Abstract
The t(15;17) translocation in acute promyelocytic leukemia (APL) yields a PML/RAR-α fusion messenger RNA species that can be detected by reverse transcription–polymerase chain reaction (RT-PCR) amplification. Breakpoints within intron 3 of PML produce a short PML/RAR-α isoform, whereas breakpoints within intron 6 result in a longer form. Using RT-PCR, serial evaluations were performed on the bone marrow of 82 patients with APL (median follow-up, > 63 months) who received retinoic acid (RA) induction followed by postremission treatment with chemotherapy, RA, and biologic agents. Sixty-four patients attained a clinical complete remission and had at least 2 RT-PCR assays performed after completing therapy. Forty of 47 patients (85%) with newly diagnosed APL who were induced using RA had residual disease detectable by RT-PCR before additional therapy. After 3 cycles of consolidation therapy, residual disease was found in only 4 of 40 evaluable patients (10%). Among newly diagnosed patients who had 2 or more negative RT-PCR assays, only 3 of 41 (7%) had a relapse, whereas all 4 patients (100%) who had 2 or more positive results had a relapse. Among 63 newly diagnosed patients, those who expressed the short isoform appeared to have shorter disease-free and overall survival durations than patients who expressed the long isoform. These data indicate that 2 or more negative RT-PCR assays on bone marrow, performed at least 1 month apart after completing therapy, are strongly associated with long-term remissions. Conversely, a confirmed positive test is highly predictive of relapse.
Introduction
Acute promyelocytic leukemia (APL) is characterized by a specific translocation that fuses the promyelocytic leukemia gene(PML) on chromosome 15 with a gene encoding a retinoic acid receptor (RAR-α) on chromosome 17,1-4 and by responsiveness to differentiation therapy with all-transretinoic acid (RA).5-8 Molecular detection of PML/RAR-α fusion messenger RNA (mRNA) by reverse transcription and polymerase chain reaction (RT-PCR) amplification is useful in diagnosing APL, in predicting responsiveness to RA, and in detecting minimal residual disease after remission induction.9-12Preliminary studies by us and others suggest that this assay may also be useful in predicting relapse and in identifying patients who require further antileukemic therapy.13-19
Although the breakpoint on chromosome 17 invariably disrupts theRARA gene in the second intron, rearrangement of thePML gene may occur at 2 major breakpoints. Breakpoints within PML intron 3 (bcr 3) consistently yield the short fusion mRNA transcript that joins PML exon 3 and RAR-α exon 3. Breakpoints within PML intron 6 (bcr 1) consistently produce the long transcript from the joining of PML exon 6 to RAR-α exon 3. Additionally, breakpoints within PML exon 6 (bcr 2) are associated with a variable length transcript in a small number of patients.10,12,20,21 The influence of breakpoint site on patient outcome remains controversial. Early reports suggested that patients who express the short isoform have an increased incidence of clinical relapse and shorter survival compared to patients with the long isoform.15,22 Others have reported no difference in disease-free survival between patients with the long or short isoforms after adjusting for the association of the short form with a higher leukocyte count at presentation.23
As a follow-up to our preliminary report,14 we have prospectively evaluated 82 patients treated with RA and chemotherapy for the presence or absence of PML/RAR-α fusion mRNA over time using serial RT-PCR analyses of bone marrow. In this study, we show that elimination of the RT-PCR signal is strongly associated with long-term remission, whereas a confirmed positive assay after completion of therapy is highly predictive of relapse. In addition, we report that patients with the short isoform appear to have a shorter disease-free and overall survival compared to patients with the long isoform, independent of the initial leukocyte count.
Patients, materials, and methods
Patients
We analyzed the course of all newly diagnosed and relapsed patients with APL treated consecutively on protocols at Memorial Sloan-Kettering Cancer Center from June 1990 to September 1995. All patients met morphologic criteria for the diagnosis of APL (acute myelocytic leukemia [AML]-M3 or M3-variant) according to the French-American-British classification system,24 and this diagnosis was confirmed by RT-PCR analysis for PML/RAR-α rearrangements.10
Treatment
Most patients received all-trans RA as previously described7; 2 patients, including 1 with newly diagnosed disease, were induced with 9-cisRA.25 Only one newly diagnosed patient received all-trans RA concomitantly with chemotherapy because of leukocytosis. Newly diagnosed patients remained on retinoids for approximately 30 days after they had attained a clinical complete remission. After discontinuing RA, 3 cycles of idarubicin and cytarabine were administered to most patients as consolidation therapy, as previously described.26,27 Because of coexisting medical conditions or patient refusal, 4 newly diagnosed patients did not complete all 3 cycles of consolidation chemotherapy, and 1 patient received no consolidation therapy. Six newly diagnosed patients received postremission therapy with the recombinant humanized anti-CD33 monoclonal antibody HuM195 in addition to chemotherapy.28Two pediatric patients received 5 or 6 cycles of chemotherapy as consolidation. Most patients who had relapsed from previous chemotherapy were maintained solely on all-trans RA unless they were candidates for bone marrow transplantation or other investigational therapy. Seven patients in second remission received postremission therapy with 131I-labeled M195,29 and one patient received unconjugated HuM195.28 One patient in early relapse underwent allogeneic bone marrow transplantation.
RT-PCR analysis
We analyzed serial bone marrow aspirates by RT-PCR to detect PML/RAR-α rearrangements. This method, which has been previously described,10,14 has a sensitivity of approximately 1 in 104 cells. We examined samples before patients began treatment, after they achieved clinical complete remission by standard criteria,30 and periodically thereafter.
Statistical analysis
For inclusion in this analysis, we required patients to have achieved both a clinical complete remission and to have 2 or more RT-PCR assays performed from 1 to 3 months apart after completion of therapy. We estimated the probability of disease-free and overall survival using the Kaplan-Meier method. The log-rank test was used to compare relapse-free and overall survival durations between patient groups. The χ2 test was used to compare complete remission rates between patients expressing the long or short isoforms of PML/RAR-α. Multivariate analyses of disease-free and overall survival were performed using proportional hazards regression.
Results
Patients
We studied 82 patients (median age, 45 years; range, 7-80 years) whose median duration of follow-up exceeds 63 months (range, 1-119+ months). These patients received 63 courses of treatment for newly diagnosed APL and 28 courses for relapsed APL (Table1). Patients had a mean of 4 bone marrow samples analyzed after treatment.
Treatment administered to patients with acute promyelocytic leukemia
| Treatment . | Number of courses . | |
|---|---|---|
| Newly diagnosed . | Relapsed . | |
| Induction therapy | 63 | 28 |
| All-trans RA | 61 | 23 |
| 9-cisRA | 1 | 1 |
| All-trans RA + chemotherapy | 1 | 0 |
| Chemotherapy | 0 | 3 |
| BMT | 0 | 1 |
| Postremission therapy | 54 | 25 |
| Chemotherapy | 54 | 0 |
| 1 cycle | 1 | |
| 2 cycles | 3 | |
| 3 cycles | 42 | |
| 3 cycles + HuM195 | 6 | |
| 5 cycles | 1 | |
| 6 cycles | 1 | |
| All-trans RA maintenance | 0 | 11 |
| 131I-M195 | 0 | 7 |
| BMT | 0 | 4 |
| AuBMT | 0 | 2 |
| HuM195 maintenance | 0 | 1 |
| Treatment . | Number of courses . | |
|---|---|---|
| Newly diagnosed . | Relapsed . | |
| Induction therapy | 63 | 28 |
| All-trans RA | 61 | 23 |
| 9-cisRA | 1 | 1 |
| All-trans RA + chemotherapy | 1 | 0 |
| Chemotherapy | 0 | 3 |
| BMT | 0 | 1 |
| Postremission therapy | 54 | 25 |
| Chemotherapy | 54 | 0 |
| 1 cycle | 1 | |
| 2 cycles | 3 | |
| 3 cycles | 42 | |
| 3 cycles + HuM195 | 6 | |
| 5 cycles | 1 | |
| 6 cycles | 1 | |
| All-trans RA maintenance | 0 | 11 |
| 131I-M195 | 0 | 7 |
| BMT | 0 | 4 |
| AuBMT | 0 | 2 |
| HuM195 maintenance | 0 | 1 |
BMT indicates bone marrow transplantation; AuBMT, autologous bone marrow transplantation; 131I-M195, iodine-131–labeled M195 (anti-CD33 monoclonal antibody); HuM195, recombinant humanized M195.
Molecular remission induction
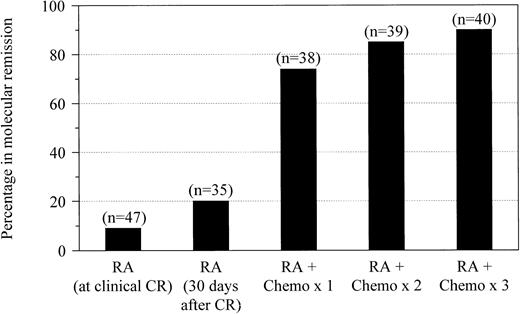
We monitored the effect of therapy using serial RT-PCR analyses of bone marrow throughout treatment (Figure1). Forty-seven newly diagnosed patients who received induction therapy with RA had assays performed at the time they attained clinical complete remission. After retinoid therapy alone, only 4 patients (9%) had no evidence of residual leukemia detectable by RT-PCR. Among 35 patients who were maintained on RA for at least 30 days after achieving remission and who underwent RT-PCR evaluation before consolidation chemotherapy, 7 (20%) became negative. Molecular evidence of residual disease was eliminated in 28 of 38 patients (74%) who were evaluated after one cycle of consolidation with idarubicin and cytarabine. After the second and third cycles of chemotherapy, RT-PCR–detectable disease was eliminated in 33 of 39 patients (85%) and 36 of 40 patients (90%) who were evaluated, respectively. None of the patients who achieved molecular complete remissions converted to RT-PCR positivity while still receiving consolidation therapy.
Molecular remission after RA and chemotherapy.
Molecular remission rates are shown for patients with newly diagnosed APL after treatment with all-trans RA at the time of documented clinical complete remission and after 30 days of maintenance with RA followed by postremission therapy with 1, 2, or 3 cycles of idarubicin and cytarabine.
Molecular remission after RA and chemotherapy.
Molecular remission rates are shown for patients with newly diagnosed APL after treatment with all-trans RA at the time of documented clinical complete remission and after 30 days of maintenance with RA followed by postremission therapy with 1, 2, or 3 cycles of idarubicin and cytarabine.
Within this limited patient sample, the time at which patients achieved molecular remission during their treatment course did not appear to affect long-term disease-free survival. Among the 7 patients who were RT-PCR negative after induction with RA alone, 1 (14%) relapsed. Of the 21 patients who achieved molecular remission after one cycle of consolidation therapy, 4 (19%) had relapses. In contrast, none of the 8 patients who achieved molecular remission after second or third cycles of chemotherapy had a relapse.
Prognostic significance of the RT-PCR assay
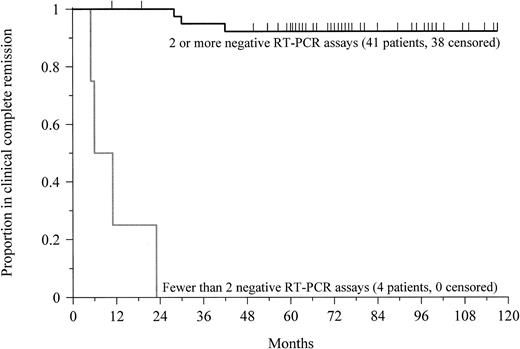
Forty-five patients with newly diagnosed APL achieved a clinical complete remission and had 2 or more RT-PCR assays performed between 1 and 3 months apart following the completion of therapy. All 4 patients (100%) who had at least 2 positive RT-PCR determinations had relapse (Table 2). The median disease-free survival was 9 months (range, 5-23 months) from the time of documented clinical complete remission (Figure 2). The prognostic value of the test decreased if only a single assay was performed. Among the 10 patients who had at least one positive assay following therapy, 7 patients (70%) had relapse.
Prognostic significance of RT-PCR
| . | No. of relapses/no. of patients . | ||
|---|---|---|---|
| Newly diagnosed (%) . | Relapsed (%) . | Overall (%) . | |
| < 2 negative assays | 4/4 (100) | 18/18 (100) | 22/22 (100) |
| ≥ 2 negative assays | 3/41 (7) | 1/7 (14) | 4/48 (8) |
| . | No. of relapses/no. of patients . | ||
|---|---|---|---|
| Newly diagnosed (%) . | Relapsed (%) . | Overall (%) . | |
| < 2 negative assays | 4/4 (100) | 18/18 (100) | 22/22 (100) |
| ≥ 2 negative assays | 3/41 (7) | 1/7 (14) | 4/48 (8) |
Prognostic significance of RT-PCR in APL.
This Kaplan-Meier plot shows the probability of disease-free survival for newly diagnosed patients with at least 2 negative RT-PCR assays or fewer than 2 negative assays after completion of therapy. Tick marks indicate the time of last follow-up. The probability of remaining leukemia-free after 5 years for patients with at least 2 negative assays was 92%. All patients with a confirmed positive test relapsed after a median of 9 months (P < .0001).
Prognostic significance of RT-PCR in APL.
This Kaplan-Meier plot shows the probability of disease-free survival for newly diagnosed patients with at least 2 negative RT-PCR assays or fewer than 2 negative assays after completion of therapy. Tick marks indicate the time of last follow-up. The probability of remaining leukemia-free after 5 years for patients with at least 2 negative assays was 92%. All patients with a confirmed positive test relapsed after a median of 9 months (P < .0001).
Conversely, among the 41 patients with newly diagnosed APL who had 2 or more negative RT-PCR assays performed following therapy, only 3 patients (7%) relapsed (Table 2). The median remission duration for this group exceeds 75 months (range, 11-117+ months;P < .0001; Figure 2). Again, the prognostic value of the assay decreased if a single test was performed. Six of 40 patients (15%) with only one negative assay relapsed. Among patients with at least 3 negative assays following treatment, however, only 1 of 34 patients (3%) had a relapse, similar to the outcomes of patients with at least 2 negative determinations.
Analogous results were found for patients in second or greater remission. All 18 patients (100%) with at least 2 positive RT-PCR determinations performed after the completion of therapy relapsed after a median of 6 months (range, 2-39 months). Of the 7 patients beyond first remission with 2 or more negative assays, 1 patient (14%) relapsed after 14 months. If only one RT-PCR assay was performed, the prognostic significance of the test decreased. For the 13 patients with at least one negative assay, 7 patients (53%) relapsed. Nevertheless, among the 6 patients with at least 3 negative assays, only 1 patient (17%) relapsed. This was comparable to the outcomes of patients with only 2 negative determinations.
Time from molecular relapse detection to overt clinical relapse
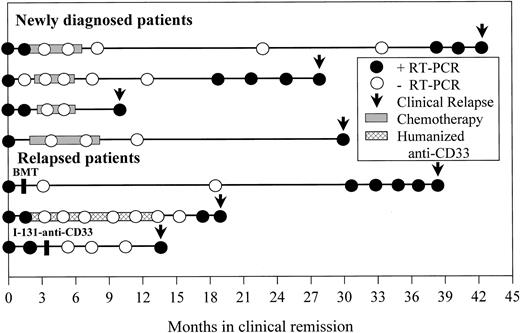
Seven patients (4 newly diagnosed, 3 relapsed) who attained a molecular remission established by at least 2 negative RT-PCR bone marrow determinations performed at least 1 month apart later relapsed (Figure 3). Four of these patients had positive RT-PCR assays 1, 4, 8, and 9 months before the time of clinical relapse, consistent with our previously reported results.15 Despite serial monitoring with RT-PCR every 3 to 6 months in 2 of the remaining 3 patients, the assay failed to predict clinical relapse in these individuals who were found to be RT-PCR positive only at the time of overt clinical relapse.
Time from molecular relapse detection to clinical relapse.
Longitudinal RT-PCR results from bone marrow aspirates of 7 patients who attained a molecular remission but later relapsed are shown. Each circle represents an assay performed at the indicated time after achieving clinical complete remission. Only samples with adequate RNA quality for amplification of control RNA are included. ● denotes a positive assay; ○ denotes a negative assay. Periods of consolidation therapy with chemotherapy or humanized anti-CD33 monoclonal antibody HuM195 are shown by the shaded bars. Arrows indicate the time of clinical relapse.
Time from molecular relapse detection to clinical relapse.
Longitudinal RT-PCR results from bone marrow aspirates of 7 patients who attained a molecular remission but later relapsed are shown. Each circle represents an assay performed at the indicated time after achieving clinical complete remission. Only samples with adequate RNA quality for amplification of control RNA are included. ● denotes a positive assay; ○ denotes a negative assay. Periods of consolidation therapy with chemotherapy or humanized anti-CD33 monoclonal antibody HuM195 are shown by the shaded bars. Arrows indicate the time of clinical relapse.
Prognostic significance of PML/RAR-α isoform type
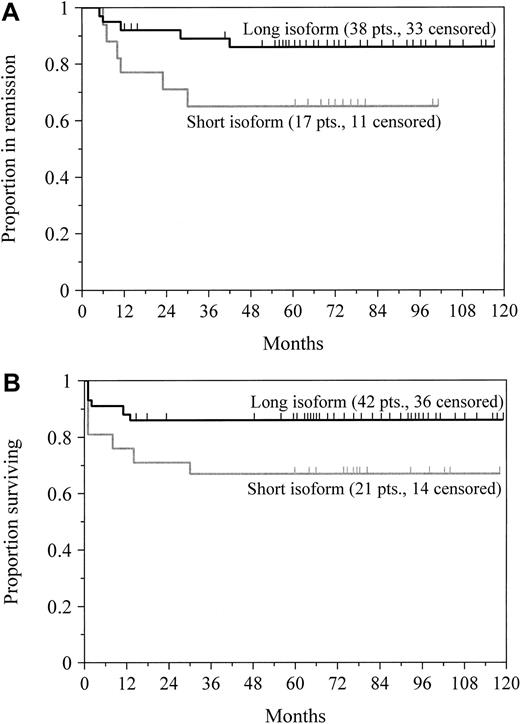
To determine the influence of PML/RAR-α breakpoint patterns on disease-free and overall survival, we included only the 63 patients who were treated for newly diagnosed disease. There was no significant difference in the incidence of clinical complete remission in the patients who expressed the short (81%; n = 21) or long (90%; n = 42) isoform of PML/RAR-α (P = .28). Nevertheless, the breakpoint location appeared to influence both disease-free and overall survival. Among the 55 patients who attained remission, the estimated probability of remaining disease-free after 5 years was 86% for the patients expressing the long isoform and 65% for those expressing the short isoform; this difference was borderline significant at P = .06 (Figure4A). For all 63 patients, the probability of 5-year survival was 86% for patients with the long transcript and 67% for patients with the short transcript (Figure 4B;P = .08). We found a strong trend toward improved disease-free survival (P = .08) and overall survival (P = .31) for patients with an initial leukocyte count of fewer than 5000 cells/μL in this series, but not a highly significant association as others have reported.8,23,31,32A multivariate proportional hazards model showed that the leukocyte count at presentation (P = .007) affected disease-free survival to a greater extent than PML/RAR-α isoform type (P = .08; Table 3), as previously reported by Gallagher and coworkers.23 In a similar analysis, however, the breakpoint location (P = .08), but not the leukocyte count (P = .93), tended to influence overall survival (Table 3).
Survival by PML/RAR-α isoform type.
Probabilities of disease-free survival for newly diagnosed patients achieving clinical complete remission (A) and overall survival (B) in patients who expressed either the short or long transcript of PML/RAR-α are shown. Tick marks indicate the time of last follow-up. Patients with the short transcript had an inferior remission duration (P = .06) and overall survival (P = .08) compared with patients expressing the long isoform.
Survival by PML/RAR-α isoform type.
Probabilities of disease-free survival for newly diagnosed patients achieving clinical complete remission (A) and overall survival (B) in patients who expressed either the short or long transcript of PML/RAR-α are shown. Tick marks indicate the time of last follow-up. Patients with the short transcript had an inferior remission duration (P = .06) and overall survival (P = .08) compared with patients expressing the long isoform.
Prognostic factors in multivariate proportional hazards model
| . | P . | Risk ratio . | 95% CI . |
|---|---|---|---|
| Disease-free survival | |||
| Isoform type3-150 | .08 | 2.86 | 0.88 -9.80 |
| Presenting WBC3-151 | .007 | 2.53 | 1.01 -1.06 |
| Survival | |||
| Isoform type3-150 | .08 | 2.47 | 0.88 -7.81 |
| Presenting WBC3-151 | .93 | 1.63 | 0.98 -1.03 |
| . | P . | Risk ratio . | 95% CI . |
|---|---|---|---|
| Disease-free survival | |||
| Isoform type3-150 | .08 | 2.86 | 0.88 -9.80 |
| Presenting WBC3-151 | .007 | 2.53 | 1.01 -1.06 |
| Survival | |||
| Isoform type3-150 | .08 | 2.47 | 0.88 -7.81 |
| Presenting WBC3-151 | .93 | 1.63 | 0.98 -1.03 |
CI indicates confidence interval; WBC, white blood cell count.
Short versus long.
Continuous variable.
Discussion
Reverse transcription PCR for the detection of mRNA product of the PML/RAR-α fusion gene is a useful diagnostic and prognostic tool for patients with APL because the elimination of residual disease detectable by the currently used methods of RT-PCR is necessary for patients to achieve long-term remission. In this study, all patients who had 2 positive tests after treatment relapsed. Although the prognostic significance of a single negative assay is uncertain, newly diagnosed patients with at least 2 negative assays after the completion of therapy had only a 7% risk of relapse. Reduction of minimal residual disease below the level of detection by RT-PCR without complete eradication of the APL clonogenic cells may explain clinical relapse in this group of patients. Diverio and colleagues reported that among 142 patients with newly diagnosed APL who tested negative in 2 or more assays after consolidation therapy, only 8 (6%) relapsed.33 These data, along with previously reported studies,13-19 31 support the use of additional therapy for all patients with a confirmed positive RT-PCR assay after treatment.
Despite achieving clinical complete remission, most patients treated solely with RA for induction had persistent evidence of leukemia detectable by RT-PCR before consolidation therapy in this study. After 3 courses of consolidation chemotherapy, 90% of patients were RT-PCR negative. No differences in presenting leukocyte count, isoform type, or additional cytogenetic abnormalities were identified between the small group of patients who never achieved molecular remission and the patients who became RT-PCR negative. Using RA and chemotherapy together, Mandelli and colleagues reported that 60% of patients with newly diagnosed APL were RT-PCR negative after induction and that 98% of patients tested negative after 3 cycles of consolidation therapy.34 These data suggest that by carefully monitoring residual disease during treatment, the requirement for postremission chemotherapy may be reduced or eventually eliminated in APL, particularly as newer agents, such as arsenic trioxide,35liposomal all-trans RA,36 and antibody-based therapies,28 are integrated into treatment strategies. Unlike the Medical Research Council,31 we found that within this small series, patients who attained molecular remission earlier in their course of treatment did not have a reduced risk of relapse. Among the 28 patients who were RT-PCR negative after RA alone or RA induction and one cycle of consolidation chemotherapy, 5 (18%) relapsed, whereas none of the 8 patients who achieved molecular remission after a second or third course of consolidation therapy relapsed.
In patients who attained molecular remission determined by 2 or more negative RT-PCR assays but who later relapsed, the time from molecular to clinical relapse varied from 1 to 9 months. Diverio and colleagues found that among 20 similar patients, the median time from a positive RT-PCR determination to overt relapse was 3 months (range, 1-14 months).33 Based on these data, we recommend RT-PCR evaluations of bone marrow every 3 months during the initial 2 years after remission is achieved, when the risk of relapse is greatest. Additional studies will be needed to assess the impact of molecular monitoring in patients receiving maintenance therapy with RA alone or in combination with chemotherapy.18 37 At present, however, we recommend serial RT-PCR testing every 3 months even in patients receiving maintenance therapy.
The role of RT-PCR for the detection of minimal residual disease has been investigated in other types of leukemia. The prognostic significance of RT-PCR for bcr/abl in chronic myelogenous leukemia (CML) depends on the clinical setting in which it is used. Early after allogeneic bone marrow transplantation, a significant proportion of patients may continue to express thebcr/abl fusion transcript.38-40Spontaneous conversion to RT-PCR negativity forbcr/abl transcripts could occur either as long-lived nonclonogenic cells (such as B lymphocytes, which may carry the bcr/abl transcript) die after transplantation, or as a T-cell–mediated graft-versus-leukemia effect eliminates residual host leukemia.38 Conversely, the persistence of bcr/abl mRNA, particularly in the setting of mixed donor/host T-cell chimerism,41 has correlated with a high incidence of relapse. Another study has indicated that RT-PCR positivity in patients with CML receiving interferon is not necessarily associated with immediate disease recurrence.42 The prognostic value of RT-PCR for AML1/ETO in t(8;21) AML is less clear. Several groups have detected this rearrangement in long-term survivors, possibly because this genetic alteration is only one of several required for leukemogenesis.43-45 More recently, however, Morschhauser and colleagues reported that negative RT-PCR results strongly correlated with long-term remissions in patients with t(8;21) AML when a standardized one-step RT-PCR technique was used to analyze bone marrow samples.46
Because the sensitivity of bcr/abl testing by RT-PCR (ie, 1 cell in 106)47 considerably exceeds that achieved by most groups for PML/RAR-α testing, the incidence of false-negative results may be higher for APL, particularly for patients in second or greater remission. Frequent bone marrow evaluations may increase the predictive value of this assay. Preliminary data also suggest that quantitative real-time RT-PCR techniques may provide important prognostic information for patients with APL.48 Nevertheless, the assay used in this study10 and similar assays9,11 12 provide a level of sensitivity that is clinically useful for assessing response to therapy and for predicting relapse.
Previously, we22 and others15 reported that the location of the PML/RAR-α breakpoint affects prognosis in APL (Table 4). In the present study, patients with the short isoform appeared to have a shorter remission duration and overall survival compared to patients with the long isoform. With a median follow-up of 5 years, 86% of the patients with the long isoform remain in first remission, compared with only 65% of those with the short isoform in the present study (P = .06). Although 86% of patients with the long isoform remain alive, only 67% of those with the short isoform are alive (P = .08). Unlike Gallagher and colleagues,23 we could not attribute these apparent differences solely to the higher initial leukocyte counts in patients expressing the short isoform. Presenting white blood cell counts, however, have been reported to significantly influence disease-free survival in several studies.23,31 32Ultimately, longer follow-up and greater patient numbers will be needed to clarify the influence of the breakpoint location on patient outcomes.
Prognostic significance of PML/RAR-α isoform type
| Study . | No. of remissions/no. of patients . | No. of relapses/no. of patients after 2 y . | ||
|---|---|---|---|---|
| Long isoform (%) . | Short isoform (%) . | Long isoform (%) . | Short isoform (%) . | |
| Huang15 | 32/33 (97) | 16/19 (84) | 1/32 (3) | 3/16 (19) |
| Gallagher4-15023 | 44/60 (73) | 31/38 (82) | 8/44 (18) | 9/31 (29) |
| Current study | 38/42 (90) | 17/21 (81) | 3/38 (8) | 5/17 (29) |
| Study . | No. of remissions/no. of patients . | No. of relapses/no. of patients after 2 y . | ||
|---|---|---|---|---|
| Long isoform (%) . | Short isoform (%) . | Long isoform (%) . | Short isoform (%) . | |
| Huang15 | 32/33 (97) | 16/19 (84) | 1/32 (3) | 3/16 (19) |
| Gallagher4-15023 | 44/60 (73) | 31/38 (82) | 8/44 (18) | 9/31 (29) |
| Current study | 38/42 (90) | 17/21 (81) | 3/38 (8) | 5/17 (29) |
Includes only patients receiving induction with RA.
Differences in the biologic activities of the long and short isoforms may also offer some insight into clinical outcomes. When the long and short isoforms were constitutively expressed in a human erythroleukemia cell line, the long isoform, but not the short, inhibited growth of these cells in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF).49 In the absence of GM-CSF, the short isoform partially protected against apoptosis, whereas the long isoform accelerated cell death. Moreover, inhibition of cell growth by all-trans RA was greater in cells expressing the long isoform than the short.49 These data suggest that APL cells expressing the short isoform may be more resistant to differentiation therapy with RA, to cytotoxic chemotherapy, or to intrinsic biologic or immunologic control of the residual clone than cells with the long isoform. Similar in vitro studies examining the impact of isoform type on the activity of arsenic trioxide are needed. Prospective trials examining the use of newer agents, such as arsenic trioxide35 or antibody-based therapies,28 should be investigated in high-risk patients who express the short isoform or have elevated initial leukocyte counts. Additionally, correlation of clinical outcomes and PML/RAR-α isoform types will be needed in patients receiving arsenic trioxide.
We wish to thank Dr Kristi Levine for her laboratory assistance and Drs Ellin Berman, Peter Maslak, Mark Weiss, and Mark Heaney for their clinical assistance.
Supported in part by grants from the Orphan Products Division, Food and Drug Administration (FD-R-000764 and FD-R-000925); the National Cancer Institute (CA 33049); the National Institutes of Health (CA 05826); the American Cancer Society (EDT-47); and the Lymphoma Foundation. J.G.J. is the recipient of a Clinical Oncology Career Development Award from the American Cancer Society. W.H.M. is the recipient of a scholarship of the Medical Research Council of Canada. D.A.S. is a Translational Investigator of the Leukemia Society of America and the recipient of a Doris Duke Distinguished Clinical Scientist Award.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Joseph G. Jurcic, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10021; e-mail: jurcicj@mskcc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal