Here, we compared the ability of MV-Edm to replicate in neoplastic myeloma cells and normal cells, and we investigated its potential as an antitumor agent in human myeloma xenografts in vivo. We report that the virus replicated selectively in a panel of 6 myeloma cell lines and in CD138-sorted myeloma cells from 6 patients and that it caused potent cytopathic effects. When administered intratumorally or intravenously into mice bearing established myeloma xenografts, MV-Edm caused growth inhibition or total regression of 2 different myeloma xenograft models.
Materials and methods
Cell culture
The multiple myeloma ARH-77 cell line (ATCC CRL-1621) was obtained from American Type Culture Collection (Rockville, MD). The rest of the multiple myeloma cell lines were kind gifts of Dr John Lust (RPMI 8226), Dr Diane Jelinek (KAS-6/1), and Dr Rafael Fonseca (KMS-11, MM1, JJN-3) from the Mayo Clinic (Rochester, MN). All the human myeloma cell lines were maintained in RPMI-1640 (Gibco BRL, Rockville, MD) supplemented with 10% fetal bovine serum (FBS) except for KAS-6/1, which was grown in media supplemented with 1 ng/mL interleukin (IL)-6 (Sigma, St Louis, MO). Bone marrow aspirates were obtained after institutional review board approval and informed patient consent. Bone marrow samples were drawn into a tube containing heparin and centrifuged on a Ficoll-Hypaque gradient (Amersham Pharmacia, Piscataway, NJ) to enrich for mononuclear cells. Mononuclear cells were then incubated with MACS CD138 microbeads (Miltenyi Biotech, Auburn, CA) for 15 minutes in a 8°C water bath. CD138+ primary myeloma cells were then isolated, washed, and maintained in 10% FBS-RPMI supplemented with 1 ng/mL IL-6. Peripheral blood lymphocytes (PBLs) obtained from healthy volunteers were activated overnight with 2.5 μg/mL phytohemagglutinin (PHA; ICN Pharmaceuticals, Costa Mesa, CA) before infection with MV-Edm. Vero African green monkey kidney cells (ATCC CCL-81) were maintained in Dulbecco modified Eagle medium (Gibco BRL) supplemented with 5% FBS.
Viruses and infection assays
Parental MV-Edm and recombinant MV-Edm carrying enhanced green fluorescent protein reporter gene (MV-eGFP) were propagated on Vero cells as described previously.9 Titers of the resultant viral stocks were determined by 50% end-point dilution assays (TCID 50) on Vero cells.10 For virus infection assays, 2 × 105 myeloma cells or PHA-stimulated PBLs were incubated with MV-eGFP at a multiplicity of infection (MOI) of 0.1, 1.0, or 10.0 (virus titer determined on Vero cells) in 0.5 to 1.0 mL Opti-MEM (Gibco-BRL) for 3 hours at 37°C. At the end of the incubation period, the virus was removed and the cells were maintained in 10% FBS-RPMI (myeloma cell lines), 10% FBS-RPMI containing 2.5 μg/mL PHA (PBLs), or 10% FBS-RPMI containing 1 ng/mL IL-6 (primary myeloma cells, KAS-6/1). At various time points (48 hours, 72 hours, 96 hours) after infection, the cells were photographed under blue light, and the percentage of measles virus infection or syncytia was noted. Cell viability was determined using trypan blue exclusion assay and the Promega (Madison, WI) CellTiter 96 nonradioactive cell proliferation assay according to manufacturer's instructions. The CellTiter 96 assay is based on the conversion of a tetrazolium salt by viable cells into an insoluble blue formazan product that can be quantitated using a spectrophotometer at 570 nm.
In vivo experiments
All procedures involving animals were approved by and performed according to guidelines of the Institutional Animal Care and Use Committee of the Mayo Foundation. Female athymic nude or C-B.17 scid/scid (SCID) mice (5-7 weeks of age; Harlan Sprague Dawley) were maintained in the barrier facilities of Mayo Clinic. These mice were given whole body irradiation at 1.5 Gy 2 days before implantation of the tumor cells. To test the antitumorigenic activity of MV-Edm, each mouse received 107 ARH-77 myeloma cells premixed with 107 plaque-forming units (pfu) MV-Edm or UV-inactivated MV-Edm (MOI, 1.0). Thus, for a group of 16 mice, 1.6 × 108 ARH-77 cells were mixed with 1.6 × 108 pfu MV-Edm in a total volume of 1.6 mL Opti-MEM. One hundred microliters of this mixture was then immediately injected subcutaneously into the hind flanks of C-B.17 SCID mice. The control group of 16 mice received ARH-77 cells premixed with UV-inactivated MV-Edm (MOI, 1.0). Mice were examined daily for tumor growth. For the therapy experiments, tumors were established by inoculating ARH-77 and RPMI 8226 cells (107 cells/100 μL per site) into the right flanks of C-B.17 SCID or athymic mice, respectively. The animals were examined daily until the tumors were palpable, after which tumor size was measured daily in 2 dimensions using calipers. For intratumoral administration, MV-Edm (107 pfu in 100 μL Opti-MEM) was injected into ARH-77 tumors (0.4-0.6 cm in diameter) using a 27-gauge needle twice a week for a total of 7 doses. Control mice were treated with an equivalent dose of virus inactivated by UV irradiation. For intravenous administration, tumor-bearing mice were injected through the tail using a 27-gauge needle with MV-Edm (107 pfu in 100 μL Opti-MEM). Each mouse received 1 dose or 7 doses of active or UV-inactivated MV-Edm administered twice a week. Animals were euthanized at the end of the experiment or when tumor burden reached 10% of body weight. Tumor volume is calculated asa2 × b × 0.5, wherea is the width and b is the length of the tumor.
In situ hybridization for MV-Edm nucleocapsid mRNA
ARH-77 tumor xenografts were harvested and fixed in 10% formalin. Paraffin-embedded tissue sections (5 μm) were deparaffinized and were stained with hematoxylin and eosin or probed for the presence of measles virus nucleocapsid (N)–specific mRNA using digoxigenin (DIG)-labeled nucleocapsid RNA of negative polarity.11 Briefly, tissue sections were pretreated with proteinase K (22 μg/mL) for 10 minutes at room temperature, washed twice with phosphate-buffered saline, and prehybridized overnight at 37°C. The next day, the sections were washed and incubated overnight with DIG-labeled N RNA probe (1 μg) at 65°C in a humidified chamber. Immunologic detection was performed with DIG-nucleic acid detection kit (Roche Diagnostics, Indianapolis, IN). Briefly, the sections were washed with phosphate-buffered saline, incubated with anti-DIG antibody conjugated to alkaline phosphatase (1:500 dilution), and probed with a color substrate for alkaline phosphatase.11 Tumor sections were subsequently counterstained with hematoxylin.
Statistical methods
Tumor volumes were calculated as mean ± SEM. Repeated measures analysis of variance (SAS general linear models procedure) was used to determine the statistical significance of differences between the growth of tumors by comparing tumor volumes at the start and end of treatment in the experimental groups. P ≤ .05 indicates that the groups are significantly different.
Results
MV-Edm replicates efficiently and selectively in myeloma cells and induces potent cytopathic effects
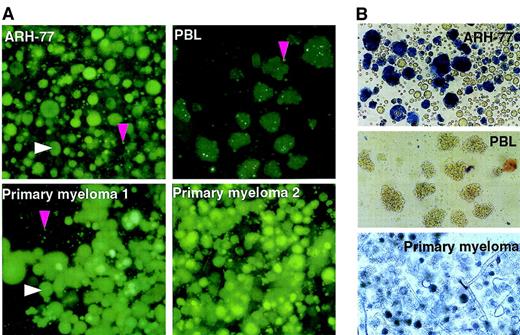
We first infected the ARH-77 myeloma cell line and PHA-stimulated PBLs with MV-eGFP (recombinant MV-Edm carrying the enhanced GFP reporter gene) and compared the ability of the virus to spread within the culture. MV-eGFP grew efficiently in the ARH-77 cells but not in the PHA-stimulated PBLs. Rapid replicative spread of the virus in the culture gave rise to the characteristic measles virus-induced cytopathic effect of cell-to-cell fusion with the formation of large multinucleated syncytia (Figure 1). These MV-eGFP infected syncytia were at different stages of viability. Viable syncytia were highly fluorescent under blue light because of GFP expression and were able to exclude trypan blue. There were also nonviable syncytia that had lost the ability to exclude trypan blue and were no longer fluorescent. This selectivity of MV-Edm for neoplastic cells was tested against more myeloma cell lines—JJN-3, RPMI 8226, MM1, KAS-6/1, KMS-11—and against primary human myeloma cells isolated by CD138 sorting of bone marrow aspirates from 6 patients. We found that MV-eGFP grew efficiently and selectively in all the myeloma cells tested, whereas virus growth was severely restricted in primary cultures of PHA-stimulated PBLs (Table1), normal dermal fibroblasts, and vascular smooth muscle cells (data not shown). Ninety-six hours after infection at an MOI of 1.0, an advanced cytopathic effect involving more than 50% of the cells in the culture was noted in all myeloma cell lines and in all primary myeloma cell cultures (Figure 1). Cell viability in these cultures was determined by a cell proliferation assay that measures the ability of viable cells to convert a tetrazolium compound into a blue formazan product. Ninety-six hours after infection, 70% to 90% of cells/syncytia in the MV-eGFP–infected cultures were no longer viable (Table2). In agreement with previously published studies characterizing the mechanism of cell killing by measles virus,12 apoptotic death of syncytia was confirmed by TUNEL staining (data not shown). In contrast, MV-eGFP infection in PHA-stimulated PBLs was restricted (Figure 1). Syncytium formation remained minimal in PBL cultures, and no cytopathic effect was seen with prolonged culture to 10 days after infection. Thus, MV-Edm replication is restricted in nontransformed human cells but proceeds efficiently in human myeloma cell lines and in primary human myeloma cell cultures that are selectively killed by the virus.
Edmonston-B vaccine strain of measles virus (MV-Edm) replicates selectively in myeloma cells and induces potent cytopathic effects.
The ARH-77 myeloma cell line, human primary myeloma cells, and PHA-stimulated PBLs were infected with MV-eGFP (MOI, 1.0), photographed, and stained with trypan blue 96 hours later. The white arrow indicates a fluorescent syncytium, and the pink arrow indicates a single infected fluorescent cell. (A) At 48 hours after infection, the viable MV-infected ARH-77 cells and primary myeloma cells are identifiable as large fluorescent syncytia because of GFP expression under blue light. (B) Phase contrast reveals that at 96 hours after infection, many syncytia in the ARH-77 and primary myeloma cultures have died and lost the ability to exclude trypan blue. In comparison, MV-Edm replication in PHA-stimulated PBLs is restricted, and cytopathic effects are minimal.
Edmonston-B vaccine strain of measles virus (MV-Edm) replicates selectively in myeloma cells and induces potent cytopathic effects.
The ARH-77 myeloma cell line, human primary myeloma cells, and PHA-stimulated PBLs were infected with MV-eGFP (MOI, 1.0), photographed, and stained with trypan blue 96 hours later. The white arrow indicates a fluorescent syncytium, and the pink arrow indicates a single infected fluorescent cell. (A) At 48 hours after infection, the viable MV-infected ARH-77 cells and primary myeloma cells are identifiable as large fluorescent syncytia because of GFP expression under blue light. (B) Phase contrast reveals that at 96 hours after infection, many syncytia in the ARH-77 and primary myeloma cultures have died and lost the ability to exclude trypan blue. In comparison, MV-Edm replication in PHA-stimulated PBLs is restricted, and cytopathic effects are minimal.
MV-Edm infection induced extensive syncytial formation in ARH-77 myeloma cell line and primary myeloma cells but not in PHA-activated PBLs
| Cells . | Percentage of infected cells/syncytia (size of syncytia) . | ||
|---|---|---|---|
| MOI 0.1 . | MOI 1.0 . | MOI 10.0 . | |
| ARH-77 | 50% (+++) | > 90% (+++) | > 90% (+++) |
| Patient 1 | 10% (+++) | 60% (+++) | > 90% (++) |
| Patient 2 | 10% (+++) | 60% (++) | 80% (++) |
| Patient 3 | ND | 40% (+++) | 70% (+) |
| Patient 4 | ND | 30% (++) | 60% (+) |
| Patient 5 | ND | 50% (+++) | 80% (+++) |
| Patient 6 | 30% (+++) | 50% (+++) | 30% (+++) |
| PBLs | ND | 24% (±) | 11% (±)* |
| Cells . | Percentage of infected cells/syncytia (size of syncytia) . | ||
|---|---|---|---|
| MOI 0.1 . | MOI 1.0 . | MOI 10.0 . | |
| ARH-77 | 50% (+++) | > 90% (+++) | > 90% (+++) |
| Patient 1 | 10% (+++) | 60% (+++) | > 90% (++) |
| Patient 2 | 10% (+++) | 60% (++) | 80% (++) |
| Patient 3 | ND | 40% (+++) | 70% (+) |
| Patient 4 | ND | 30% (++) | 60% (+) |
| Patient 5 | ND | 50% (+++) | 80% (+++) |
| Patient 6 | 30% (+++) | 50% (+++) | 30% (+++) |
| PBLs | ND | 24% (±) | 11% (±)* |
The percentage of fluorescent cells/syncytia was noted 3 to 4 days after infection by MV-eGFP. Average syncytial size was scored by estimating the average number of cells per syncytium.
+++ indicates more than 50 cells per syncytium; ++, 21-50 cells per syncytium; +, 6-20 cells per syncytium; ±, 5 or fewer cells per syncytium; ND, not done.
MOI = 100.
Cell viability in MV-Edm–infected cultures of myeloma cell lines and PHA-stimulated PBLs
| Cells . | Percentage of viable cells . | |
|---|---|---|
| Uninfected (%) . | MV-eGFP infected (%) . | |
| PBLs | 100 | 104 |
| JJN-3 | 100 | 7.4 |
| ARH-77 | 100 | 7.9 |
| RPMI 8226 | 100 | 18.5 |
| MM1 | 100 | 20.2 |
| KAS-6/1 | 100 | 36.3 |
| KMS-11 | 100 | 37.8 |
| Cells . | Percentage of viable cells . | |
|---|---|---|
| Uninfected (%) . | MV-eGFP infected (%) . | |
| PBLs | 100 | 104 |
| JJN-3 | 100 | 7.4 |
| ARH-77 | 100 | 7.9 |
| RPMI 8226 | 100 | 18.5 |
| MM1 | 100 | 20.2 |
| KAS-6/1 | 100 | 36.3 |
| KMS-11 | 100 | 37.8 |
Cells were infected with MV-eGFP (MOI, 1.0), and cell viability was determined 96 hours after infection. The percentage of viable cells in the infected culture is compared against that in the uninfected culture (100% viable). Values are representative of 3 experiments.
MV-Edm has potent antitumorigenic and antineoplastic activity against human myeloma xenografts
We first tested the antitumorigenic ability of MV-Edm in a “premix” experiment. Ten million ARH-77 myeloma cells were premixed with MV-Edm (MOI, 1.0) or UV-inactivated MV-Edm just before the cells were injected subcutaneously into the flanks of immunocompromised SCID mice. Only 1 of the 16 mice that received MV-Edm–treated cells developed a palpable tumor, and the rest of the mice remained tumor free at 6 weeks after implantation. In the group that received cells premixed with UV-inactivated MV-Edm, 15 of 16 mice developed established tumors with diameters greater than 6.0 mm 2 weeks after implantation of the cells.
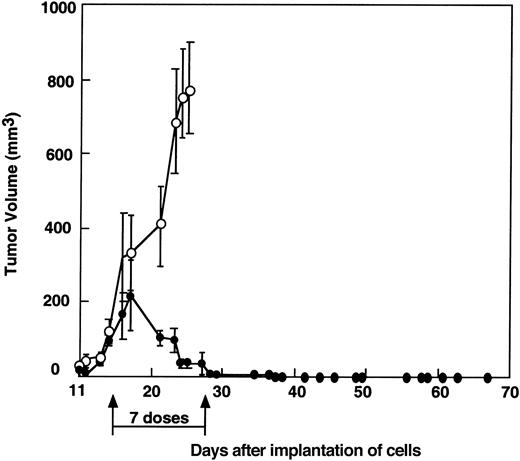
To test the potency of MV-Edm as a tumor therapy, human ARH-77 myeloma cells were established as subcutaneous xenografts in the hind flanks of SCID mice. Tumors were allowed to grow until they reached diameters between 4.3 and 6.4 mm (volume, 50-169 μL). Tumor-bearing mice were treated by intratumoral inoculation of 107infectious units of MV-Edm in 100 μL Opti-MEM administered twice a week for a total of 7 doses. Control tumors were injected with equivalent amounts of UV-inactivated virus. As shown in Figure2, the ARH-77 myeloma xenografts were highly sensitive to this dose of MV-Edm, and all treated tumors regressed completely. One ARH-77 tumor regrew after therapy but regressed completely when injected with a single dose of 107 pfu/100 μL MV-Edm. Multinucleated syncytia were detected in hematoxylin and eosin-stained sections of tumors inoculated 3 days earlier with 107 pfu MV-Edm (data not shown). Analysis of these sections of MV-Edm–inoculated tumors by in situ hybridization for measles virus nucleocapsid (N) mRNA confirmed that the syncytia in these tumors contained abundant MV-Edm RNA (Figure3). Control tumors injected with UV-inactivated virus showed no syncytia and did not stain positive for MV-N RNA (Figure 3).
Complete regression of ARH-77 myeloma xenografts in SCID mice after intratumoral injection of MV-Edm.
Tumors were injected directly with 107 pfu active (●) or UV-inactive MV-Edm (○) in 100 μL twice a week for a total of 7 doses. Each group consisted of 6 animals. All tumors treated with active MV-Edm showed complete response. One tumor in the MV-Edm–treated group regrew but regressed completely after another dose of 107 pfu MV-Edm.
Complete regression of ARH-77 myeloma xenografts in SCID mice after intratumoral injection of MV-Edm.
Tumors were injected directly with 107 pfu active (●) or UV-inactive MV-Edm (○) in 100 μL twice a week for a total of 7 doses. Each group consisted of 6 animals. All tumors treated with active MV-Edm showed complete response. One tumor in the MV-Edm–treated group regrew but regressed completely after another dose of 107 pfu MV-Edm.
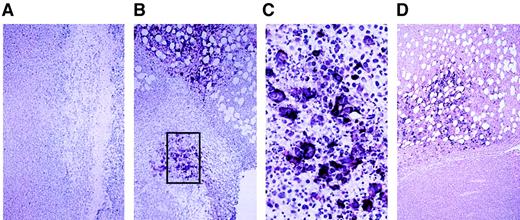
In situ hybridization for MV nucleocapsid (N) mRNA in ARH-77 tumor xenografts treated with MV-Edm.
(A) Tumor injected with UV-inactivated measles virus stained negative for MV-N mRNA. (B) Tumor injected directly with a single dose of 107 pfu active MV-Edm and harvested 3 days later showed positive (dark purple) staining in tumor cells/syncytia, indicating viral replication. (C) Higher magnification of boxed area. (D) Tumor harvested 7 days after mouse was injected intravenously through the tail vein with 1 dose of 107 pfu active MV-Edm also showed positive staining for MV-N mRNA in the tumor tissue.
In situ hybridization for MV nucleocapsid (N) mRNA in ARH-77 tumor xenografts treated with MV-Edm.
(A) Tumor injected with UV-inactivated measles virus stained negative for MV-N mRNA. (B) Tumor injected directly with a single dose of 107 pfu active MV-Edm and harvested 3 days later showed positive (dark purple) staining in tumor cells/syncytia, indicating viral replication. (C) Higher magnification of boxed area. (D) Tumor harvested 7 days after mouse was injected intravenously through the tail vein with 1 dose of 107 pfu active MV-Edm also showed positive staining for MV-N mRNA in the tumor tissue.
Intravenous administration of MV-Edm caused regression of myeloma xenografts
Intratumoral therapy is of limited value for the treatment of disseminated malignancy. We therefore tested the antineoplastic potential of systemically administered MV-Edm against 2 subcutaneous myeloma xenograft models, RPMI 8226 and ARH-77. In the first model, 107 RPMI-8226 myeloma cells were implanted subcutaneously into the flanks of athymic mice. Eleven days later (tumor volume 21 ± 5 μL; n = 16), the mice were treated by intravenous administration of 107 infectious units of MV-Edm in 100 μL Opti-MEM administered on alternate days for a total of 7 doses. Control tumor-bearing mice were injected with equivalent amounts of UV-inactivated virus. As shown in Figure4, tumor growth in the MV-Edm–treated animals (mean tumor volume, 404 ± 131 μL, n = 8) was significantly inhibited (P = .002) compared to control animals that were treated with UV-inactivated MV-Edm (mean tumor volume, 1667 ± 295 μL, n = 8). Thirteen days after the initiation of treatment, all 8 animals in the control group that received UV-inactivated MV-Edm had to be euthanized because of a large tumor burden. Survival of mice in the MV-Edm–treated group was considerably longer, and complete tumor regression was achieved in 1 of the 8 treated mice.
Systemic MV-Edm therapy by intravenous administration into mice bearing RPMI 8226 myeloma xenografts.
Intravenous administration of 7 doses of active MV-Edm (●) caused growth inhibition of RPMI 8226 myeloma xenografts, and 1 of 8 mice in the group showed complete regression. In the group treated with equivalent doses of UV-inactivated MV-Edm (○), all 8 mice were euthanized because of tumor burden 13 days after initial therapy. The difference in tumor growth in the 2 groups was significantly different (P = .002).
Systemic MV-Edm therapy by intravenous administration into mice bearing RPMI 8226 myeloma xenografts.
Intravenous administration of 7 doses of active MV-Edm (●) caused growth inhibition of RPMI 8226 myeloma xenografts, and 1 of 8 mice in the group showed complete regression. In the group treated with equivalent doses of UV-inactivated MV-Edm (○), all 8 mice were euthanized because of tumor burden 13 days after initial therapy. The difference in tumor growth in the 2 groups was significantly different (P = .002).
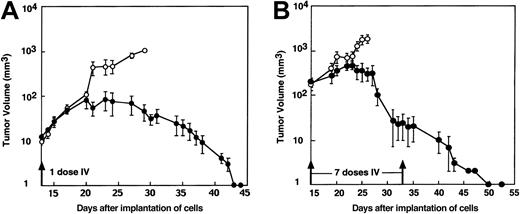
We next tested the potency of intravenous MV-Edm against the ARH-77 model of multiple myeloma. Human ARH-77 myeloma cells were established as subcutaneous xenografts in SCID mice, and the mice were treated with one or multiple doses of MV-Edm. Intravenous administration of a single dose of MV-Edm caused complete regression of small (12 μL) ARH-77 xenografts in all 4 treated animals (Figure5A). Complete regression of larger ARH-77 xenografts (195 μL) could not be achieved with a single dose but was achieved in all 6 animals by repeated intravenous administration of 107 pfu MV-Edm (Figure 5B). We were unable to detect any treatment-related toxicity, but it should be recognized that the mouse lacks MV receptors and, hence, is a poor model in which to assess the toxicity of this agent.13-15 No antitumor effect was seen after treatment with UV-inactivated virus. In situ hybridization for MV-N mRNA in ARH-77 tumor sections from mice treated 7 days earlier with intravenous MV-Edm confirmed the presence of MV-Edm–infected tumor cells that were patchily distributed throughout the tumor (Figure3D).
Systemic MV-Edm therapy by intravenous administration into mice bearing ARH-77 myeloma xenografts.
Mice bearing ARH-77 myeloma xenografts were treated intravenously with a single dose (A) or 7 doses (B) of 107 pfu MV-Edm/100 μL (●) or UV-inactivated MV-Edm (○). The number of mice in each treatment group that received 1 dose or 7 doses of virus was 4 and 6, respectively. For both dosing regimes, the difference in tumor growth between the treatment group and the control group was significantly different (P = .0001).
Systemic MV-Edm therapy by intravenous administration into mice bearing ARH-77 myeloma xenografts.
Mice bearing ARH-77 myeloma xenografts were treated intravenously with a single dose (A) or 7 doses (B) of 107 pfu MV-Edm/100 μL (●) or UV-inactivated MV-Edm (○). The number of mice in each treatment group that received 1 dose or 7 doses of virus was 4 and 6, respectively. For both dosing regimes, the difference in tumor growth between the treatment group and the control group was significantly different (P = .0001).
Discussion
This study brings to light the previously unrecognized antimyeloma activity of MV-Edm and its selectivity for myeloma cells. Live attenuated measles virus vaccines afford a high level of protection against infection and are routinely administered to preschool children throughout the world. The appeal of measles vaccine as an antineoplastic agent is greatly enhanced by its excellent safety record.16 In the current study, MV-Edm was administered by intravenous or intratumoral inoculation at a dose of 107infectious units compared to the recommended dose for immunization of 103 to 104 infectious units administered subcutaneously.7 The therapeutic potency of the virus at doses significantly lower than 107 infectious units was substantially reduced (Peng and Russell, unpublished data). Additional studies are required to determine whether the antineoplastic activity of the MV-Edm is amplified at higher challenge doses and to characterize the toxicity profile of MV-Edm administered to human subjects at the higher doses and alternative routes required for antineoplastic activity.
In light of its selectivity for myeloma cells and its potent antineoplastic activity against myeloma xenografts, MV-Edm appears to be a promising agent that merits further evaluation in clinical trials. However, several outstanding issues pertinent to this proposed use of MV-Edm remain that have not been addressed in the current study. First, the mechanisms underlying the selective antineoplastic activity of MV-Edm have not been elucidated. Possibilities that we are exploring include a relative abundance of measles virus receptors on myeloma cells, higher concentrations of intracellular factors supporting virus replication, and specific defects in the antiviral responses of the myeloma cells. A second concern is that certain theoretical toxicities of MV-Edm could not be evaluated with the SCID mouse model used in the current study because these mice do not express receptors for the virus. Thus, MV-Edm infection of human monocytes17 or osteoblasts18 might lead to the up-regulation of IL-6, a known inhibitor of myeloma cell apoptosis that can also stimulate myeloma cell proliferation.19 In addition, measles virus can infect and activate human osteoclast precursors and has been implicated in the pathogenesis of Paget disease of bone.20,21 MV-Edm might, therefore, have the potential to exacerbate the osteolytic bone disease characteristic of multiple myeloma. Another potential toxicity is MV-Edm–mediated immune suppression. Measles vaccination is known to be mildly immunosuppressive, leading to transient suppression of delayed-type hypersensitivity responses,22 and this effect may be greatly amplified after the administration of the large doses of MV-Edm required for antineoplastic therapy. Transgenic mice expressing human CD46 are susceptible to MV-Edm infection and may facilitate the experimental study of some of these potential toxicities,11,23 as may the SCID-Hu model of multiple myeloma in which primary myeloma cells are grown in vivo in a human bone marrow microenvironment, provided by a transplanted fragment of fetal bone.24 25
Another concern associated with the use of replicating viruses as antitumor agents is the theoretical risk that the therapeutic virus might evolve to become highly pathogenic and transmissible, thereby causing disease in the contacts of the patient.26 For MV-Edm the risk is negligible because most of the patient's contacts will have pre-existing immunity from childhood measles vaccination or infection. It is possible that, in addition to providing a barrier to horizontal virus transmission, the high prevalence of measles virus antibody seropositivity might compromise the value of MV-Edm for cancer therapy. However, considerable evidence suggests that this may not be the case given that asymptomatic measles virus infection has been well documented in vaccinated children.27 Recently, it was demonstrated that macaques vaccinated with live attenuated virus or DNA can experience subclinical infection when challenged with measles virus.28 Studies have shown that the therapeutic efficacy of conditionally replicating adenoviruses,29herpesviruses,30 or reoviruses4 was not negated by pre-existing antiviral antibodies. In addition, Grote et al (manuscript submitted) recently demonstrated that the infusion of neutralizing MV-Edm antibodies into immunocompromised mice did not compromise the antitumor efficacy of MV-Edm. It is also worth noting that profound suppression of serum immunoglobulin levels is a characteristic feature of multiple myeloma. Indeed, efficacy from vaccination of myeloma patients against influenza, Streptococcus pneumoniae, and Haemophilus influenza type B is poor, and only a small percentage of patients achieved protective antibodies.31 Therefore, multiple myeloma may represent the most attractive target for oncolytic virotherapy using MV-Edm.
Intracellular restriction of virus replication was the basis for tumor selectivity of all previously studied oncolytic viruses.2-6 Viruses whose host range is restricted at the level of attachment and entry into the target cell have not previously been tested, primarily because it has not been feasible to target viruses in this way. Our demonstration that MV-Edm can eliminate CD46-receptor–positive tumors from CD46-receptor–negative mice when administered intravenously suggests that receptor-mediated targeting is a worthwhile strategy to pursue. Toward this goal, we have recently demonstrated the feasibility of targeting MV-Edm entry. High-affinity ligands including epidermal growth factor, insulin-like growth factor, and a single-chain antibody against carcinoembryonic antigen were displayed on the surface of MV-Edm as C-terminal extensions of the H glycoprotein.32,33 Targeted attachment and entry of these recombinant viruses was confirmed on rodent cells expressing the relevant cognate receptors, but human cells were infected promiscuously through their natural receptors. To optimize this engineering strategy for efficient receptor-mediated targeting of human tumor cells, we are now attempting to eliminate the binding of the hybrid H glycoprotein to the natural receptors, CD4613,14 (regulator of complement activation) and SLAM15 (signaling lymphocyte-activation molecule; CDw150; B- and T-cell–specific protein).
In conclusion, we have demonstrated that MV-Edm administered by the intravenous route can target, infect, and destroy human myeloma cells in immunocompromised mice. Possible advantages of MV-Edm as an antineoplastic virus include its excellent safety record and the potential for further modification of the viral genome to enhance its selectivity and efficacy against specific tumor types. In light of its natural lymphotropism7 and its potent activity against myeloma cell lines, primary myeloma cells, and myeloma xenografts, we conclude that MV-Edm is particularly promising as a novel agent for the treatment of multiple myeloma.
We thank Sue Facteau and Jill Ludvigson for expert technical assistance with the in vivo studies, Dr Shane Pankratz for help with statistical analysis, Maureen Craft for secretarial support, and Drs Richard Vile, Eric Poeschla, and Greg Poland for helpful comments on the manuscript.
Supported through grants from the Harold W. Siebens and George W. Eisenberg Foundations and by NIH grant CA62242.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Stephen J. Russell, Molecular Medicine, Mayo Foundation, Guggenheim 18, 200 First St SW, Rochester, MN 55905; e-mail: sjr@mayo.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal