HVMNE is a novel Epstein-Barr (EBV)–like virus isolated from a Macaca nemestrina with CD8+ T-cell mycosis fungoides–cutaneous T-cell lymphoma. Here it is demonstrated that intravenous inoculation of irradiated HVMNE-infected T cells or cell-free virus from the J94356PBMC cell line in New Zealand White rabbits results in seroconversion to the viral capsid antigen (VCA) of EBV; all animals that seroconverted to VCA developed malignant lymphoma within months of inoculation. In contrast, control rabbits, inoculated with heat-inactivated culture supernatants from the same cell line, failed to seroconvert to VCA and did not develop disease. Disseminated lymphoma cells of mixed origin were detected in most vital organs, including the spleen, liver, lungs, kidneys, and heart of the affected rabbits. Neoplastic infiltrates were also observed in lymph nodes, thymus, skin, and subcutaneous tissues. HVMNE DNA and EBV-like RNA expression was demonstrated in the lymphomatous organs and in 2 transformed T-cell lines, one established from the lymph node and the other from the blood of the 2 lymphomatous animals. Analysis of one of these T-cell lines demonstrated the persistence of HVMNEDNA, expression of an LMP1-like protein, and acquisition of interleukin-2 independence, and constitutive activation of the Jak/STAT pathway. Thus, HVMNE in rabbits provides a valuable animal model for human T-cell lymphoma whereby genetic determinants for T-cell transformation by this EBV-like animal virus can be studied.
Introduction
DNA viruses such as Epstein-Barr virus (EBV) and human herpesvirus 8 (HHV8), in addition to the retrovirus human T-cell leukemia–lymphoma virus type I (HTLV-I), have been causally linked to lymphoma in humans.1,2 EBV was originally discovered in the cultured cells of a Ugandan child with Burkitt lymphoma.3 EBV infection is highly prevalent worldwide,4 and in most patients it is asymptomatic. Occasionally, primary exposure to EBV results in infectious mononucleosis.5 Evidence supporting a causative role of EBV in human cancers, such as Burkitt lymphoma and nasopharynx carcinoma,6,7 is supported by the observation that EBV induces the immortalization of primary human B cells in vitro and that EBV infection induces malignant B-cell proliferation in New World nonhuman primate species.8 The clinical relevance of EBV as a causative agent of lymphoma, including a subset of Hodgkin lymphoma,9 is further demonstrated by the introduction of iatrogenic practices such as bone marrow and solid organ transplantation whereby the induced immune suppression results in de novo infection or reactivation of pre-existing EBV infection and lymphoma development.6,10 11
EBV infects B lymphocytes through the C3d complement receptor CD21, which also binds 3 other known ligands, the p53 tumor-suppressor protein, the p68 calcium-binding protein, and the p120 ribonucleoprotein.12 Although for many years EBV has been associated mainly with B-cell malignancies, several recent reports have suggested its association with T-cell malignancies as well.13-24 Indeed, EBV infection of human T lymphocytes occurs even in the absence of surface expression of the EBV CD21 receptor or in the presence of the anti-CR2 monoclonal antibody that inhibits virus attachment, alluding to the existence of an alternative EBV receptor on T cells.25
The in situ hybridization technique, developed to detect the presence of EBV, has revealed immediate-early EBV transcripts in CD4+ and CD8+ T lymphocytes,26suggesting that EBV could contribute to T-cell proliferation and T-cell malignancies. In humans, EBV-harboring T cells have been detected in chronic active EBV infection,27 EBV-associated hemophagocytic syndrome,28 angiocentric lymphomas,29 and extranodal lymphomas, involving especially the nose, skin, and gastrointestinal tract.30Although EBV has been found in cutaneous T-cell lymphoma and Sézary syndrome lesions,16,31 the causative role of EBV in this disease remains controversial.32 Injection of peripheral blood mononuclear cells (PBMCs) from EBV-infected humans into HuSCID mice results in uncontrolled proliferation of human B cells.3 In marmosets, inoculation of EBV induces mainly B-cell lymphomas, though polyclonal proliferation of T cells can also develop.8
EBV-like herpesviruses have been found in most Old World nonhuman primate species studied.34-37 HVMNE was originally isolated from the CD8+ T cells of a Macaca nemestrina with mycosis fungoides,38 whereas HVMA (from Macaca arctoides) and cyno-EBV (from cynomolgus monkeys) were isolated from macaques with B-cell lymphoma. Interestingly, although human EBV is not oncogenic in rabbits, both HVMA and cyno-EBV induce B-cell lymphoma in them.39 40 Here we report that HVMNE, isolated from the neoplastic CD8+ T cells of M nemestrinawith cutaneous T-cell lymphoma, induces lymphoma in all rabbits that seroconvert to viral antigens and that transformed interleukin-2 (IL-2)–independent T-cell lines of rabbit origin can be obtained in vitro from the lymphomatous rabbits. Because all infected rabbits develop lymphoma within a few months of inoculation, this model can be exploited to study genetic determinants of HVMNEoncogenicity in T cells. These studies may be instrumental in defining EBV genes involved in T-cell proliferation.
Materials and methods
Cell cultures and supernatants
The establishment of 2 CD8+ T-cell lines (J94356PBMC and J94356Skin) from the PBMCs and skin lesions of an STLV-I/II-negative M nemestrina with mycosis fungoides was previously described.38 These cell lines express EBV-related antigens and harbor herpesvirus-like particles. The J94356PBMC cell line was cultured with medium containing RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Gibco BRL, Gaithersburg, MD) with penicillin–streptomycin (500 U/mL and 500 U/mL, respectively) (Gibco BRL), L-glutamine (2 mM) (Gibco BRL), and recombinant IL-2 (20 U/mL; Boehringer Mannheim, Indianapolis, IN). Cells (109) from the J94356PBMC cell line were incubated in medium containing Na-butyrate (1 μM) for 48 hours and lethally irradiated with 100 Gy for 10 minutes.
Supernatants obtained from J94356PBMC culture after Na-butyrate stimulation were centrifuged at 8000g for 30 minutes to remove cell debris, filtered using a 0.45 μm filter, and centrifuged again at 19 000 rpm for 2.5 hours to obtain a virus pellet. Half the virus pellet was heat inactivated at 56°C for 30 minutes before inoculation.
Rabbit inoculation and serology
Eighteen specific-pathogen–free juvenile New Zealand White rabbits (each weighing 2-3 kg) were obtained from Covance Research (Alice, TX). Malignant lymphoma does not develop spontaneously in this breed of animal at a young age. Ten animals were inoculated intravenously with 1.275 × 108 J94356PBMCcells, 4 with the cell-free virus obtained from 200 mL supernatants from the J94356PBMC cell culture. Four animals received equivalent amounts of heat-inactivated cell-free virus (56°C for 30 minutes).
Antibody responses to viral capsid antigen of EBV-related virus in rabbits
Titers of anti–viral capsid antigen (VCA) immunoglobulin G in sera from all rabbits before and after inoculation were determined by ELISA (Diasorin, Stillwater, MN).
Establishment of rabbit tumor cell lines
Cell lines were established from the PBMCs or tissues from 8 tumor-bearing rabbits. Normal PBMCs from 4 non–tumor-bearing rabbits were cultured as controls.
PBMCs were isolated by density-gradient centrifugation on lymphocyte separation medium (Organon Teknika, Durham, NC) from anticoagulated rabbit blood. Buffer-coat cells were washed twice in Dulbecco phosphate-buffered saline (PBS; Gibco BRL) and were suspended in RPMI (Gibco BRL) with 20% heat-inactivated FBS (Gibco BRL) with penicillin–streptomycin (500 U/mL and 500 U/mL, respectively) (Gibco BRL) and L-glutamine (2 mM) (Gibco BRL) and stimulated with phytohemagglutinin (PHA; 5 μg/mL) (Murex Diagnostics, Norcross, GA). At 72 hours, the cells were washed twice in Dulbecco PBS and resuspended in fresh RPMI with 20% heat-inactivated FBS, penicillin–streptomycin, and L-glutamine; a separate set was supplemented with recombinant IL-2 (20 U/mL; Boehringer Mannheim). Fresh neoplastic tissues from diseased animals were homogenized to release single cells, which were banded on lymphocyte separation medium and placed in culture as described above. All cells were incubated at 37°C with 5% CO2; fresh media were added periodically, as needed, to sustain growth. After 6 weeks in culture, the PBMCs and lymph node-derived cells of 2 tumor-bearing rabbits showed evidence of increased proliferation and clustering; cells from these animals have been cultured for more than 20 months.
Electrophoretic mobility shift assay
PHA-stimulated human and rabbit PBMCs were cultured in the presence of IL-2 (20 U/mL) for 8 days and used as a control in this assay. In starvation experiments, 1 × 107 stimulated PBMCs and IL-2–dependent and –independent C2174 rabbit cell lines were resuspended in 20 mL RPMI 1640 with 1% FBS after they were washed twice with 1× PBS and incubated for 21 hours at 37°C in 5% CO2. Protein lysates were prepared in 20 mM/L HEPES (pH 7.9), 450 mM NaCl, 0.4 mM EDTA, 0.5 mM dithiothreitol (DTT), 25% glycerol, 1 mM Na3VO4, 1 mM AEBSF, 20 μg/mL aprotinin, and 20 μg/mL leupeptin. The binding reaction was performed by preincubating 5 μg nuclear extracts with 1 μg poly(dI-dC) in a buffer containing 5.9 mM HEPES (pH 7.9), 30 mM KCl, 5.9 mM Tris (pH 7.4), 0.7 mM DTT, 0.6 mM EDTA, 8.9% glycerol, 0.1 mM Na3VO4, 1 mM AEBSF, 20 μg/mL aprotinin, and 20 μg/mL leupeptin in ice for 20 minutes. A 32P-labeled probe (20 000 cpm) corresponding to the MGF binding site in the β-casein gene promoter (5′-TAGATTTCTAGGAATTCG-3′) was added to the reaction mixture and incubated on ice for 30 minutes. For the supershift assay, STAT-5 antibody (N-20) (Santa Cruz Biotechnology, Santa Cruz, CA) was incubated with cell extracts on ice for 20 minutes after the addition of a radiolabeled probe. Complexes were resolved on 4.5% polyacrylamide gels.
Histology
Formalin-fixed, paraffin-embedded tissue blocks were sectioned (3-5 μm thick) and stained for conventional histopathology with hematoxylin and eosin.
DNA extraction, polymerase chain reaction amplification, and DNA sequence analysis
Cell pellets of PBMCs obtained by Ficoll gradient separation were incubated for 1 hour at 37°C in a buffer containing 0.5% sodium dodecyl sulfate, 10 mM Tris (pH 8.0), 1 mM EDTA (pH 8.0), and RNAse (20 μg/mL) (Boehringer Mannheim) before Proteinase K (100 μg/mL) (Boehringer Mannheim) was added. After overnight incubation at 37°C, total DNA was extracted from the lysates with phenol and chloroform. Frozen tissues that had been maintained at −80°C were thawed on ice and finely minced with sterile blades. Tissue was resuspended in ice-cold Dulbecco PBS and was washed twice before lysis and extraction, as detailed above. DNA was initially amplified using the primer-pools DFASA/GDTD1B, described by Rose et al,41 corresponding to a conserved region in the herpesvirus family that encodes conserved primer-binding sites within the DNA polymerases. An aliquot of the first polymerase chain reaction (PCR) products was amplified using the HVMNE sequence-specific primers EDR 8/97 and MGF/2B. PCR conditions were described previously by Rivadeneira et al.38 Amplified DNA was transferred to nylon membrane and hybridized with 32P-labeled specific probes complementary to the sequences of HVMNE. Nested PCR products were analyzed by gel electrophoresis and cloned into the PCR 2.1 (Invitrogen, Carlsbad, CA), and the DNA sequence was obtained. Each DNA was first assessed for its amplification competence using DNA primers for cytochrome B (MVZ03: GCT TCC ATC CAA CAT CTC AGC ATG ATG; MVZ04: GCA GCC CCT CAG AAT GAT ATT TGT CCT CY).
Phenotypic analysis
Pelleted rabbit cell lines C2174 and C2180 and PBMCs derived from a naive rabbit were immunostained using antibodies against specific rabbit antigens (CD3, CD4, CD8 [Spring Valley Laboratories, Woodbine, MD], LMP1, CD25 [Pharmingen International, San Diego, CA], CD11a, CD11b, CD5, and IgM [Biosource International, Camarillo, CA]) and were analyzed using FACS.
EBV-like RNA in situ hybridization
Cells derived from rabbit tissues and peripheral blood were formalin fixed, pelleted, and paraffin embedded. Sections (3-5 μm) were cut onto glass slides and hybridized with a cocktail of fluorescein isothiocyanate (FITC)–conjugated probes reactive with EBV-like RNA (EBER), which is abundantly transcribed in all cells latently infected with EBV (Novocastra Laboratories, Newcastle, United Kingdom). Hybridization was detected using an alkaline–phosphatase-conjugated anti-FITC antibody (Dako, High Wycombe, United Kingdom) and nitro-blue tetrazolium as chromogenic substrate (Dako). Subsequently, the EBER in situ hybridization was performed using sections of tissue biopsy samples obtained from 5 rabbits affected with lymphoma. Hybridization using a poly-dT probe (Novocastra) was used to confirm the integrity of the RNA in all tissue sections.
Results
Induction of lymphoma in New Zealand White rabbits after inoculation with HVMNE
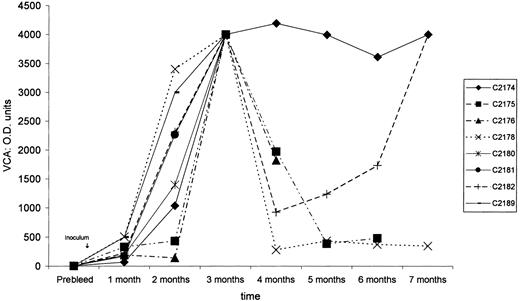
After treatment with sodium butyrate, 1.2 × 108HVMNE-infected J94356PBMC cells were irradiated and inoculated intravenously into 10 New Zealand White rabbits. Simultaneously, equivalent amounts of HVMNE cell-free virus were inoculated as such or after heat inactivation in 4 rabbits each (Table 1). Seven of the 10 animals inoculated with irradiated cells seroconverted to VCA, as demonstrated by the detection of specific serum antibodies to the VCA of EBV. Of the 4 animals inoculated with virus pellet, 1 of 4 seroconverted, whereas none of the 4 animals that received the heat-inactivated virus pellet did (Figure 1). The kinetics of seroconversion varied among animals, and in most animals the serum antibodies to VCA were highest 2 and 3 months after inoculation (Figure1). Lymphoma was first observed 3 months after inoculation in rabbits C2180 and C2181, which received irradiated cells, and in rabbit C2189, inoculated with cell-free virus. In the remaining rabbits, lymphomas were observed within 9 months of inoculation (Table 1). Lymphoma development correlated with seroconversion to VCA—all animals that seroconverted developed lymphoma, whereas no neoplasms were observed in the animals that failed to seroconvert (Table 1).
Time to development of malignant lymphoma New Zealand White rabbits
| Rabbit . | Inoculum (intravenous) . | Lymphoma . | Time of lymphoma detection (mo) . | Serum antibody to VCA . |
|---|---|---|---|---|
| C2174 | 1.275 × 108irradiated cells | + | 9 | + |
| C2175 | 1.275 × 108 irradiated cells | + | 6 | + |
| C2176 | 1.275 × 108 irradiated cells | + | 6 | + |
| C2177 | 1.275 × 108 irradiated cells | — | NA | — |
| C2178 | 1.275 × 108 irradiated cells | + | 9 | + |
| C2179 | 1.275 × 108 irradiated cells | — | NA | — |
| C2180 | 1.275 × 108 irradiated cells | + | 3 | + |
| C2181 | 1.275 × 108 irradiated cells | + | 3 | + |
| C2182 | 1.275 × 108 irradiated cells | + | 9 | + |
| C2183 | 1.275 × 108 irradiated cells | — | NA | — |
| C2188 | Cell-free virus | — | NA | — |
| C2189 | Cell-free virus | + | 3 | + |
| C2190 | Cell-free virus | — | NA | — |
| C2191 | Cell-free virus | — | NA | — |
| C2184 | Cell-free virus (heat-inactivated) | — | NA | — |
| C2185 | Cell-free virus (heat-inactivated) | — | NA | — |
| C2186 | Cell-free virus (heat-inactivated) | — | NA | — |
| C2187 | Cell-free virus (heat-inactivated) | — | NA | — |
| Rabbit . | Inoculum (intravenous) . | Lymphoma . | Time of lymphoma detection (mo) . | Serum antibody to VCA . |
|---|---|---|---|---|
| C2174 | 1.275 × 108irradiated cells | + | 9 | + |
| C2175 | 1.275 × 108 irradiated cells | + | 6 | + |
| C2176 | 1.275 × 108 irradiated cells | + | 6 | + |
| C2177 | 1.275 × 108 irradiated cells | — | NA | — |
| C2178 | 1.275 × 108 irradiated cells | + | 9 | + |
| C2179 | 1.275 × 108 irradiated cells | — | NA | — |
| C2180 | 1.275 × 108 irradiated cells | + | 3 | + |
| C2181 | 1.275 × 108 irradiated cells | + | 3 | + |
| C2182 | 1.275 × 108 irradiated cells | + | 9 | + |
| C2183 | 1.275 × 108 irradiated cells | — | NA | — |
| C2188 | Cell-free virus | — | NA | — |
| C2189 | Cell-free virus | + | 3 | + |
| C2190 | Cell-free virus | — | NA | — |
| C2191 | Cell-free virus | — | NA | — |
| C2184 | Cell-free virus (heat-inactivated) | — | NA | — |
| C2185 | Cell-free virus (heat-inactivated) | — | NA | — |
| C2186 | Cell-free virus (heat-inactivated) | — | NA | — |
| C2187 | Cell-free virus (heat-inactivated) | — | NA | — |
NA indicates not applicable.
Anti-VCA–immunoglobulin G titers.
After HVMNE inoculation into New Zealand White rabbits, anti-VCA–immunoglobulin G titers show the seroconversion of rabbits to EBV VCA.
Anti-VCA–immunoglobulin G titers.
After HVMNE inoculation into New Zealand White rabbits, anti-VCA–immunoglobulin G titers show the seroconversion of rabbits to EBV VCA.
Clinical and histologic features of lymphoma
Rabbits that seroconverted experienced a decline in physical well-being, including loss of appetite and emaciation. At necropsy, macroscopic analysis revealed splenomegaly or hepatomegaly or both in most tumor-bearing animals. White nodules were frequently found in kidneys, heart, lungs, and, with less frequency, gall bladder, eyes, skeletal muscles, and sacculus rotundus. In some cases, lymph node enlargement and skin involvement was also observed.
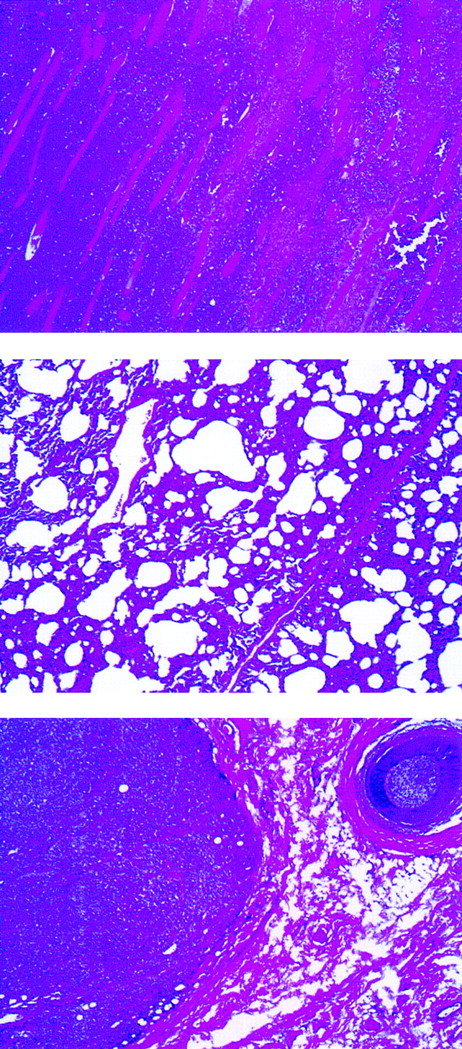
Histologic examination of rabbit tissues revealed diffuse mixed lymphocyte infiltrates involving many organs. An example is shown in Figure 2 of rabbit C2182, in which poorly differentiated neoplastic cells were predominant in skeletal muscle and lungs. Subcutaneous infiltrates were observed in animals C2182 (Figure2, bottom panel) and in rabbits C2174 and C2178 (data not shown).
Abnormal findings of lymphomatous rabbit C2182.
Lymphomatous cell infiltration in the abdominal muscles (top), lung (center), and subcutaneous mass (bottom) (original magnification, ×40).
Abnormal findings of lymphomatous rabbit C2182.
Lymphomatous cell infiltration in the abdominal muscles (top), lung (center), and subcutaneous mass (bottom) (original magnification, ×40).
Presence of HVMNE viral DNA and RNA in neoplastic tissues of lymphomatous rabbits
To further confirm that lymphoma induction was linked to the inoculated HVMNE, the presence of viral DNA and RNA was assessed in several tissues from the lymphomatous animals. DNA primers, previously used to amplify the HVMNE, were used to amplify viral sequences from tissue DNA.41 The identity of the 236-bp PCR product was demonstrated by hybridization with the specific probe HVMNE p536.38 As demonstrated in Figure3 and Table2, such analysis revealed HVMNE DNA in several tissues from rabbits C2174, C2178, and C2182. Controls for the amplification competence of each DNA were performed using primers for the cytochrome B gene (data not shown). DNA sequences of amplicons from rabbits C2174 and C2180 confirmed identity with HVMNE (data not shown).
Detection of HVMNE DNA by PCR and Southern blot hybridization using p536, HVMNE-specific probe, in the tissues of 3 lymphomatous rabbits.
C2182 (lung, spleen, lymphoid tissues); C2174 (cultured PBMCs with and without IL-2, heart, thymus); C2178 (heart, lymphoid tissues, subcutaneous mass, spleen, thigh mass, PBMCs, and uterine mass). In rabbit C2174, PBMCs cultured with and without IL-2 were also analyzed. Each DNA was also assessed for amplification competence using DNA primers for cytochrome B (data not shown).
Detection of HVMNE DNA by PCR and Southern blot hybridization using p536, HVMNE-specific probe, in the tissues of 3 lymphomatous rabbits.
C2182 (lung, spleen, lymphoid tissues); C2174 (cultured PBMCs with and without IL-2, heart, thymus); C2178 (heart, lymphoid tissues, subcutaneous mass, spleen, thigh mass, PBMCs, and uterine mass). In rabbit C2174, PBMCs cultured with and without IL-2 were also analyzed. Each DNA was also assessed for amplification competence using DNA primers for cytochrome B (data not shown).
Detection of HVMNE DNA and RNA in the tissues of the lymphomatous rabbits
| Rabbit . | HVMNE DNA positivity by PCR . | EBER-like expression . |
|---|---|---|
| C2174 (irradiated cells) | LN, PBMC, heart, thymus | ND |
| C2175 | Retro-orbital mass, lung | Liver, spleen, heart, lymphoid tissues, eye |
| C2176 | LN, spleen | Lung |
| C2178 | Heart, uterine mass, lymphoid tissues, subcutaneous mass, spleen, thigh mass, PBMC | ND |
| C2180 | Kidneys, lung, heart, appendix, LN | Spleen, kidney, muscle |
| C2181 | Heart, spleen, liver, kidney | Spleen |
| C2182 | Lung, spleen, lymphoid tissues | ND |
| C2189 (cell-free pellet) | Heart, spleen | Lung |
| Rabbit . | HVMNE DNA positivity by PCR . | EBER-like expression . |
|---|---|---|
| C2174 (irradiated cells) | LN, PBMC, heart, thymus | ND |
| C2175 | Retro-orbital mass, lung | Liver, spleen, heart, lymphoid tissues, eye |
| C2176 | LN, spleen | Lung |
| C2178 | Heart, uterine mass, lymphoid tissues, subcutaneous mass, spleen, thigh mass, PBMC | ND |
| C2180 | Kidneys, lung, heart, appendix, LN | Spleen, kidney, muscle |
| C2181 | Heart, spleen, liver, kidney | Spleen |
| C2182 | Lung, spleen, lymphoid tissues | ND |
| C2189 (cell-free pellet) | Heart, spleen | Lung |
LN indicates lymph node; ND, not done.
To assess HVMNE expression, in situ hybridization was performed on several preserved rabbit tissues. Staining of tissues of 5 rabbits with the EBER probe was performed on at least 2 occasions using standard and longer hybridization times. In each tissue, oligo-dT staining was performed to assess the integrity of the RNA. As expected, not all cells within the lymphoid infiltrates expressed EBER-specific viral RNA (Table 2), and the intensity of the EBV EBER staining varied among cells of the same tissue, as shown in Figure4 in tissues from animals C2175 and C2180. Possibly the low-level expression found using EBER was related to a suboptimal sensitivity of this technical approach owing to the incomplete homology of the probe used because the DNA sequence encoding EBER from HVMNE is unknown. Thus, these results suggest an etiological role for HVMNE in lymphoma induction.
EBER expression.
Demonstration of EBER-like reactivity in formalin-fixed, paraffin embedded sections of malignant lymphomas by in situ hybridization using labeled oligonucleotide probe. Panels A and C show low- and high-power views, respectively, of positive nuclear staining in the ocular tumor of lymphomatous rabbit C2175, and panels B and D show low- (original magnification, ×40) and high-power (original magnification, ×400) views of the tumor infiltrates of the heart from rabbit C2180.
EBER expression.
Demonstration of EBER-like reactivity in formalin-fixed, paraffin embedded sections of malignant lymphomas by in situ hybridization using labeled oligonucleotide probe. Panels A and C show low- and high-power views, respectively, of positive nuclear staining in the ocular tumor of lymphomatous rabbit C2175, and panels B and D show low- (original magnification, ×40) and high-power (original magnification, ×400) views of the tumor infiltrates of the heart from rabbit C2180.
Retention of HVMNE DNA and constitutive activation of STAT-5 in T cells transformed in vivo
The J94356 T-cell line, originally obtained from a M nemestrina with mycosis fungoides, used to inoculate the rabbits in this study had a CD8+ T-cell phenotype.38Therefore, we investigated whether HVMNE infection could also transform rabbit T cells in vivo. PBMCs of animal C2174 and lymph-node cells derived from animal C2180 were cultured in the presence of IL-2. PBMCs and lymph node cells from both animals rapidly grew in culture and became IL-2 independent. Both cell lines expressed the CD25 activation marker (IL-2Rα chain) and expressed T-cell antigens, as summarized in Table 3. Changes in phenotypes and viral status were followed in the C2174PBMC cell lines over time. By month 6, only one fourth of the cell population expressed T-cell markers. By month 12, most cells became positive for the CD4 and the CD8 markers (data not shown). At month 20, most cells expressed both CD4 and CD8 markers (Table 3). Further confirmation of the T-cell origin of these cell lines was obtained using the anti-CD11a antibody, a specific antibody able to immunoprecipitate proteins from RL-5 T cells (rabbit T-cell line) that stained 90% of the cells, and by the lack of staining for IgM antibody, a specific B-cell marker (Figure 6D). To assess whether HVMNE DNA sequences were retained over time in these T-cell lines, DNA was obtained from the C2174PBMC rabbit T-cell line at 6- and 12-month culture and was analyzed for the presence of HVMNE sequence. At both time points, the specific 236-bp HVMNE DNA frequency was detected by PCR on the cell line DNA (Figure 5A).
Phenotypic markers of the 2 independent transformed rabbit T-cell lines
| Antibody3-150 . | Cell line (6 mo) C2180LN . | Cell line (6 mo) C2174PBMC . | Cell line (20 mo) C2174PBMC . | Normal rabbit PBMCs . |
|---|---|---|---|---|
| CD3 | 2.7 | 22.12 | 84 | 39.4 |
| CD4 | 42.2 | 18.1 | 79 | 57.4 |
| CD8 | 2.2 | 16.58 | 75 | 42 |
| CD25 | 47 | 95.4 | 94 | ND |
| CD11a | 34 | 19.5 | 90 | ND |
| CD11b | 0.17 | 7.8 | 0.1 | ND |
| CD5 | 23 | 5.75 | 0.3 | ND |
| IgM | 0.81 | 2.1 | 0 | ND |
| CD4/CD8 | ND | ND | 37.153-151 | ND |
| CD3/CD4 | ND | ND | 52.33 | ND |
| CD8/CD3 | ND | ND | 17.213-151 | ND |
| LMP 1 | ND | ND | 50 | ND |
| LMP 1/CD3 | ND | ND | 54.74 | ND |
| LMP 1/CD4 | ND | ND | 62.72 | ND |
| LMP 1/CD8 | ND | ND | 50.33 | ND |
| Antibody3-150 . | Cell line (6 mo) C2180LN . | Cell line (6 mo) C2174PBMC . | Cell line (20 mo) C2174PBMC . | Normal rabbit PBMCs . |
|---|---|---|---|---|
| CD3 | 2.7 | 22.12 | 84 | 39.4 |
| CD4 | 42.2 | 18.1 | 79 | 57.4 |
| CD8 | 2.2 | 16.58 | 75 | 42 |
| CD25 | 47 | 95.4 | 94 | ND |
| CD11a | 34 | 19.5 | 90 | ND |
| CD11b | 0.17 | 7.8 | 0.1 | ND |
| CD5 | 23 | 5.75 | 0.3 | ND |
| IgM | 0.81 | 2.1 | 0 | ND |
| CD4/CD8 | ND | ND | 37.153-151 | ND |
| CD3/CD4 | ND | ND | 52.33 | ND |
| CD8/CD3 | ND | ND | 17.213-151 | ND |
| LMP 1 | ND | ND | 50 | ND |
| LMP 1/CD3 | ND | ND | 54.74 | ND |
| LMP 1/CD4 | ND | ND | 62.72 | ND |
| LMP 1/CD8 | ND | ND | 50.33 | ND |
These antibodies recognize specifically only rabbit markers.
CD8 down-regulation occurred by double staining with permeabilized cells with αCD3 and αCD4 antibodies but not with LMP1 antibodies.
ND indicates not done.
Establishment of a primary cell-line from the PBMCs of rabbit C2174.
(A) Detection, by PCR and Southern blot hybridization using HVMNE (p536)-specific probe, of HVMNE DNA in the rabbit cell-line C2174 both at 6 and 12 months after culture (lane 1, C2174 after 6 months of culture; lane 2, PCR-negative control; lane 3, cell line J94356 as PCR-positive control; lane 4, C2174 after 12 months of culture). (B) Electrophoretic mobility shift assay to evaluate the Jak/STAT status of the rabbit T-cell line derived from primary culture of rabbit C2174 PBMCs. Transition to IL-2 independence of the C2174PBL cell line was associated with STAT-5 activation. On the top panel, the plus and minus signs represent the use of IL-2 pulse after overnight ligand starvation. αSTAT-5 indicates the addition to the lysate of specific αSTAT-5 antibodies in lanes 2, 4, 6, 8, and 10. Resting PBMCs (lanes 1-2) and the C2174PBL cell line cultured in the presence of IL-2 (lanes 3-6) and in the absence of IL-2 (lanes 7-10) after 8 months of culture.
Establishment of a primary cell-line from the PBMCs of rabbit C2174.
(A) Detection, by PCR and Southern blot hybridization using HVMNE (p536)-specific probe, of HVMNE DNA in the rabbit cell-line C2174 both at 6 and 12 months after culture (lane 1, C2174 after 6 months of culture; lane 2, PCR-negative control; lane 3, cell line J94356 as PCR-positive control; lane 4, C2174 after 12 months of culture). (B) Electrophoretic mobility shift assay to evaluate the Jak/STAT status of the rabbit T-cell line derived from primary culture of rabbit C2174 PBMCs. Transition to IL-2 independence of the C2174PBL cell line was associated with STAT-5 activation. On the top panel, the plus and minus signs represent the use of IL-2 pulse after overnight ligand starvation. αSTAT-5 indicates the addition to the lysate of specific αSTAT-5 antibodies in lanes 2, 4, 6, 8, and 10. Resting PBMCs (lanes 1-2) and the C2174PBL cell line cultured in the presence of IL-2 (lanes 3-6) and in the absence of IL-2 (lanes 7-10) after 8 months of culture.
Because the transformation of T or B cells by HTLV-I and EBV, respectively, is often associated with constitutive activation of the Jak/STAT pathways42-44 and because, in HVMNEtransformation of the CD8+ T cells of monkey J94356, growth factor independence was previously demonstrated to be associated with the constitutive activation of the Jak/STAT pathway,38 we investigated whether the constitutive activation of STAT-5 protein also correlated with the acquisition of IL-2 independence of the C2174 rabbit T-cell line. Normal uninfected rabbit PBMCs after PHA stimulation and PBMCs from rabbit C2174 were cultured in the absence or presence of IL-2, and cellular extracts were obtained. Constitutive binding of STAT-5 to the MGF probe was clearly evident in the cell extracts from the C2174 PBMCs at 8 months of culture (Figure 5B). Similar results were obtained using SIE probe specific for STAT-1 and STAT-3 proteins (data not shown). Thus, as in the case of the J94356 original cell lines,38 HVMNE infection and transformation of rabbit T cells also are associated with the constitutive activation of STAT-5.
HVMNE infection of T cells is associated with typical herpesvirus-induced morphologic changes and expression of LMP1-like protein
Herpes simplex virus (HSV), herpesvirus-6 (HHV-6), and human EBV infection often induce multinucleated giant cells. Analysis of cytospin preparations by Giemsa staining of the HVMNE-infected C2174PBMC T cells after 20 months in culture revealed the presence of multinucleated giant cells (Figure6A) containing up to 18 nuclei (Figure6B), demonstrating that, like other herpesviruses, HVMNEinduces similar morphologic changes. To assess whether an LMP1-like protein was expressed in the C2174PBMC T cells, an antibody able to recognize the human EBV LMP1 was used. As shown in Figure 6D, half the cell population expressed LMP1. To quantitate the percentage of LMP1-positive cells that also expressed other T-cell markers, initial gating was performed on the whole population of cells. Quadrant analysis was performed for the CD3, CD4, and CD8 markers in conjunction with LMP1. We were able to demonstrate that 54.74% of the cells expressed CD4 and LMP1 and that 50.33% expressed CD8 and LMP1 (Figure 6D). Furthermore, we visualized these cells using a Leica DMRA microscope at the NCI LRBGE Fluorescence Imaging Facility (Bethesda, MD) (program QFISH) (Figure 6C).
Morphologic and phenotypical characterization of the C2174PBL cells.
(A,B) C2174PBL cell line cytospins after 20 months of culture; note the presence of multinucleated giant cells (Giemsa staining). Original magnification of each, × 40. (C) An aliquot of the C2174PBL cultured PBMCs permeabilized and then stained with rabbit specific antibodies (CD3 FITC, CD8 FITC) and EBV-LMP1 antibody (LMP1 PE) was fixed in paraformaldehyde placed in mounting media and analyzed using a Leica DMRA microscope (using program QFISH). Original magnification, × 63. The upper portion of panel C shows a double-positive cell for LMP1 and CD3. The dent present on the upper right corner clearly shows evidence of an adjacent double-negative cell. The middle panel shows an LMP1-positive and a CD8−cell, whereas the lower panel shows a CD3+ and an LMP1-negative cell. (D) Histogram plots from the FACS analysis of the C2174 cultured PBMCs at 20 months of culture. Blue lines indicate specific isotypic control.
Morphologic and phenotypical characterization of the C2174PBL cells.
(A,B) C2174PBL cell line cytospins after 20 months of culture; note the presence of multinucleated giant cells (Giemsa staining). Original magnification of each, × 40. (C) An aliquot of the C2174PBL cultured PBMCs permeabilized and then stained with rabbit specific antibodies (CD3 FITC, CD8 FITC) and EBV-LMP1 antibody (LMP1 PE) was fixed in paraformaldehyde placed in mounting media and analyzed using a Leica DMRA microscope (using program QFISH). Original magnification, × 63. The upper portion of panel C shows a double-positive cell for LMP1 and CD3. The dent present on the upper right corner clearly shows evidence of an adjacent double-negative cell. The middle panel shows an LMP1-positive and a CD8−cell, whereas the lower panel shows a CD3+ and an LMP1-negative cell. (D) Histogram plots from the FACS analysis of the C2174 cultured PBMCs at 20 months of culture. Blue lines indicate specific isotypic control.
Discussion
Although humans are the only known natural hosts for EBV, EBV-like agents have been described in Old World nonhuman primates, including the chimpanzee, baboon, African green monkey, gorilla, and macaque species. A pre-existing, cross-reactive immunity for EBV is thought to prevent the development of B-cell lymphoma in Old World primates. In fact, the inoculation of HVMNE-infected J94356 monkey blood in other M nemestrina did not induce lymphoma (M.G.F. et al, unpublished results, January 1997). In studies of immunodeficiency, however, approximately one third of Macaca fascicularis (HVMF-1) infected with another EBV-like virus developed B-cell lymphomas.45 In New World monkeys, EBV can induce B-cell lymphomas. In addition, a high prevalence of a novel EBV-like gamma herpesvirus46 in a captive population of common marmosets was recently shown to be associated with lymphoma.
EBV-like viruses have also been described in M arctoides and cynomolgus monkeys (HVMA and cyno-EBV, respectively); both these viral strains cause B-cell lymphoma in rabbits.39 40Cyno-EBV also induces occasional tumors expressing T-cell markers. Thus, it appears that, contrary to what occurs with human EBV, several of the EBV-like viruses present in nonhuman primates may be oncogenic in rabbits. Genetic differences among EBV and EBV-like virus genes may help to further the understanding of species specificity of disease induction.
Eight of 14 exposed rabbits became infected with HVMNE, as judged by the presence of serum antibodies to VCA and by the presence of viral HVMNE DNA in blood and tissues. The remaining 6 exposed rabbits failed to produce antibodies to VCA, and DNA from circulating PBMCs was consistently negative for the HVMNEsequence. In addition, postmortem PCR analysis on DNA extracted from several tissues of these apparently uninfected animals failed to demonstrate an HVMNE DNA sequence. Nevertheless, the data presented here also indicate that once HVMNE infection is established, lymphoma occurs in all animals, thereby providing a model to study the impact of EBV-related genes in tumor development and in the transformation of T cells. Knowledge of the DNA sequence of the entire HVMNE will be instrumental in defining similarities with or differences from human EBV in genes involved in transformation. Of particular interest will be the study of the LMP1 equivalent of HVMNE given that the human EBV LMP1 protein affects several cellular pathways in B cells,12 including Jak/STAT signaling, by direct binding and activation of Jak3.47Because HVMNE infection is associated with the constitutive activation of the same pathway in T cells, likely the same mechanism may lead to STAT-5 activation induced by HVMNE. However, differences may exist in other genes of HVMNE because EBV does not cause lymphoma in rabbits.
We thank Drs Robert L. Harrod, Julie M. Johnson, and Christophe Nicot for critical reading of the manuscript and Steven Snodgrass for editorial assistance.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Genoveffa Franchini, Basic Research Laboratory, National Cancer Institute, 41/D804, Bethesda, MD 20892; e-mail:veffa@helix.nih.gov.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal