Abstract
Radioimmunotherapy with anti-CD20 monoclonal antibodies is a promising new treatment approach for patients with relapsed B-cell lymphomas. However, the majority of patients treated with conventional radiolabeled anti-CD20 antibodies eventually have a relapse because the low tumor-to-blood and tumor-to–normal organ ratios of absorbed radioactivity limit the dose that can be safely administered without hematopoietic stem cell support. This study assessed the ability of a streptavidin-biotin “pretargeting” approach to improve the biodistribution of radioactivity in mice bearing Ramos lymphoma xenografts. A pretargeted streptavidin-conjugated anti-CD20 1F5 antibody was infused, followed 24 hours later by a biotinylated N-acetylgalactosamine–containing “clearing agent” and finally 3 hours later by 111In-labeled DOTA-biotin. Tumor-to-blood ratios were 3:1 or more with pretargeting, compared with 0.5:1 or less with conventional 111In-1F5. Tumor-to–normal organ ratios of absorbed radioactivity up to 56:1 were observed with pretargeting, but were 6:1 or less with conventional 111In-1F5. Therapy experiments demonstrated that 400 μCi (14.8 MBq) or more of conventional 90Y-1F5 was required to obtain major tumor responses, but this dose was associated with lethal toxicity in 100% of mice. In marked contrast, up to 800 μCi (29.6 MBq)90Y-DOTA-biotin could be safely administered by the pretargeting approach with only minor toxicity, and 89% of the mice were cured. These data suggest that anti-CD20 pretargeting shows great promise for improving current therapeutic options for B-cell lymphomas and warrants further preclinical and clinical testing.
Introduction
Non-Hodgkin lymphomas afflict 58 000 Americans each year and are rapidly increasing in incidence.1 Only one third of patients with B-cell lymphoma are cured with conventional chemotherapy and radiotherapy; therefore, innovative new treatments are a high priority for this malignancy. Monoclonal antibodies (mAbs) directed against tumor-associated antigens have emerged as effective new reagents for lymphomas and are being tested extensively in the laboratory and in clinical trials. Although many B-cell surface antigens have been targeted with antibodies, to date anti-CD20 antibodies have been the most widely tested and have achieved the best clinical results.2-5 CD20 is a 35 000-kd nonglycosylated phosphoprotein expressed on the surface of nearly all mature B-lymphoid cells and on 95% of B-cell lymphomas.6 The CD20 antigen appears to have many favorable attributes that commend its use as an immunotherapeutic target. CD20 is not shed into the bloodstream, is not rapidly internalized, and is expressed at a high surface density on the vast majority of lymphomas.6-8 Rituximab, a chimeric anti-CD20 antibody, induces remissions in 50% to 70% of patients with newly diagnosed follicular lymphomas, 48% to 60% of patients with relapsed follicular lymphomas, 30% to 35% of those with relapsed diffuse large B-cell lymphomas, 30% to 35% of patients with relapsed mantle cell lymphomas, and 12% of those with relapsed small lymphocytic lymphomas.2,4,5 Unfortunately, only 6% to 20% of patients achieve complete remissions (CRs) and no convincing evidence has yet shown that anti-CD20 antibodies alone are curative. To enhance CR rates and remission durations many investigators have conjugated 131I or 90Y to anti-CD20 antibodies.9-14 Radioimmunotherapy (RIT) response rates have been substantially higher than those obtained with “naked” antibodies, with 96% of patients with newly diagnosed disease and 65% to 80% of patients with relapsed B-cell lymphomas responding.9-13 A recent randomized study documented superior overall and complete response (CR) rates for patients treated with a 90Y-anti-CD20 antibody (overall 80%, CR 30%) compared with patients treated with a corresponding unconjugated chimeric anti-CD20 antibody (overall 56%, CR 16%).14 Despite these promising results, most patients treated with nonmyeloablative doses of radiolabeled anti-CD20 antibodies eventually have a relapse. Our group has escalated doses of RIT to myeloablative levels and relied on stem cell transplantation to reconstitute normal hematopoiesis. With this aggressive approach, 85% to 90% of patients with relapsed lymphoma achieved objective remissions, including 75% to 80% with CRs.15-18 Some of these patients have remained in continuous CR for up to 12 years, suggesting that some may be permanently cured.17 Although this myeloablative approach appears to markedly enhance therapeutic efficacy, the attendant toxicity is substantial, hospitalization time is prolonged, and the cost of transplantation is significant. We therefore are testing methods that might achieve the excellent outcomes of high-dose RIT without the toxicities and expense.
To increase the dose of RIT delivered to tumor cells while preserving hematopoietic function, it is necessary to improve the specificity of targeting. Conventional RIT approaches rely on intravenous injection of antibodies directly labeled with radionuclides. The exquisite specificity of the radioimmunoconjugate for its antigen is compromised by the exposure of nonantigen-bearing tissues to radiation during the long period (24-48 hours) required for the radioimmunoconjugate to circulate through the body, accumulate at tumor sites, and penetrate to the center of tumor masses. In theory, strategies that dissociate the antibody distribution phase from the delivery of radiation should improve the tumor-to–normal organ ratios of absorbed radioactivity by markedly diminishing the exposure of normal tissues to radioactivity, thereby enhancing the therapeutic index.19-25 Several pilot clinical trials have validated the rationale of pretargeting and underscored its promise in relatively radioresistant solid tumor models.26-29 We hypothesized that CD20-expressing lymphomas should be an ideal setting for this approach, in view of their slow rate of CD20 internalization, the radiosensitivity of B-cell lymphomas, and the impressive success of chimeric and directly radiolabeled anti-CD20 antibodies.
Several strategies for pretargeting have been described.19-25 We elected to investigate a pretargeting approach using streptavidin-conjugated anti-CD20 antibodies, followed 24 to 48 hours later by administration of a “clearing agent” (CA) to remove unbound antibody from the bloodstream, and then followed 1 to 3 hours later with radiolabeled biotin. This report describes a model system in which we demonstrate superior biodistributions of a pretargeted anti-CD20 antibody compared with a conventional directly labeled anti-CD20 antibody as well as reduced toxicity and markedly enhanced therapeutic efficacy with the pretargeting method.
Materials and methods
Cell lines
The human Ramos B lymphoma cell line (American Type Culture Collection, Bethesda, MD) was maintained in log-phase growth in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum in a 5% CO2 incubator.
Antibodies
The murine anti–human CD20 IgG2a mAb 1F5 was produced in a hollow fiber bioreactor system in the Fred Hutchinson Cancer Research Center Monoclonal Antibody Production Facility (Seattle, WA). The 1F5 hybridoma was a gift from Dr Clay Siegall (Seattle Genetics, Seattle, WA). The isotype-matched NR-LU-10 and G3G6 murine mAbs were used as controls. The G3G6 antibody recognizes an idiotypic immunoglobulin on a single patient's B-cell lymphoma, but does not bind to Ramos cells or to other B-cell lymphomas. The G3G6 hybridoma was a gift from Dr Dana Matthews (Fred Hutchinson Cancer Research Center). NR-LU-10 antibody recognizes a 40-kd epithelial antigen known as Ep-CAM, which is expressed on many carcinoma cells but not on lymphoma cells,30 and was a gift from NeoRx (Seattle, WA).
Preparation of 1F5-streptavidin conjugates
Conjugates were prepared according to the method of Hylarides et al.31 The disulfides of 1F5 were reduced to thiols by addition of sufficient dithiothreitol (DTT; Aldrich, Milwaukee, WI) to a solution of 1F5 to bring the DTT concentration to 20 mM. Recombinant streptavidin (SA; Boehringer Mannheim, Mannheim, Germany) was functionalized by addition of a 3-fold molar excess of succinimidyl 4-(N-maleimidomethyl) cyclohexane-1-carboxylate (SMCC; Pierce Chemical, Rockford, IL) in dimethylsulfoxide to an SA solution, at pH 8.0. The SA-mal and reduced 1F5 solutions were then each isolated by elution through a sanitized (5 column volumes each of 1 M AcOH and 1 M NaOH) medium or coarse Sephadex G25 column (Pharmacia, Peapack, NJ) in phosphate-buffered saline containing 1 mM diethylenetriaminepentaacetic acid (DTPA, Aldrich). Concentrations were determined spectrophotometrically using optical density (OD) 280 extinctions of 1.4 and 3.4 for 1 mg/mL solutions of 1F5 and SA, respectively. Maleimides on SA-mal were assayed by treating an aliquot of SA-mal with excess cysteine then detecting residual cysteine with 5,5′-dithiobis[2-nitrobenzoic acid] (DTNB; Eastman Chemical, Kingsport, TN). Thiols on reduced 1F5 were assayed by treating an aliquot of reduced 1F5 with DTNB. The concentration of DTNB (reflecting the concentration of thiol) was determined spectrophotometrically at pH 8.0 using a molar extinction coefficient of 1.36 × 104at 412 nm. Suitable molar ratios were 1.4 to 1.8 mal/SA and 8.5 to 11 thiols/1F5 molecule.
Equimolar quantities of SA-mal and reduced 1F5 were combined for conjugation and agitated gently. The reaction was monitored by size exclusion chromatography (SEC) using either a TSK column (Tosohaas USA, Montgomeryville, PA) or S-300 column (Waters, Milford, MA). Optimal reaction times were typically 35 to 45 minutes. The reaction was stopped by adding to the conjugation reaction sufficient solid sodium tetrathionate to make the solution 1 mM. The conjugation mixture was prepared for loading by adding solid glycine and NaCl to make the solution 50 mM and 500 mM, respectively, then adjusting the pH to 9.0. The conjugation mixture was loaded onto an iminobiotin column containing 500 mM NaCl at pH 9.0, then washed with iminobiotin loading buffer until the OD 280 returned to baseline. The flow-through was assayed by SEC to ensure that all conjugate was retained on the column. The iminobiotin column was eluted with 0.2 M NaOAc, pH 4.0.
Purification of 1F5-SA conjugate
The postiminobiotin conjugation mixture was purified by cation exchange chromatography using a Fractogel EMD SO3 650 (S) column (EM Separations Technology, Gibbstown, NJ). The column was equilibrated in 20 mM sodium phosphate, pH 6.5. The conjugation mixture was prepared for loading by dilution by buffer exchange to a conductivity to less than 2.5 mS/cm and a pH of 6.5. The conjugation solution was loaded on the column, washed with equilibration buffer, and the desired 1:1 and 1:2 conjugates eluted with a step gradient of 20 mM sodium phosphate, 90 mM NaCl, pH 6.5, and collected as a single fraction. High-molecular-weight byproducts were eluted with 20 mM sodium phosphate, 200 mM NaCl, pH 6.5. All fractions were assayed by SEC. The concentration of the desired conjugate was determined spectrophotometrically at 280 nm using an extinction of 2.0 for a 1-mg/mL solution. Maximal yields were approximately 35% (mg protein product/mg protein starting materials). The biotin-binding capacity of the conjugate was determined by displacement of 2-(4′-hydroxyphenylazo)-benzoic acid (HABA; Aldrich) from SA by biotin as previously described.32
Radioiodination of mAbs and mAb-SA conjugates
The mAbs and mAb-SA conjugates were radiolabeled with125I or 131I (NEN Life Science Products, Boston, MA) by the chloramine T method as previously published.33
111In90Y-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid radiolabeling
The mAbs were radiolabeled with 111In or90Y (NEN Life Science Products) usingP-isothiocyanatobenzyl-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (p-SCN-Bz-DOTA; Macrocyclics, Richardson, TX) by the method of Mirzadeh and coworkers.34 Buffer solutions were prepared using “low metal” reagents and storage vials and the mAbs were demetallated using Chelex 100 resin (Bio-Rad Laboratories, Hercules, CA). The specific activity was typically 1.8 μCi/μg (0.066 MBq/μg).
Biotin-galactose CA
A proprietary CA from Neorx (Seattle, WA) was used to remove excess unbound mAb-SA conjugates from the bloodstream prior to radiobiotin administration. The synthesis and characterization of this reagent has been published separately.35,36 Each polymeric molecule contains 16 N-acetyl-galactosamine residues and one biotin moiety appended to a dendrimeric backbone. The biotin moiety binds avidly to SA-mAb conjugates. The N-acetyl-galactosamine residues have a high affinity for hepatic asialoglycoprotein receptors, which mediate the rapid hepatic clearance of residual 1F5-SA conjugates from the bloodstream and their endocytosis into liver cells.25
111ln-DOTA-biotin
The bifunctional ligand DOTA-biotin was synthesized as described.37 Carrier-free 111In or90YCl3, 0.02 to 0.5 mL in 0.04 M HCl, was diluted with 0.5 mL 2 M ammonium acetate pH 5. DOTA-biotin, 0.1 to 1 mg, was added, and the solution was heated for 30 minutes at 80°C. DTPA was added to chelate any unbound 111In. Radiochemical purity was more than 99% by C18 reverse-phase gradient high-performance liquid chromatography (HPLC; A:5 mM aqueous DTPA, B: 50% acetonitrile in A) with flow-through gamma detection. Labeling efficiency was typically 93%.
90Y-DOTA-biotin
Carrier-free 90Y-Cl3, 0.02 to 0.2 mL in 0.05 M HCL, was diluted with 2 M ammonium acetate, pH 5, to a total volume of 0.4 mL. Ascorbic acid, 0.05 mL of a 0.5-g/mL solution and 0.1 mL of 10 mg/mL DOTA-biotin, was added, and the solution was heated at 80°C for 1 hour. DTPA, 0.05 mL of a 0.1-M solution, was added to chelate any unbound radiometal.
Lymphoma xenograft model in immunodeficient mice
Six- to 10-week-old female BALB/c nude mice (Simonsen Laboratories, Gilroy, CA or B & K Universal, Kent, WA) were injected with 20 × 106 Ramos cells subcutaneously in each flank. In biodistribution experiments, mice were preirradiated with either 3 or 6 Gy gamma irradiation to facilitate engraftment of Ramos cells. Mice were monitored until palpable tumor nodules appeared (7-10 days) and mice with similar tumor sizes (∼5 mm diameter) were selected for experimentation. Tumor-bearing mice were placed on a biotin-free diet (Harlan Teklad, Madison, WI) for 4 to 7 days prior to injection of mAb-SA conjugates and radiobiotin. Groups of 4 to 6 mice each were injected either in the tail vein or intraperitoneally with 4 to 400 μg mAbs or mAb-SA conjugates. In most experiments, mice were coinjected with 400 μg of a nonspecific IgG2a irrelevant antibody (G3G6) to block nonspecific binding of the 1F5 and NR-LU-10 IgG2a antibodies to Fc receptors in the spleen, marrow, and liver. Animals in pretargeted groups were injected intraperitoneally or intravenously with 50 μg (5.8 nmol) of CA 24, 48, or 72 hours later.111In or 90Y biotin was injected 0.5, 1, 2, or 3 hours after CA and mice were killed 2, 24, 48, 72, 96, or 120 hours after injection of radiobiotin or directly labeled mAbs. At each time point, blood samples were obtained from the retro-orbital venous plexus and tumors and normal organs (lungs, stomach, small intestine, colon, spleen, bone marrow, quadriceps muscle, kidneys, and liver) were excised, weighed, and gamma counted for 131I,125I, 111In, or 90Y. In double-label experiments, counts were corrected for 131I crossover into the 125I channel, or 111In crossover into the 125I channel. Counts were also corrected for radioactive decay using an aliquot of the injectate. The percent injected dose of radionuclide per gram (% ID/g) of tumor or organ was calculated as well as the tumor-to–normal organ ratios of absorbed radioactivity. The mean values and SEs for each time point were plotted to generate biodistribution curves for each tissue. Control groups were injected with radiolabeled isotype-matched, nonbinding control mAb NR-LU-10 or NR-LU-10-SA conjugates. Animal studies were conducted under the supervision of veterinarians from the University of Washington Comparative Medicine Department.
RIT of lymphoma xenografts
To compare the therapeutic efficacy of pretargeted and conventional radiolabeled antibodies, groups of 8 to 10 tumor-bearing mice were placed on biotin-free chow for 9 to 10 days and injected with 1.4 nmol (215 μg, 200-400 μCi [7.4-14.8 MBq]) of directly labeled 90Y-DOTA-1F5,90Y-DOTA-NR-LU-10, or equimolar amounts (300 μg) of 1F5-SA or NR-LU-10-SA conjugates followed 24 hours later by 5.8 nmol (50 μg) CA and 3 hours later by 1.2 nmol (1 μg)90Y-DOTA-biotin labeled with 400 or 800 μCi (14.8-29.6 MBq) 90Y. Mice for therapy experiments were not preirradiated prior to implantation of Ramos tumors to avoid confounding myelosuppressive effects of external beam preirradiation. Mice were monitored every other day for general appearance, tumor volume measurements, and body weight. Mice were euthanized if tumors grew large enough to cause obvious discomfort or impair ambulation.
Results
Reagent synthesis
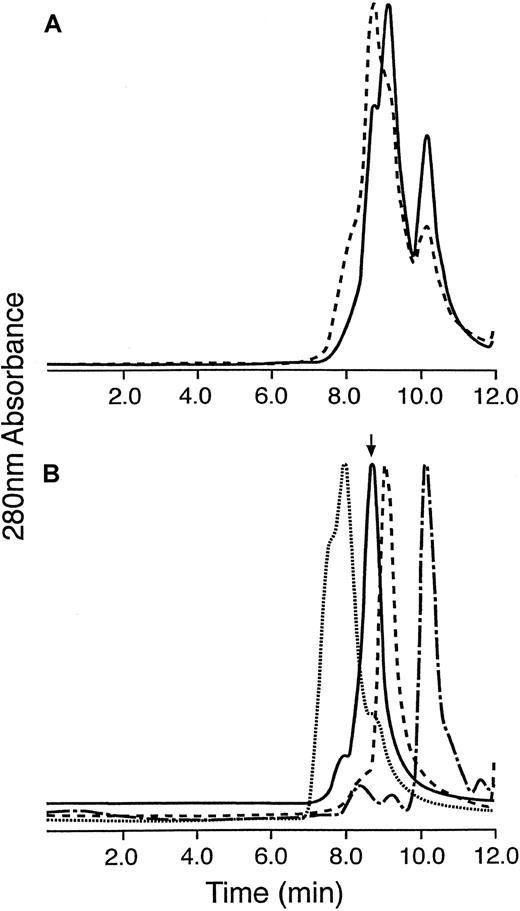
Four batches of 1F5-SA were prepared using the heterobifunctional SMCC cross-linker as previously described.24,31 Yields of 28% to 37% and purities of 95% or higher were obtained after purification with iminobiotin and cation exchange chromatography (Figure 1). The final 1F5-SA conjugate contained 80% to 85% 1:1 1F5:SA conjugates, 5% to 10% 1:2 1F5:SA conjugates, and 6% to 10% molecules of higher molecular weight as assessed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and HPLC. The biotin-binding capacity of the conjugate was calculated to be 5 moles of biotin per mole of conjugate mixture as determined by HABA displacement from SA by biotin.32 This is slightly higher than the expected capacity of 4:1 reflecting the presence of conjugates containing 2 or more SA molecules per mAb moiety.
Synthesis and purification of the 1F5-SA anti-CD20 immunoconjugate.
(A) Reaction progress. Size exclusion chromatograms of the conjugation reaction of reduced 1F5 with SMCC-functionalized streptavidin at an early time point (∼5 minutes; solid trace) and a late time point (∼45 minutes; dashed trace). Components present are: 8.0 minute, high-molecular-weight products; 8.7 minutes, desired 1:1 and 1:2 1F5-SA conjugate; 9.0 minute, unreacted 1F5; 10.2 minutes, unreacted streptavidin. (B) Composite purification profile of the 1F5-SA anti-CD20 immunoconjugate monitored by sequential HPLC analysis. Size exclusion chromatograms of the conjugation reaction components isolated during the purification process. Dotted trace, high-molecular-weight byproducts eluted with high salt from the cation exchange column; solid trace, purified 1:1 and 1:2 1F5-SA conjugate eluted from the cation exchange column with 90 mM NaCl; dashed trace, unreacted 1F5 from the “flow-through” of the iminobiotin affinity column; dot-dash trace, residual SA from the “flow-through” of the cation exchange column. The figure illustrates the separation of each nondesired component from the desired conjugate (solid trace), and the amount of desired conjugate lost with each undesired component. The only significant unrecoverable loss of product on purification is in the high-molecular-weight byproduct peak (dotted trace).
Synthesis and purification of the 1F5-SA anti-CD20 immunoconjugate.
(A) Reaction progress. Size exclusion chromatograms of the conjugation reaction of reduced 1F5 with SMCC-functionalized streptavidin at an early time point (∼5 minutes; solid trace) and a late time point (∼45 minutes; dashed trace). Components present are: 8.0 minute, high-molecular-weight products; 8.7 minutes, desired 1:1 and 1:2 1F5-SA conjugate; 9.0 minute, unreacted 1F5; 10.2 minutes, unreacted streptavidin. (B) Composite purification profile of the 1F5-SA anti-CD20 immunoconjugate monitored by sequential HPLC analysis. Size exclusion chromatograms of the conjugation reaction components isolated during the purification process. Dotted trace, high-molecular-weight byproducts eluted with high salt from the cation exchange column; solid trace, purified 1:1 and 1:2 1F5-SA conjugate eluted from the cation exchange column with 90 mM NaCl; dashed trace, unreacted 1F5 from the “flow-through” of the iminobiotin affinity column; dot-dash trace, residual SA from the “flow-through” of the cation exchange column. The figure illustrates the separation of each nondesired component from the desired conjugate (solid trace), and the amount of desired conjugate lost with each undesired component. The only significant unrecoverable loss of product on purification is in the high-molecular-weight byproduct peak (dotted trace).
Optimizing reagent doses and dosing intervals
Initial experiments focused on defining the optimal doses of each component of the pretargeting regimen and determining the optimal time intervals for administration of each reagent. Pilot studies demonstrated significant nonspecific uptake of IgG2a antibodies (1F5, NR-LU-10, anti-B1, G3G6) but not of IgG1 mAbs (eg, the BC8 anti–human CD45 mAb) in reticuloendothelial organs, especially the spleen and marrow, of both tumor-bearing and non–tumor-bearing mice. Nonspecific retention of IgG2a antibodies in spleen and marrow resulted from binding to Fc receptors and could be blocked by preinjection (or coinjection) of a nonlabeled IgG2a antibody. Blocking was maximal with coinjection of 400 μg of the G3G6 antibody (data not shown) and this dose was used in subsequent experiments. Optimal biodistributions of 1F5-SA were achieved after injections of 300 μg (1.4 nmol) of the conjugate, 5.8 nmol (50 μg) CA administered 24 hours after the 1F5-SA conjugate, and 1.2 nmol (1 μg) radiobiotin administered 0.5 to 3 hours after the CA (data not shown).
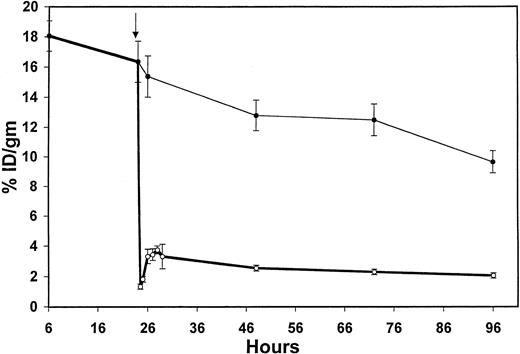
Effects of a biotinylated polymeric, N-acetylgalactosamine–containing CA on circulating 1F5-SA conjugate
A synthetic CA designed to eliminate biotin-binding molecules from the circulation via hepatic clearance35 36 reproducibly depleted 80% to 95% of circulating 1F5-SA from the bloodstream within 30 minutes of injection (Figure 2). In a representative experiment, the blood concentration of125I-1F5-SA dropped precipitously after injection of 5.8 nmol CA from 16.4% ± 1.4% of the injected radioactivity per gram of blood to 1.3% ± 0.2% 30 minutes later (Figure 2). A slight rebound rise of 125I-1F5-SA conjugate to 3.4% ± 0.4% ID/g was observed in the blood over the next 3 hours, presumably due to re-equilibration with conjugate in the extravascular compartment (Figure 2). The CA decreased the blood concentration by 92% within 30 minutes of injection and decreased the blood area under the curve by 61% when administered 24 hours after the 1F5-SA conjugate. Injection of radiobiotin 30 minutes to 3 hours after the CA yielded optimal tumor uptake of radiobiotin (data not shown).
Effects of a biotinylated polymeric, N-acetyl-galactosamine–containing CA on circulating 1F5-SA conjugate.
Six BALB/c mice were injected intraperitoneally with 1.4 nmol of the125I-1F5 (anti-CD20)–streptavidin (SA) conjugate at time 0. Three mice were injected intraperitoneally 24 hours later with 5.8 nmol CA (bold line), whereas the other 3 mice did not receive CA (thin line). Serial blood samples were obtained from the retro-orbital venous plexus 6, 24, 24.5, 25, 26, 27, 28, 29, 48, 72, and 96 hours after the injection of 125I-1F5-SA and were analyzed by gamma counting.
Effects of a biotinylated polymeric, N-acetyl-galactosamine–containing CA on circulating 1F5-SA conjugate.
Six BALB/c mice were injected intraperitoneally with 1.4 nmol of the125I-1F5 (anti-CD20)–streptavidin (SA) conjugate at time 0. Three mice were injected intraperitoneally 24 hours later with 5.8 nmol CA (bold line), whereas the other 3 mice did not receive CA (thin line). Serial blood samples were obtained from the retro-orbital venous plexus 6, 24, 24.5, 25, 26, 27, 28, 29, 48, 72, and 96 hours after the injection of 125I-1F5-SA and were analyzed by gamma counting.
Comparative biodistributions of radioactivity after conventional 1-step versus 2-step pretargeted RIT
After defining the optimal doses and time intervals for pretargeted RIT in this model system, comparative biodistribution studies were undertaken. Twelve groups of 5 mice each were injected with 1.4 nmol of either conventional 125I-1F5 (6 groups) or pretargeted 125I-1F5-SA followed 24 hours later by 5.8 nmol CA and 3 hours after that by 1.2 nmol 111In-DOTA-biotin (6 groups). Groups of mice were killed at 6 different time points from 2 to 144 hours after injection of the radiolabeled species as indicated in Figure 3. The pretargeting approach resulted in far superior biodistributions of radioactivity compared with conventional RIT. In mice treated with the pretargeting strategy, the radioactivity in the xenograft reached a maximum of 13.5% ± 3.6% ID/g 12 hours after the111In-DOTA-biotin, decreasing gradually to 2.1% ± 1.0% ID/g 144 hours later. At all time points, the tumor-to-blood ratio exceeded 3:1 using the pretargeting approach, varying from 3.5:1 at 12 hours to 25:1 at 144 hours. Ratios with other normal tissues were even better (Table 1). In contrast, mice treated with conventional RIT accrued a maximum of 7.2% ± 1.3% ID/g in tumors at 12 hours decreasing to 1.5% ± 0.3% ID/g at 144 hours. Tumor-to-blood ratios varied from 0.5:1 at 48 hours to a low of 0.2:1 at 144 hours using conventional RIT. Two other experiments yielded concordant results (not shown).
Biodistributions of radioactivity in blood, tumor, and normal organs of Ramos xenograft-bearing athymic mice injected with either directly labeled 1F5 antibody or pretargeted 1F5-SA conjugate.
Mice were injected either with 1.4 nmol conventional trace-labeled125I-1F5 (A) or 1.4 nmol 1251F5-SA (B) followed 24 hours later by 5.8 nmol CA and 3 hours after that by 1.2 nmol111In-DOTA-biotin (B). Groups of 5 mice were euthanized 2, 12, 24, 48, 96, and 144 hours after injection of the radiolabeled species and the radioactivity in blood, tumor, and normal organs was quantified by gamma counting and expressed as the percent of the injected dose of radioactivity present per gram of tissue. Tumor (●, heavy solid line), blood (○, dashed line), kidney (▪), liver (▴), lung (×), muscle (*), small intestine (♦).
Biodistributions of radioactivity in blood, tumor, and normal organs of Ramos xenograft-bearing athymic mice injected with either directly labeled 1F5 antibody or pretargeted 1F5-SA conjugate.
Mice were injected either with 1.4 nmol conventional trace-labeled125I-1F5 (A) or 1.4 nmol 1251F5-SA (B) followed 24 hours later by 5.8 nmol CA and 3 hours after that by 1.2 nmol111In-DOTA-biotin (B). Groups of 5 mice were euthanized 2, 12, 24, 48, 96, and 144 hours after injection of the radiolabeled species and the radioactivity in blood, tumor, and normal organs was quantified by gamma counting and expressed as the percent of the injected dose of radioactivity present per gram of tissue. Tumor (●, heavy solid line), blood (○, dashed line), kidney (▪), liver (▴), lung (×), muscle (*), small intestine (♦).
Tumor-to–normal organ ratios of absorbed radiation in Ramos xenograft-bearing mice treated with standard radiolabeled anti-CD20 antibodies or pretargeted radiolabeled biotin
| Tissue . | Time . | |||||
|---|---|---|---|---|---|---|
| 2 h . | 12 h . | 24 h . | 48 h . | 96 h . | 144 h . | |
| Blood | ||||||
| PT | 3.5 ± 0.3 | 4.1 ± 0.9 | 5.7 ± 1.3 | 8.1 ± 1.7 | 15.3 ± 2.9 | 31.9 ± 8.0 |
| Std | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.2 | 0.2 ± 0.1 |
| Kidney | ||||||
| PT | 5.7 ± 0.7 | 7.9 ± 2.5 | 7.8 ± 1.1 | 8.0 ± 4.2 | 5.5 ± 1.1 | 3.4 ± 1.0 |
| Std | 0.9 ± 0.3 | 1.5 ± 0.2 | 1.7 ± 0.3 | 2.4 ± 0.4 | 1.6 ± 0.6 | 0.7 ± 0.2 |
| Liver | ||||||
| PT | 8.8 ± 1.0 | 9.5 ± 2.4 | 12.5 ± 2.3 | 14.5 ± 6.6 | 9.8 ± 2.1 | 11.4 ± 3.8 |
| Std | 1.2 ± 0.4 | 1.7 ± 0.3 | 2.3 ± 0.6 | 3.5 ± 0.6 | 2.1 ± 0.8 | 1.0 ± 0.3 |
| Lung | ||||||
| PT | 7.0 ± 0.8 | 9.3 ± 2.5 | 12.0 ± 2.6 | 16.7 ± 6.2 | 14.3 ± 2.6 | 12.9 ± 4.8 |
| Std | 0.9 ± 0.3 | 1.0 ± 0.1 | 1.1 ± 0.8 | 1.4 ± 0.3 | 1.0 ± 0.4 | 0.4 ± 0.7 |
| Muscle | ||||||
| PT | 7.7 ± 1.6 | 23.1 ± 4.6 | 32.2 ± 7.0 | 49.4 ± 16.8 | 25.3 ± 4.8 | 21.6 ± 8.4 |
| Std | 0.7 ± 0.4 | 2.1 ± 0.4 | 4.8 ± 0.8 | 6.1 ± 1.1 | 5.9 ± 2.4 | 2.4 ± 0.7 |
| Intestine | ||||||
| PT | 22.5 ± 1.3 | 39.2 ± 14.7 | 38.2 ± 11.4 | 56.0 ± 32.8 | 33.2 ± 5.7 | 24.7 ± 7.4 |
| Std | 1.4 ± 0.5 | 3.0 ± 0.5 | 3.1 ± 0.8 | 4.3 ± 0.7 | 3.6 ± 1.4 | 1.6 ± 0.4 |
| Colon | ||||||
| PT | 22.0 ± 2.6 | 26.7 ± 9.8 | 43.1 ± 10.2 | 45.8 ± 22.4 | 28.9 ± 5.4 | 18.0 ± 4.7 |
| Std | 1.3 ± 0.4 | 2.7 ± 0.5 | 1.3 ± 0.6 | 4.7 ± 1.1 | 3.8 ± 1.6 | 1.4 ± 0.4 |
| Spleen | ||||||
| PT | 12.5 ± 1.6 | 13.9 ± 4.2 | 16.7 ± 3.2 | 15.8 ± 6.0 | 9.2 ± 1.6 | 6.1 ± 1.7 |
| Std | 0.6 ± 0.1 | 1.1 ± 0.2 | 1.3 ± 0.3 | 1.9 ± 0.3 | 1.5 ± 0.5 | 0.8 ± 0.2 |
| Stomach | ||||||
| PT | 21.2 ± 2.0 | 35.5 ± 12.2 | 37.6 ± 6.4 | 38.2 ± 10.0 | 26.8 ± 4.7 | 24.0 ± 6.5 |
| Std | 1.7 ± 0.6 | 3.0 ± 0.5 | 2.8 ± 0.3 | 3.6 ± 0.7 | 3.5 ± 1.2 | 1.3 ± 0.3 |
| Marrow | ||||||
| PT | 8.0 ± 0.7 | 14.2 ± 3.6 | 12.3 ± 2.1 | 14.9 ± 5.2 | 9.0 ± 2.2 | 6.6 ± 1.6 |
| Std | 0.6 ± 0.1 | 1.4 ± 0.4 | 1.3 ± 0.2 | 2.2 ± 0.4 | 1.4 ± 0.5 | 0.6 ± 0.2 |
| Tissue . | Time . | |||||
|---|---|---|---|---|---|---|
| 2 h . | 12 h . | 24 h . | 48 h . | 96 h . | 144 h . | |
| Blood | ||||||
| PT | 3.5 ± 0.3 | 4.1 ± 0.9 | 5.7 ± 1.3 | 8.1 ± 1.7 | 15.3 ± 2.9 | 31.9 ± 8.0 |
| Std | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.2 | 0.2 ± 0.1 |
| Kidney | ||||||
| PT | 5.7 ± 0.7 | 7.9 ± 2.5 | 7.8 ± 1.1 | 8.0 ± 4.2 | 5.5 ± 1.1 | 3.4 ± 1.0 |
| Std | 0.9 ± 0.3 | 1.5 ± 0.2 | 1.7 ± 0.3 | 2.4 ± 0.4 | 1.6 ± 0.6 | 0.7 ± 0.2 |
| Liver | ||||||
| PT | 8.8 ± 1.0 | 9.5 ± 2.4 | 12.5 ± 2.3 | 14.5 ± 6.6 | 9.8 ± 2.1 | 11.4 ± 3.8 |
| Std | 1.2 ± 0.4 | 1.7 ± 0.3 | 2.3 ± 0.6 | 3.5 ± 0.6 | 2.1 ± 0.8 | 1.0 ± 0.3 |
| Lung | ||||||
| PT | 7.0 ± 0.8 | 9.3 ± 2.5 | 12.0 ± 2.6 | 16.7 ± 6.2 | 14.3 ± 2.6 | 12.9 ± 4.8 |
| Std | 0.9 ± 0.3 | 1.0 ± 0.1 | 1.1 ± 0.8 | 1.4 ± 0.3 | 1.0 ± 0.4 | 0.4 ± 0.7 |
| Muscle | ||||||
| PT | 7.7 ± 1.6 | 23.1 ± 4.6 | 32.2 ± 7.0 | 49.4 ± 16.8 | 25.3 ± 4.8 | 21.6 ± 8.4 |
| Std | 0.7 ± 0.4 | 2.1 ± 0.4 | 4.8 ± 0.8 | 6.1 ± 1.1 | 5.9 ± 2.4 | 2.4 ± 0.7 |
| Intestine | ||||||
| PT | 22.5 ± 1.3 | 39.2 ± 14.7 | 38.2 ± 11.4 | 56.0 ± 32.8 | 33.2 ± 5.7 | 24.7 ± 7.4 |
| Std | 1.4 ± 0.5 | 3.0 ± 0.5 | 3.1 ± 0.8 | 4.3 ± 0.7 | 3.6 ± 1.4 | 1.6 ± 0.4 |
| Colon | ||||||
| PT | 22.0 ± 2.6 | 26.7 ± 9.8 | 43.1 ± 10.2 | 45.8 ± 22.4 | 28.9 ± 5.4 | 18.0 ± 4.7 |
| Std | 1.3 ± 0.4 | 2.7 ± 0.5 | 1.3 ± 0.6 | 4.7 ± 1.1 | 3.8 ± 1.6 | 1.4 ± 0.4 |
| Spleen | ||||||
| PT | 12.5 ± 1.6 | 13.9 ± 4.2 | 16.7 ± 3.2 | 15.8 ± 6.0 | 9.2 ± 1.6 | 6.1 ± 1.7 |
| Std | 0.6 ± 0.1 | 1.1 ± 0.2 | 1.3 ± 0.3 | 1.9 ± 0.3 | 1.5 ± 0.5 | 0.8 ± 0.2 |
| Stomach | ||||||
| PT | 21.2 ± 2.0 | 35.5 ± 12.2 | 37.6 ± 6.4 | 38.2 ± 10.0 | 26.8 ± 4.7 | 24.0 ± 6.5 |
| Std | 1.7 ± 0.6 | 3.0 ± 0.5 | 2.8 ± 0.3 | 3.6 ± 0.7 | 3.5 ± 1.2 | 1.3 ± 0.3 |
| Marrow | ||||||
| PT | 8.0 ± 0.7 | 14.2 ± 3.6 | 12.3 ± 2.1 | 14.9 ± 5.2 | 9.0 ± 2.2 | 6.6 ± 1.6 |
| Std | 0.6 ± 0.1 | 1.4 ± 0.4 | 1.3 ± 0.2 | 2.2 ± 0.4 | 1.4 ± 0.5 | 0.6 ± 0.2 |
Std indicates standard radiolabeled anti-CD20 antibodies; PT, pretargeted radiolabeled biotin.
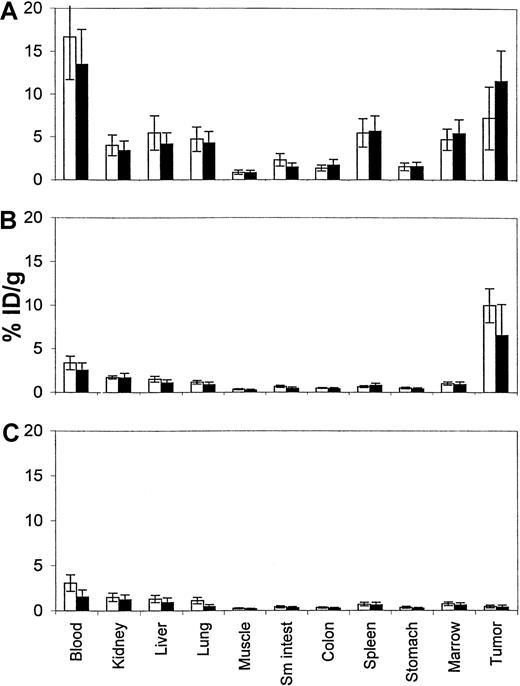
Although these results appear convincing, it is possible that some of the observed difference between the 2 systems resulted from a difference in retention of 111In-biotin and125I-mAb by tumor cells, because 111In is retained better than 125I in some model systems, particularly when internalizing antibodies are used.38 To address this criticism, we repeated these experiments using directly labeled 111In-1F5 mAb and compared it with111In-DOTA-biotin pretargeted using 1F5-SA followed 24 hours later by CA. Figure 4 displays results from 1 of 5 concordant experiments demonstrating significantly superior tumor-to-blood ratios and tumor-to–normal organ ratios for the pretargeting method as compared with conventional one-step111In-1F5 targeting. With pretargeting, the tumor content of 111In-DOTA-biotin was 10.0% ± 2.0% ID/g at 24 hours, whereas the blood content was only 3.4% ± 0.8% ID/g at 24 hours. In contrast, the tumor content of conventional111In-1F5 was 7.2% ± 3.7% at 24 hours with a blood content of 16.7% ± 5.0% at 24 hours. Tumor-to-blood ratios were 3:1 at 24 hours with pretargeting compared with 0.4:1 at 24 hours with conventional targeting. Control animals given NR-LU-10-SA followed by CA and 111In-DOTA-biotin had negligible tumor uptake of radiobiotin (0.42% ± 0.15% ID/g at 24 hours), demonstrating the specificity of targeting in these experiments (Figure 4C). These results also demonstrate that in this model system,111In-1F5 gives similar results to 125I-1F5, as would be expected for a noninternalizing antibody.38
Comparative biodistributions of radioactivity in the blood, tumors, and normal organs.
Ramos xenograft-bearing nude mice were injected with either directly labeled 111In-1F5 antibody (A), pretargeted 1F5-SA conjugate followed by CA and 111In-DOTA-biotin (B), or a pretargeted nonspecific control conjugate NR-LU-10-SA followed by CA and 111In-DOTA-biotin (C). Groups of 5 mice were euthanized at 24 (□) and 48 hours (■) after injection of the radiolabeled species and the radioactivity in blood, tumor, and normal organs was quantified by gamma counting and expressed as the percent of the injected dose of radioactivity present per gram of tissue.
Comparative biodistributions of radioactivity in the blood, tumors, and normal organs.
Ramos xenograft-bearing nude mice were injected with either directly labeled 111In-1F5 antibody (A), pretargeted 1F5-SA conjugate followed by CA and 111In-DOTA-biotin (B), or a pretargeted nonspecific control conjugate NR-LU-10-SA followed by CA and 111In-DOTA-biotin (C). Groups of 5 mice were euthanized at 24 (□) and 48 hours (■) after injection of the radiolabeled species and the radioactivity in blood, tumor, and normal organs was quantified by gamma counting and expressed as the percent of the injected dose of radioactivity present per gram of tissue.
Radioimmunotherapy with either conventional or pretargeted anti-CD20 antibodies
In view of the promising findings of the comparative biodistribution experiments described above, we performed pilot RIT experiments to assess whether the superior biodistributions obtained with the pretargeting method would translate to enhanced efficacy and diminished toxicity compared with conventional one-step RIT. Experimental groups of 8 to 10 lymphoma-bearing mice were injected with 1.4 nmol 1F5-SA followed 24 hours later by 5.8 nmol CA and 3 hours later by 1.2 nmol 90Y-DOTA-biotin labeled with 400 or 800 μCi/mouse. Comparison groups were injected with 1.4 nmol directly radiolabeled 90Y-DOTA-1F5 (200 or 400 μCi/mouse). Control groups were injected with 1.4 nmol of the nonbinding NR-LU-10-SA control conjugate plus 5.8 nmol CA plus 800 μCi90Y-DOTA-biotin, or 800 μCi 90Y-DOTA-biotin alone without a first-step conjugate.
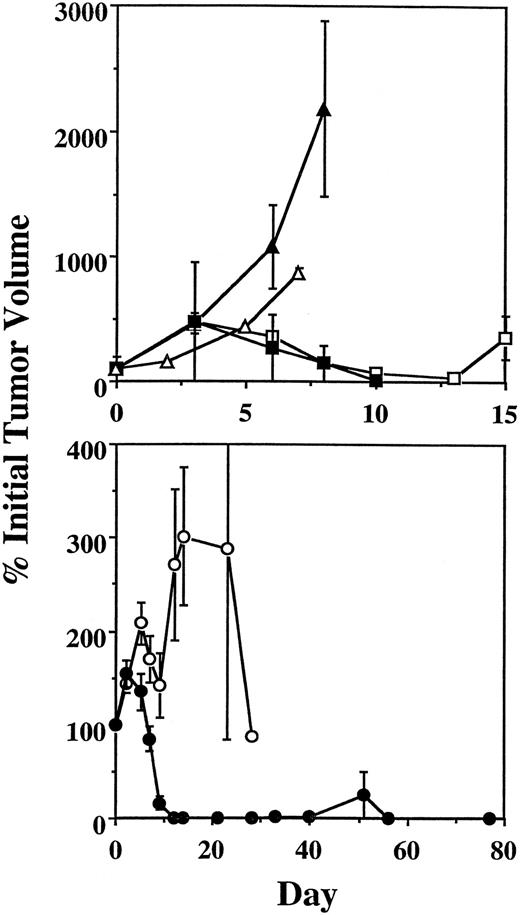
All mice in the control groups treated with 90Y-DOTA-biotin alone or with control NR-LU-10-SA conjugate plus90Y-DOTA-biotin experienced exponential growth of their lymphoma xenografts requiring euthanasia before day 10 (Figure5A). The rates of tumor growth in these control groups were indistinguishable from untreated xenograft-bearing mice (data not shown). Mice treated with conventional one-step RIT using 200 μCi 90Y-DOTA-1F5 experienced transient partial remissions with regression of tumor to 39.8% ± 67.8% of initial tumor volume by day 13 after treatment (Figure 5A). However, tumors regrew in all mice mandating euthanasia before day 20 (Figure6). Mice in this group experienced reversible toxicity, losing 10.4% ± 2.0% of their body weight by day 8 after therapy, but recovered to their baseline weight by day 13. Mice treated with 400 μCi conventional one-step 90Y-DOTA-1F5 experienced more striking tumor regressions, with xenografts shrinking to 12.1% ± 33.3% of their initial volumes by day 10 after therapy (Figure 5A). However, all mice experienced lethal toxicity, losing 19.9% ± 1.7% of their initial weight by day 6 after therapy and dying of marrow suppression and infection on day 10 (Figure 6). Gastrointestinal toxicity (diarrhea or emesis) was not evident in this study. Mice were not treated with doses of direct conjugates higher than 400 μCi because this was a lethal dose.
Regression of lymphoma xenografts after conventional or pretargeted RIT.
(A) Conventional RIT. Six- to 10-week-old BALB/c nude mice bearing Ramos lymphoma xenografts were injected intraperitoneally with either 800 μCi 90Y-biotin alone (▵), 1.4 nmol control conjugate NR-LU-10-SA, followed 24 hours later by 5.8 nmol CA and 3 hours after that with 800 μCi 90Y-DOTA-biotin (▴), or with 1.4 nmol directly radiolabeled 90Y-DOTA-1F5 labeled with either 200 μCi (■) or 400 μCi of 90Y (▪). (B) Pretargeted RIT. Six- to 10-week-old BALB/c nude mice bearing Ramos lymphoma xenografts were injected intraperitoneally with 1.4 nmol anti-CD20 immunoconjugate 1F5-SA, followed 24 hours later by 5.8 nmol CA, and 3 hours after that with either 400 μCi (○) or 800 μCi (●) 90Y-DOTA-biotin. Notice the differences in the scales of both the tumor size and the time axes between panels A and B.
Regression of lymphoma xenografts after conventional or pretargeted RIT.
(A) Conventional RIT. Six- to 10-week-old BALB/c nude mice bearing Ramos lymphoma xenografts were injected intraperitoneally with either 800 μCi 90Y-biotin alone (▵), 1.4 nmol control conjugate NR-LU-10-SA, followed 24 hours later by 5.8 nmol CA and 3 hours after that with 800 μCi 90Y-DOTA-biotin (▴), or with 1.4 nmol directly radiolabeled 90Y-DOTA-1F5 labeled with either 200 μCi (■) or 400 μCi of 90Y (▪). (B) Pretargeted RIT. Six- to 10-week-old BALB/c nude mice bearing Ramos lymphoma xenografts were injected intraperitoneally with 1.4 nmol anti-CD20 immunoconjugate 1F5-SA, followed 24 hours later by 5.8 nmol CA, and 3 hours after that with either 400 μCi (○) or 800 μCi (●) 90Y-DOTA-biotin. Notice the differences in the scales of both the tumor size and the time axes between panels A and B.
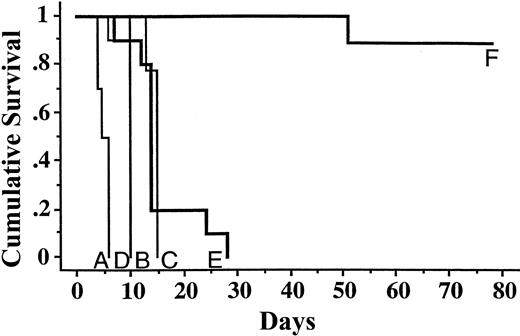
Kaplan-Meier analysis of cumulative survival of mice bearing Ramos lymphoma xenografts treated with conventional or pretargeted RIT.
Groups of 8 to 10 mice bearing 50 to 300 mm3 Ramos tumor xenografts were treated as described in the legend to Figure 5and analyzed serially for survival as a function of time. Treatment groups included 800 μCi 90Y-DOTA-biotin alone (A), 1.4 nmol control conjugate NR-LU-10-SA, followed 24 hours later by 5.8 nmol CA and 3 hours after that with 800 μCi 90Y-DOTA-biotin (B), with 1.4 nmol directly radiolabeled 90Y-DOTA-1F5 labeled with either 200 μCi (C) or 400 μCi of 90Y (D), or with 1.4 nmol anti-CD20 immunoconjugate 1F5-SA followed 24 hours later by 5.8 nmol CA, and 3 hours after that with 400 μCi (E) or 800 μCi (F) 90Y-DOTA-biotin (lines B and D overlap extensively).
Kaplan-Meier analysis of cumulative survival of mice bearing Ramos lymphoma xenografts treated with conventional or pretargeted RIT.
Groups of 8 to 10 mice bearing 50 to 300 mm3 Ramos tumor xenografts were treated as described in the legend to Figure 5and analyzed serially for survival as a function of time. Treatment groups included 800 μCi 90Y-DOTA-biotin alone (A), 1.4 nmol control conjugate NR-LU-10-SA, followed 24 hours later by 5.8 nmol CA and 3 hours after that with 800 μCi 90Y-DOTA-biotin (B), with 1.4 nmol directly radiolabeled 90Y-DOTA-1F5 labeled with either 200 μCi (C) or 400 μCi of 90Y (D), or with 1.4 nmol anti-CD20 immunoconjugate 1F5-SA followed 24 hours later by 5.8 nmol CA, and 3 hours after that with 400 μCi (E) or 800 μCi (F) 90Y-DOTA-biotin (lines B and D overlap extensively).
Experimental mice pretargeted with 1F5-SA fared much better than other groups in terms of toxicity, tumor responses, and survival (Figures 5B and 6). Transient tumor responses were seen in mice receiving pretargeted 1F5-SA plus 400 μCi 90Y-DOTA-biotin with a maximal response seen 9 days after treatment; however, tumors rapidly regrew in all mice, leading to death by day 28 (Figures 5B and 6). In contrast, all 9 mice receiving pretargeted 1F5-SA plus 800 μCi90Y-DOTA-biotin achieved CRs by day 12. One of the 9 mice achieving a CR with 800 μCi relapsed on day 33 and was euthanized on day 51 due to progressive tumor growth (Figure 6). The other 8 appear to have been cured, without any recurrences during the observation period of the experiment (>140 days). Experience with this xenograft model suggests that relapses do not occur after this time interval. There was minimal toxicity in mice pretargeted with 1F5-SA followed by 400 or 800 μCi 90Y-DOTA-biotin, with 5% ± 1% weight loss by day 2 in both groups; all mice regained pretreatment weight by day 7.
Discussion
This report convincingly demonstrates that pretargeted anti-CD20 RIT achieves superior biodistributions of radioactivity, is less toxic, and induces more durable CRs than conventional RIT in this mouse lymphoma xenograft model. The differences in biodistribution were striking. Conventional radiolabeled antibodies achieved low tumor-to-blood ratios of 0.5:1 or less and tumor-to–normal organ ratios of 6:1 or less (Table 1). In marked contrast, the pretargeted approach maintained the absolute tumor content of 111In or90Y at the same levels as observed with standard radiolabeled antibodies, while simultaneously decreasing the content of111In or 90Y in the blood by 79% to 95% and the levels in normal organs by 76% ± 13%. As a consequence, the tumor-to–normal organ ratios of absorbed radioactivity were far superior in the pretargeted groups in every experiment conducted, with tumor-to-blood ratios of 3:1 or higher and tumor-to-normal ratios up to 56:1. Because the “therapeutic index” of an antineoplastic agent depends on the balance between toxic effects on normal tissues and cytotoxic effects on tumor cells, it is not surprising that the pretargeted approach was superior to conventional RIT. The rapid clearance of excess circulating immunoconjugate from the vasculature by the CA and the rapid urinary excretion of excess90Y-DOTA-biotin minimized nonspecific irradiation of normal organs. Consequently mice experienced negligible toxicity when treated with the pretargeted approach, even after administration of 800 μCi90Y, which was twice the lethal dose when given directly conjugated to antibody. In contrast, the prolonged circulatory half-life of directly labeled 1F5 led to substantial toxicity at 200 μCi and fatal toxicities at 400 μCi 90Y. These normal organ toxicities limited the amount of directly radiolabeled immunoconjugate that could be administered to doses that were incapable of inducing CRs or cures. On the other hand, pretargeted RIT could be given with impunity at twice the lethal dose with minimal toxicity and with apparent cures in about 90% of mice. The CA appears to be a crucial contributor to the success of this pretargeting approach because the elimination of protracted bloodstream radioactivity is largely responsible for the improvement in tumor-to–normal organ ratios, and consequently the enhancement in the therapeutic index.
Several other investigators have presented preclinical data in solid tumor models showing that (strept)avidin-biotin “pretargeting” protocols can effectively circumvent the major pharmacokinetic limitations of conventional “one-step” RIT. Hnatowich, Goodwin, Meares, and Paganelli were among the first to describe the theoretic rationale for a pretargeting approach and to demonstrate its promise in preclinical experiments using 2-step and 3-step protocols with biotin and avidin or streptavidin.21-23 Paganelli has conducted extensive murine and human experiments using pretargeting approaches with solid tumors using biotinylated antibodies, followed by (strept)avidin and then radiolabeled biotin.21,27,29 Barbet and colleagues have recently developed a novel pretargeting approach using an “affinity enhancement system” consisting of bispecific antibodies recognizing both a tumor-specific antigen and a radiolabeled hapten.20 28
D.A. and colleagues at Neorx have conducted extensive experiments and pilot clinical trials using the pretargeted NR-LU-10 anticarcinoma antibody.24,26,30 All of these approaches have confirmed the advantages of pretargeting, affording rapid effective blood clearance, have improved tumor-to–normal organ ratios of absorbed radioactivity, and have demonstrated clinical responses. However, most of the published clinical trials have targeted solid tumors using antibodies directed against antigens with a wide distribution in normal tissues. Solid tumors are relatively radioresistant and probably require the localization of very high levels of radioactivity to achieve an antitumor response. This has been difficult to achieve when targeting antigens expressed widely on normal tissues. Consequently the clinical impact of pretargeting in patients with solid tumors has been modest so far, despite excellent tumor localization.26-29The current study demonstrates that these concepts can be successfully applied to non-Hodgkin lymphomas, which are exquisitely radiosensitive and which respond readily to anti-CD20 directed RIT, even when targeted by conventional means.9-13,15-18 Similar conclusions have recently been reached by Schultz and coworkers using an anti-CD20–streptavidin fusion protein.25 A recent pilot clinical trial testing rituximab conjugated to SA followed by CA and pretargeted radiobiotin has documented the feasibility and efficacy of this approach in patients with lymphomas, with 6 of 7 patients treated with 30 mCi/m2 radiobiotin or more experiencing tumor regressions, including 2 CRs and 2 partial remissions.39It is therefore reasonable to hypothesize that extending the pretargeting approach to B-cell lymphomas will allow a significant improvement in the percentage of patients achieving CRs and in the duration of these remissions. In view of the large magnitude of benefit observed in our murine studies, it is possible that pretargeting will permit substantial dose escalation of CD20-directed RIT so that durable remissions and cures might be achieved in a high percentage of patients without requiring stem cell “rescue” and with less toxicity than current regimens.
Despite the promising results obtained in pretargeting studies, we recognize that this approach also has limitations. These include (1) the complexity of pretargeting protocols, requiring multiple injections at defined time intervals, (2) the immunogenicity of SA, which may limit the ability to administer multiple cycles of therapy, (3) the relatively high doses of radiation delivered to the kidneys in some published studies,40 and (4) the presence of endogenous biotin,41 which competes with therapeutic radiolabeled biotin for binding to SA. Further, we recognize that caution must be exercised in extrapolating the current mouse xenograft studies to humans because xenografts may have improved tumor vascularity, and hence higher tumor uptakes of radioimmunoconjugates, than human tumors. In addition, normal mouse B cells do not bind the 1F5 antibody, whereas normal human B lymphocytes do, and this might affect targeting unless circulating B cells are precleared with an infusion of nonradioactive anti-CD20 antibody.3 Finally, the use of 90Y immunoconjugates in mice can be questioned because of the relatively long path length of the emitted β particles (5 mm) compared to the sizes of mice and xenografts. On the other hand, it can be argued that mouse xenograft studies using 90Y as the targeting isotope might be a conservative predictor of human results because a significant fraction of the emitted β particles emanating from tumors may deposit their energy outside the xenograft, and because toxicity might be enhanced in the mouse model through exposure to a greater volume of normal tissue.24
In summary, pretargeting methods appear superior to conventional RIT approaches because they (1) accelerate the time frame for maximizing tumor uptake of radioactivity, (2) allow faster clearance of radioactivity from the circulation, resulting in dramatic improvements in the tumor-to-marrow and tumor-to–normal organ ratios of absorbed radioactivity, (3) permit target signal amplification because 4 radioactive biotin molecules can bind to a tetravalent streptavidin molecule, (4) cause less toxicity to normal organs including the marrow, and (5) improve the CR rates and survival of tumor-bearing mice. Other theoretical advantages include minimizing the risk of radiolysis of antibody protein by high specific activity radionuclides and enhancing the feasibility of using radioisotopes with shorter half-lives for radioimmunoscintigraphy and RIT. We believe that the superiority of pretargeting in these studies and in those published by others merits further preclinical and clinical experimentation, and, eventually, randomized clinical trials comparing standard and pretargeted anti-CD20 RIT. Furthermore, the development of novel new pretargeting reagents, including molecularly engineered anti-CD20–strepatividin fusion proteins,25 new single-chain antibodies developed from phage display libraries,42 and bispecific antibodies recognizing bivalent haptens as well as tumor antigens20 suggest that future pretargeting approaches will be even more successful than the synthetic conjugates used in this report.
We are grateful for the technical assistance of Layla Adolphson, Janet Howell-Clark, Mark Derleth, and Pamela Zwolinski and the generous donation of reagents and advice by Neorx.
Supported by National Institutes of Health grants R01 CA76287 and K23 CA78346, and a gift from the Hext Family Foundation.
L.T., E.Y., R.M., and D.A. are employees of the NeoRx Corporation who have patented the clearing agent and streptavidin method used in these experiments.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Oliver W. Press, Fred Hutchinson Cancer Research Center, D3-190, 1100 Fairview, Seattle, WA 98109; e-mail:press@u.washington.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal