Intravital microscopy was used to monitor leukocyte traffic across rat mesenteric postcapillary venules induced by the inactive terminal complement (C) complex (iTCC) topically applied to ileal mesentery. Leukocytes started rolling within 15 minutes from the administration of iTCC, and by 1 hour they adhered almost completely to the endothelium emigrating from the vessels in the next 3 hours. C5a caused a similar, though less marked, effect, whereas boiled iTCC was inactive, excluding the contribution of contaminating lipopolysaccharide. The complex stimulated the migration of polymorphonuclear neutrophils (PMNs) across endothelial cells (ECs) in a transwell system after a 4-hour incubation of ECs with iTCC added to the lower chamber of the transwell, whereas a 30-minute incubation was sufficient for C5a and interleukin (IL)-8 to induce the passage of PMNs. C5a was not responsible for the effect of iTCC because this complex had no chemotactic activity and contained too small an amount of C5a to account for the transendothelial migration of PMNs. Similarly, the effect of iTCC was not mediated by IL-8 released by stimulated ECs because anti–IL-8 failed to inhibit the migration of PMNs induced by the complex. Unlike tumor necrosis factor-α, iTCC did not cause the redistribution of platelet–endothelial cell adhesion molecule-1 (PECAM-1), and PMN mobilization was partially blocked by anti–PECAM-1 antibodies.
Introduction
Terminal complement (C) complex (TCC) represents the end product of the C-activation cascade and is formed by the assembly of the 5 late components of the C system. The best-known function of this complex is to insert into the phospholipid bilayer of the cell target as membrane attack complex (MAC) causing cytolysis. However, evidence collected in the last 2 decades has convincingly proved that MAC in sublytic amounts is also able to induce noncytolytic effects on several target cells.1,2 An early manifestation of cell activation induced by MAC is the rise in Ca++caused by the rapid influx of these ions from an extracellular source and their cytoplasmic redistribution from intracellular stores.1
The endothelium is a potential target of MAC that can be formed directly on the cell membrane as a result of C activation triggered by cell-bound antibodies. Alternatively, MAC starts assembling in the circulation and subsequently interacts with endothelial cells (ECs). Cells attacked by MAC may undergo cytolysis, but, more frequently, they release and express on their surfaces molecules involved in important biologic functions. Mobilization of P-selectin,3enhancement of tumor necrosis factor (TNF)-α–induced expression of adhesion molecules,4 release of chemokines, monocyte chemotactic protein-1 (MCP-1), and interleukin (IL)-8,5and platelet-activating factor6 are examples of pro-inflammatory effects stimulated by MAC on ECs. Moreover, MAC triggers the coagulation process, stimulating ECs to express tissue factor7 and to shed MAC-enriched vesicles that support the formation of a prothrombinase complex.8 Finally, MAC has been shown to create interendothelial gaps, causing structural changes of the endothelium,9 and it contributes to the modification of vascular tone by promoting the release of the vasodilator prostaglandin I and the vasoconstrictor thromboxane A2 from ECs.11
These noncytotoxic effects on ECs require the complex to insert into the cytoplasmic membrane to signal the cell, and they have been obtained in model systems using ECs coated with C-fixing antibodies. The chances that this may happen in vivo are limited to those clinical situations characterized by the presence of cytotoxic anti-endothelial antibodies, such as hyperacute graft rejection. More often, the endothelium is exposed to TCC that starts assembling in the bloodstream in the vicinity of ECs, and that completes its formation on the cell surface. However, the ability of this complex to insert into the EC membrane as MAC is restricted by the rapid functional decay of nascent TCC from the inactivating effect of inhibitors acting both in the fluid phase (S protein and clusterin12,13) and on the cell surface (CD59).14 In many clinical conditions associated with massive C activation, a cytolytically inactive TCC (iTCC) accumulates in plasma as an apparently irrelevant byproduct of the C sequence.15 We have previously shown that the inactive complex not only binds to ECs, it stimulates these cells to express adhesion molecules and tissue factor.16
Data accumulated in recent years have shown that terminal C components are synthesized at extravascular sites by various cell types17,18 and that TCC can be formed in biologic fluids other than blood.19 20 These findings led us to investigate whether the inactive complex present at extravascular sites may interact with ECs on the abluminal side promoting inflammation. Evidence will be presented indicating that iTCC induces transendothelial migration of polymorphonuclear neutrophils (PMNs) both in vitro and in vivo.
Materials and methods
C reagents
Purified late C components—C7, C8, and C9—were purchased from Quidel (San Diego, CA), and recombinant human C5a was supplied by Sigma Chemical (Milan, Italy). C5b6 and iTCC were purified as previously reported.16
Other reagents
Bacterial lipopolysaccharide (LPS) from Escherichia coliO55:B5 and endotoxin-free gelatin were purchased from Sigma Chemical. TNF-α (2.0 × 107 U/mg) was from Bachem Biochemica GmbH (Heidelberg, Germany), and fibronectin was from Roche Diagnostics (Mannheim, Germany). IL-8 (5 × 10−5 M) was a kind gift of Prof M. Baggiolini (Berne, Switzerland).
Antibodies
Neutralizing rabbit antibodies anti-human IL-8 were obtained from Peprotech (London, England). Monoclonal antibody (mAb) M89D3 anti-platelet–endothelial cell adhesion molecule-1 (PECAM-1) inhibiting leukocyte migration across ECs was generously provided by Dr M. R. Zocchi (San Raffaele Hospital, Milan, Italy). Two mAb anti-C5a—G25/2 and C17/5, the latter of which recognized a neo-epitope exposed on this fragment—were a kind gift of Prof O. Götze (Göttingen, Germany), and mAb aE11 directed against a C9 neo-antigen was kindly provided by Prof T. Lea (Oslo, Norway). This mAb was linked to cyanogen bromide-activated Sepharose 4B (Pharmacia Biotech, Milan, Italy) to prepare an affinity column. Goat antiserum to C5 was purchased from Quidel.
Enzyme-linked immunosorbent assay
Intravital microscopy
Male Wistar Kyoto rats, each weighing 250 to 270 g, were anesthetized intraperitoneally with sodium thiobarbital (100 mg/kg). A polyethylene catheter (PE50; Intramedic Clay-Adams, Sparks, MD) was inserted into the left carotid artery, and a Statham P23AC pressure transducer (Gould, Cleveland, OH) connected to a physiograph (Ote-Biomedica, Florence, Italy) was used to monitor the mean arterial pressure and the heart rate. Another catheter (PE20) was inserted into the left femoral vein and was connected to a micropump injector (Harvard Apparatus, South Natick, MA). The fluorescent marker acridine orange (AO) (Sigma Chemical) diluted in sterile saline was slowly infused through this device over the first 3 hours of the experimental procedure at a concentration of 0.025 mg/kg per minute and at a rate of 0.5 mL/h.
The rats were placed on an adjustable stage of an upright microscope (model BX50WI; Olympus Optical, Tokyo, Japan). A 2-cm midline incision was made through the abdominal wall, and a loop of ileal mesentery was exteriorized and carefully draped over a transparent pedestal immersed in a plexiglas chamber containing temperature-controlled and sterile modified Krebs-Henseleit solution (118 mM NaCl, 4.74 mM KCl, 2.45 mM CaCl2, 1.19 mM KH2PO4, 1.19 mM MgSO4, 12.5 mM NaHCO3). Exposed tissue was superfused throughout the study with sterile buffered saline warmed at 37°C and bubbled with a mixture of 95% N2 and 5% CO2. The integrity of the microvasculature was checked by intravenous injection of fluorescein-labeled dextran 70 kd (Molecular Probes, Eugene, OR).
The mesenteric microcirculation was transilluminated with a 12-V, 100-W direct current-stabilized light source and was viewed through 10× and 40× saltwater dipping objectives and a 10× ocular lens. AO-labeled leukocytes were made visible by epifluorescence transillumination with a UIF550 filter for excitation light (Olympus Optical, Tokyo, Japan). Images were recorded by a charge-coupled device camera connected through a peripheral component interconnect interface board (SensiCam PCO, Kelheim, Germany) to a computer device, where they were stored and analyzed off-line using dedicated imaging software (Analytica Lite, Milan, Italy).
In vivo experimental procedure
The rats were allowed to rest for 15 minutes after surgery, and segments of 3 to 5 unbranched postcapillary venules (25-40 μm diameter, 200 μm length) were selected for analysis. Venular diameter and center-line red blood cell velocity were evaluated off-line using a video caliper (Image Research, Ontario, Canada) and customized frame-by-frame analogic image analysis.24 Red blood cell velocity (VRBC) and venular diameter (D) were used to calculate venular wall shear rate (g) through the formula g = 8(Vmean/D), where Vmean = VRBC/1.6.25 Sterile saline containing either 10−7 C5a or 1.5 × 10−8 M iTCC, assuming that each complex contained 4 molecules of C9, were topically applied to the mesentery for 10 minutes after baseline evaluation, and image sequences were recorded intermittently up to 240 minutes. Control animals were treated with sterile saline or boiled iTCC for 15 minutes.
Intravascular circulation of leukocytes in postcapillary venules and their extravascular emigration were analyzed off-line during playback of the digital file sequences. Labeled leukocytes were classified as rolling (if they moved more slowly than red blood cells, thus becoming visible) or adherent (if they remained stationary for more than 30 seconds). Rolling flux was expressed as the number of leukocytes seen moving past a reference point per minute, and adherence was measured by counting the number of adherent leukocytes per 200 μm venule length. Leukocyte emigration from the vessel was evaluated by counting the cells that had extravasated up to 100 μm away from the wall in parallel with 200-μm vessel segments.
Endothelial cell culture
The procedure for isolation of human umbilical vein ECs (HUVECs) from 3 to 5 normal umbilical cord veins by collagenase digestion and culture of pooled cells in tissue culture plates (Costar, Cambridge, MA) coated with 2% endotoxin-free gelatin has been reported.16 26 HUVECs were used at their first passage.
Transendothelial migration and chemotaxis assays
HUVECs (2 × 104) were seeded onto 2% gelatin- or 0.2 μM fibronectin-coated polycarbonate inserts of a 24-well Transwell system (6.5-mm diameter, 5-μm pores; Costar) and were used 5 days after plating.27 Transendothelial electrical resistance across the inserts was evaluated daily from the second day after seeding using Millicell-ERS voltammeter (Millipore, Bedford, MA). The value of resistance (Ω × cm2) increased progressively and reached a plateau at day 5, when formation of the EC monolayer was complete.
ECs were exposed for 4 hours at 37°C to the various stimuli added either to the lower or the upper compartment of the transwell. Twenty microliters PMN suspension (2.5 × 106/mL) in phosphate-buffered saline (PBS) containing 1% bovine serum albumin prepared as previously reported16 was added to the upper chamber to a final volume of 100 μL and was further incubated at 37°C for 30 minutes. Cells that had migrated to the lower chamber and those that were detached from the lower surface of the filters by gentle washing were then harvested and counted in a ZBI Coulter Counter (Coulter Electronics, Luton, United Kingdom). A similar approach was followed to assess chemotaxis of PMNs except that the inserts of the transwell were uncoated.
PMN adhesion assay
HUVECs grown to confluence in 96-well tissue culture plates were stimulated for 4 hours at 37°C with iTCC or TNF-α, and, after 3 washings with serum-free medium, 100 μL PMN suspension (105 cells) was added to each well and was further incubated at 37°C for 25 minutes. Nonadherent PMNs were removed by washing the wells twice, and the number of adherent leukocytes was evaluated by a colorimetric assay using tetramethyl benzidine (Sigma Chemical) as a substrate for myeloperoxidase, as previously described.26
Scanning electron microscopy
An approach similar to that described for the PMN adhesion assay was followed except that HUVECs were grown to confluence on gelatin-coated glass coverslips (22 mm) and the adherent PMNs were fixed with 2.5% glutaraldehyde (Boehringer Ingelheim Bioproducts, Milan, Italy) in 0.2 M cacodylate buffer, pH 7.4. After dehydration in graded ethanol from 70% to 100%, the specimens were critically point-dried under liquid CO2, mounted on aluminum stubs, and sputter coated with gold (10 nm) using sputter pulse control. Cells were examined with a scanning electron microscope (Stereoscan 430I; Leica, Milan, Italy). Five to 7 microphotographs, each showing 15 to 20 PMNs, were digitally recorded, and the surface area of at least 100 cells was analyzed using a computer device with dedicated imaging software (Analytica Lite).
Immunofluorescence
HUVECs grown to confluence on coverslips coated with 1% gelatin in PBS were treated for 2 hours with iTCC (5 nM), TNF-α (200 U/mL), or control medium, washed with PBS and fixed with 1% paraformaldehyde (Merck, Darmstadt, Germany) in Dulbecco-PBS (Sigma Chemical) for 20 minutes. The cells were incubated first with mAb M89D3 anti–PECAM-1 (10 μg/mL) and then with fluorescein isothiocyanate (FITC)–conjugated goat anti-mouse IgG (FITC-GAM; Zymed Laboratories, San Francisco, CA). Actin morphology was examined on HUVEC monolayer fixed with 1% formaldehyde and permeabilized with 0.05% Triton X-100 (Merck) for 1 minute at 0°C, as previously described.26After they were washed with PBS, the coverslips were stained for F-actin with rhodamine tetraisothiocyanate phalloidin (Sigma) for 20 minutes at room temperature and were mounted in 50% glycerol–PBS. Microscopic analysis was performed with a Bio-Rad MRC 1000 confocal scanning microscope (Bio-Rad Laboratories, Milan, Italy), and fluorescence images were recorded on Kodak T-Max 100 film using a Focus Imagecorder Plus (Focus Graphics, Foster City, CA).
Reverse transcription–polymerase chain reaction
Total RNA was extracted from resting and activated HUVECs with a commercially available solution (RNAzol B; Tel-Test, Friendswood, TX). The RNA was reverse transcribed with M-MLV reverse transcriptase (Gibco-BRL, Milan, Italy) using 1 μg total RNA and random hexanucleotide primers (Boehringer Mannheim, Germany) according to the manufacturer's instructions. Polymerase chain reaction (PCR) was performed with 0.2 mM each dNTP, 1.4 U Taq DNA polymerase (Finnzymes OY, Espoo, Finland), 0.5 μM specific 20-mer oligonucleotide primers (Amersham Pharmacia-Biotech, Milan, Italy), and cDNA in a PTC-100 thermal cycler (MJ Research, Watertown, MA). PCR products were run through a 1.5% agarose gel containing ethidium bromide for UV detection. EMBL accession number, amplified sequences, and amplified products were as follows: IL-8: XM 003501, 74-373, 299 bp28; MCP-1: Y 18933, −30 + 324, 354 bp29; β-actin: M 10278, 311-838, 528 bp.30
Statistical analysis
Results are expressed as mean ± SD. Data were compared by analysis of variance using posthoc analysis for paired multiple comparisons with Fisher corrected t test.P [le] .05 was considered statistically significant.
Results
Leukocytes adhere to endothelium and transmigrate across mesenteric venules in response to iTCC
In our initial experiments, we sought to determine whether iTCC was able to recruit leukocytes into tissue by using intravital microscopy analysis to monitor cell mobilization. Based on preliminary results obtained from a dose-response curve, a group of rats was treated with a dose of complex found to be effective in recruiting leukocytes. Included in the experimental protocol were 3 additional groups of rats that received C5a at a dose similar to that selected for iTCC treatment and saline or boiled iTCC used to exclude the effects caused by contaminating LPS.
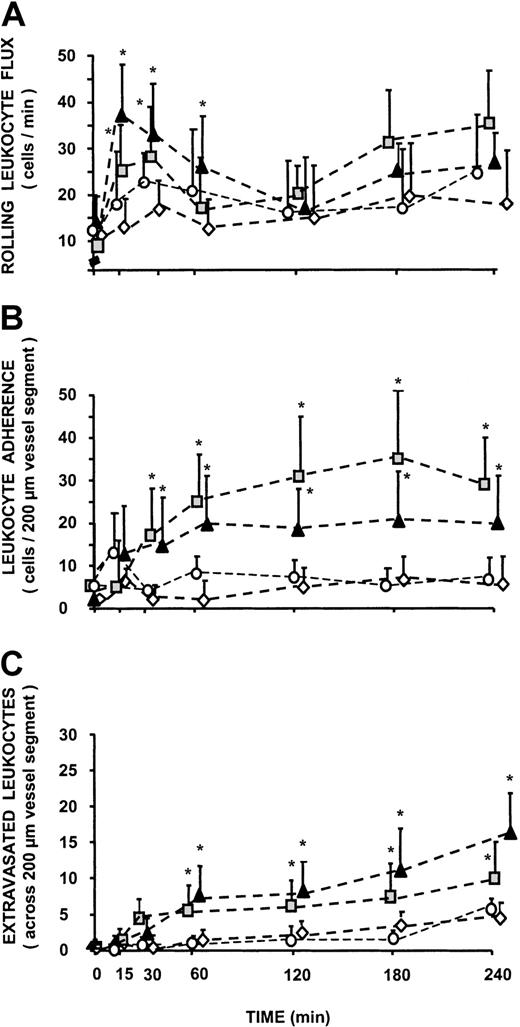
None of the rats showed overt modifications of systemic blood pressure levels and local hemodynamic parameters during the entire period of observation. Initial shear rate values in animals treated with C5a (403.7 ± 31 s−1), iTCC (395.4 ± 29 s−1), saline (411.2 ± 34.7 s−1), and boiled iTCC (387.5 ± 38.9 s−1) were not significantly different and did not change appreciably throughout the experimental procedure. Similarly, the number of rolling leukocytes and of stably adherent cells at the end of the equilibration period did not vary in the 4 groups of animals (Figure 1A). These values remained essentially unchanged in the saline and in the boiled iTCC-treated groups for the period of observation. By contrast, treatment of rats with iTCC caused a sharp increase in the leukocyte rolling flux that reached maximal value at 15 minutes after topical application of the complex (37 ± 8 cells/min). The pattern of leukocyte rolling flux through the mesentery microvessels in rats receiving C5a was essentially similar to that of iTCC-treated animals, though the maximal mean value of 28 ± 9 cells/min was lower.
Effect of iTCC on leukocyte trafficking in rat mesenteric postcapillary venules.
Traffic of leukocytes was monitored at various time intervals in response to the following stimuli: iTCC (1.5 × 10−8 M; ▴), C5a (10−7 M; ░), boiled iTCC (1.5 × 10−8 M; ○), and control saline (⋄). Measurements were performed on 4 to 6 different segments of unbranched venules (25- to 40-μm diameter, 200-μm length). (A) Values of rolling leukocyte flux defined as number of cells that become visible as bright spheres if they travel inside the venules more slowly than red blood cells. (B) Numbers of leukocytes stably adherent to the same site of postcapillary vascular endothelium of rat mesentery for at least 30 seconds. (C) Numbers of cells that migrated to the extravascular space up to 100 μm away from the vessel wall, in parallel with 200-μm vessel segments. *P < .01 versus control.
Effect of iTCC on leukocyte trafficking in rat mesenteric postcapillary venules.
Traffic of leukocytes was monitored at various time intervals in response to the following stimuli: iTCC (1.5 × 10−8 M; ▴), C5a (10−7 M; ░), boiled iTCC (1.5 × 10−8 M; ○), and control saline (⋄). Measurements were performed on 4 to 6 different segments of unbranched venules (25- to 40-μm diameter, 200-μm length). (A) Values of rolling leukocyte flux defined as number of cells that become visible as bright spheres if they travel inside the venules more slowly than red blood cells. (B) Numbers of leukocytes stably adherent to the same site of postcapillary vascular endothelium of rat mesentery for at least 30 seconds. (C) Numbers of cells that migrated to the extravascular space up to 100 μm away from the vessel wall, in parallel with 200-μm vessel segments. *P < .01 versus control.
Both iTCC and C5a induced progressive increases in the number of leukocytes stably adherent to the endothelium in the examined microvessels (Figure 1B). The mean number of leukocytes that adhered to a vessel segment 200 μm long at 3 hours after exposure to the various stimuli was significantly higher in rats treated with iTCC (22 ± 6 leukocytes) or C5a (35 ± 9 leukocytes) than in rats receiving saline (6 ± 5 leukocytes) or boiled iTCC (4 ± 3 leukocytes). Representative photomicrographs of leukocytes adherent to ECs are shown in Figure 2.
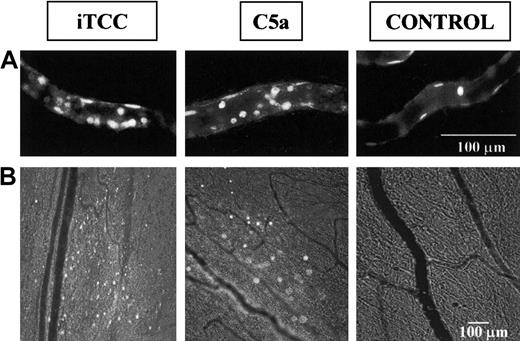
Leukocyte adherence to endothelium and extravasation.
Video photomicrographs show leukocytes adherent to endothelium (A) or migrated from rat mesenteric microvessels (B) in response to either 1.5 × 10−8 M iTCC, 10−7 M C5a, or control saline. Leukocytes labeled in vivo with AO were made visible by fluorescence epi-illumination and appeared as bright spheres. Magnifications, 400× (A) and 100× (B).
Leukocyte adherence to endothelium and extravasation.
Video photomicrographs show leukocytes adherent to endothelium (A) or migrated from rat mesenteric microvessels (B) in response to either 1.5 × 10−8 M iTCC, 10−7 M C5a, or control saline. Leukocytes labeled in vivo with AO were made visible by fluorescence epi-illumination and appeared as bright spheres. Magnifications, 400× (A) and 100× (B).
The extravascular appearance of AO-labeled cells served as a useful marker in evaluating the effects of iTCC and C5a on leukocyte extravasation (Figure 2, bottom panel). Numbers of adherent cells migrating across the wall of the mesenteric venules in the iTCC-treated mesenteric tissue started rising at 60 minutes after topical application of the complex, to a mean value of 8 ± 4 (P < .05 vs control) over a surface area of 200 × 200 μm, and it progressively increased with time to reach a mean value of 15 ± 6 at 4 hours (Figure 1C). Migration of leukocytes to the extravascular space was also seen in rats receiving C5a, but the extent of C5a-induced migration was less marked than in rats treated with iTCC, though the value of 6 ± 3.5 observed at 60 minutes was significantly different from the control value observed in the saline-treated group (P < .05 vs control).
iTCC promotes transendothelial migration of PMNs in vitro
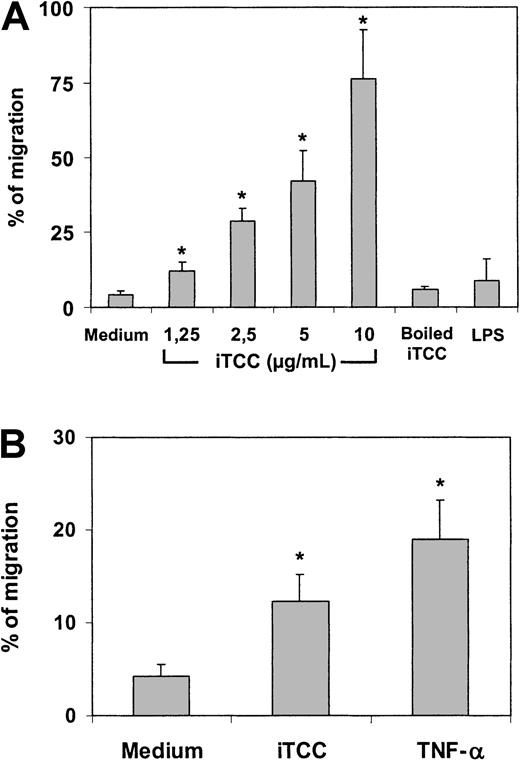
These in vivo data did not clarify whether iTCC induced transendothelial migration acting directly on ECs or through an intermediate effect on macrophages and other cell types in the peritoneal cavity, which, in turn, release cytokines and chemokines. To address this issue, HUVECs were grown to confluence on the polycarbonate insert of transwell cell culture chambers and were incubated with iTCC added to the lower compartment. PMNs were then introduced into the upper chamber and were allowed to migrate into the lower compartment. As shown in Figure 3A, the exposure of HUVECs to iTCC for 4 hours resulted in active diapedesis of a proportion of PMNs that was related to the dose of iTCC used. Attempts to induce similar effects by incubating ECs with iTCC for 30 minutes or by stimulating the cells with either LPS or boiled iTCC, as a source of contaminating endotoxin, failed. The complex, prepared with limited amounts of C9, also proved to be ineffective, as was the preparation depleted of iTCC by affinity chromatography with mAb aE11 to poly C9 (data not shown). Interestingly, the complex, like TNF-α, promoted the migration of PMNs even when added to the upper chamber of the transwell to stimulate ECs (Figure 3B).
In vitro transendothelial migration of PMNs induced by iTCC.
Transwell filters coated with first-passage HUVECs were treated for 4 hours at 37°C with increasing amounts of (A) iTCC, boiled iTCC (5 nM), LPS (0.5 μg /mL), or medium added to the lower compartment or (B) iTCC (5 nM), TNF-α (1.5 × 103 U/mL), or medium added to the upper compartment and washed after 4 hours of incubation. PMNs were added to the upper chamber, and the percentage of migrated PMNs was evaluated after an incubation of 30 minutes. Values are means ± SD of triplicate determinations of 3 separate experiments. *P < .01 versus control.
In vitro transendothelial migration of PMNs induced by iTCC.
Transwell filters coated with first-passage HUVECs were treated for 4 hours at 37°C with increasing amounts of (A) iTCC, boiled iTCC (5 nM), LPS (0.5 μg /mL), or medium added to the lower compartment or (B) iTCC (5 nM), TNF-α (1.5 × 103 U/mL), or medium added to the upper compartment and washed after 4 hours of incubation. PMNs were added to the upper chamber, and the percentage of migrated PMNs was evaluated after an incubation of 30 minutes. Values are means ± SD of triplicate determinations of 3 separate experiments. *P < .01 versus control.
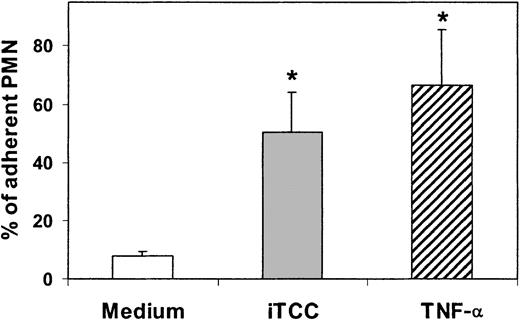
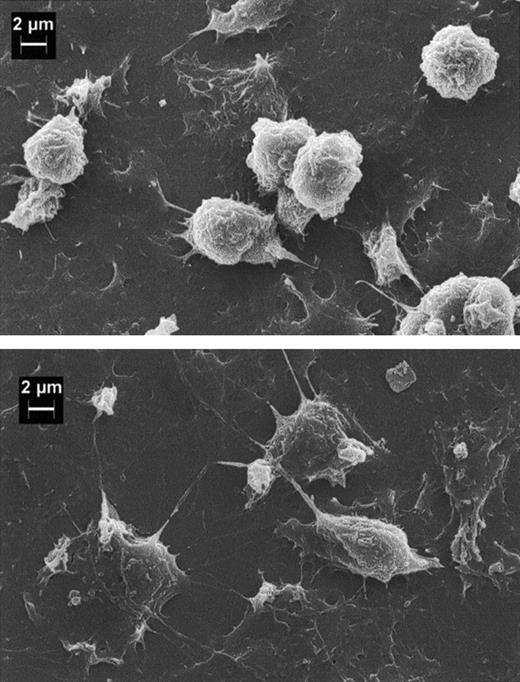
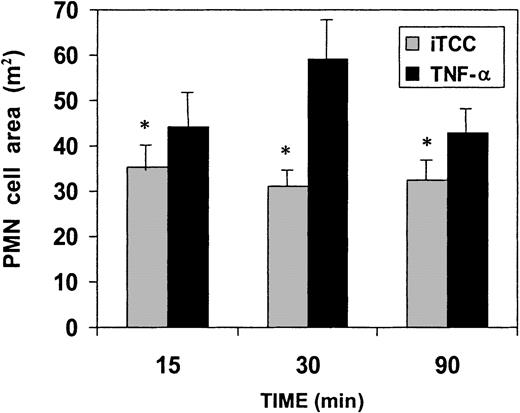
ECs exposed to concentrations of the C complex causing PMN migration exhibited increased adhesiveness for these cells (Figure4). Scanning electron microscopy analysis of adherent PMNs revealed that a large number of cells maintained a rounded shape, whereas PMNs bound to HUVECs treated with TNF-α, used as a well-established positive control, showed the morphologic appearance of flattened cells with large protrusions extending on the surfaces of ECs (Figure 5). As shown in Figure 6, PMNs attached to iTCC-stimulated ECs revealed a surface area significantly smaller than that of PMNs bound to TNF-α–treated ECs, with the maximal difference observed at 30 minutes.
Effect of iTCC and TNF-α on PMN adhesion to HUVECs.
EC monolayers were pretreated for 4 hours with medium alone or medium containing either iTCC (5 nM) or TNF-α (200 U/mL). PMNs were then added to the monolayer, and the percentage of adherent PMNs was determined after an incubation of 30 minutes at 37°C. Values are mean ± SD of triplicate determinations of 3 separate experiments. *P < .01 versus control.
Effect of iTCC and TNF-α on PMN adhesion to HUVECs.
EC monolayers were pretreated for 4 hours with medium alone or medium containing either iTCC (5 nM) or TNF-α (200 U/mL). PMNs were then added to the monolayer, and the percentage of adherent PMNs was determined after an incubation of 30 minutes at 37°C. Values are mean ± SD of triplicate determinations of 3 separate experiments. *P < .01 versus control.
Scanning electron microscopy of human PMNs adherent to HUVECs.
EC monolayers grown on a glass coverslip were pretreated for 4 hours with either iTCC (5 nM; top) or TNF-α (200 U/mL; bottom), and PMNs were added on top of the monolayers. After 30 minutes of incubation at 37°C, ECs were washed twice with PBS and then fixed with 2.5% glutaraldehyde in 0.2 M cacodylate buffer, pH 7.4.
Scanning electron microscopy of human PMNs adherent to HUVECs.
EC monolayers grown on a glass coverslip were pretreated for 4 hours with either iTCC (5 nM; top) or TNF-α (200 U/mL; bottom), and PMNs were added on top of the monolayers. After 30 minutes of incubation at 37°C, ECs were washed twice with PBS and then fixed with 2.5% glutaraldehyde in 0.2 M cacodylate buffer, pH 7.4.
Evaluation of morphologic changes of PMNs adherent to iTCC or TNF-α–stimulated HUVECs.
(See legend to Figure 5.) The surface areas of at least 100 PMNs were measured at different time intervals. Values are means ± SD of 3 experiments. *P < .01 versus TNF-α–stimulated HUVECs.
Evaluation of morphologic changes of PMNs adherent to iTCC or TNF-α–stimulated HUVECs.
(See legend to Figure 5.) The surface areas of at least 100 PMNs were measured at different time intervals. Values are means ± SD of 3 experiments. *P < .01 versus TNF-α–stimulated HUVECs.
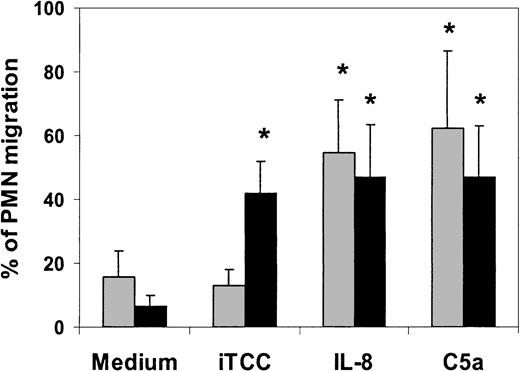
iTCC is not chemotactic for PMNs
To investigate whether iTCC exhibited chemotactic activity for PMNs, which could account, at least in part, for its ability to induce leukocyte transendothelial migration, we followed an approach similar to that used for the experiment presented in Figure 3, except that PMNs were allowed to migrate toward iTCC through the polycarbonate insert of the transwell chamber not coated with ECs. As shown in Figure7, iTCC was unable to attract PMNs chemotactically at the concentration of 5 nM, and it was inactive when tested within a concentration range between 0.5 and 10 nM. By contrast, both C5a and IL-8 caused PMN migration across transwell filters, irrespective of whether they were coated with ECs. PMN response was dose related and was no longer seen at concentrations lower than 500 nM for C5a and IL-8 (5 × 10−8 M and 0.5 × 10−9 M, respectively). Interestingly, exposure of ECs to either C5a or IL-8 for 30 minutes was sufficient to induce cell migration, whereas a longer incubation time of 4 hours was required for iTCC to be similarly effective. To exclude that C5a as a contaminant in our iTCC was responsible for the effect of the C complex, we measured C5a level using a sensitive enzyme-linked immunosorbent assay (ELISA) and found that it was approximately 1 nM. This concentration was far below the amount shown to induce the migration of PMNs across HUVECs.
Effect of iTCC on chemotaxis and transendothelial migration of PMNs.
Various stimuli, including iTCC (5 nM), IL-8 (10−8 M), or C5a (5 × 10−8 M) were added to the lower compartment of transwells, and PMNs were allowed to migrate from the upper chamber through filters coated with gelatin to test for PMN chemotaxis (░) or with first-passage HUVECs to test for PMN transmigration (▪). The percentage of migrated PMNs was determined after 30 minutes. Values are means ± SD of triplicate determinations of 5 experiments. *P < .01 versus control.
Effect of iTCC on chemotaxis and transendothelial migration of PMNs.
Various stimuli, including iTCC (5 nM), IL-8 (10−8 M), or C5a (5 × 10−8 M) were added to the lower compartment of transwells, and PMNs were allowed to migrate from the upper chamber through filters coated with gelatin to test for PMN chemotaxis (░) or with first-passage HUVECs to test for PMN transmigration (▪). The percentage of migrated PMNs was determined after 30 minutes. Values are means ± SD of triplicate determinations of 5 experiments. *P < .01 versus control.
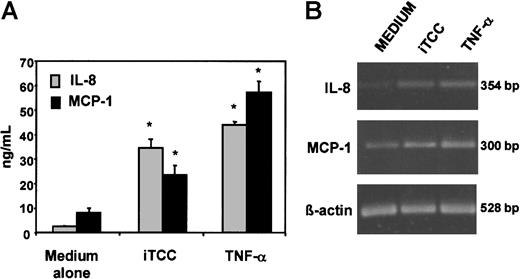
HUVECs stimulated by iTCC release IL-8 and MCP-1
The possibility that chemokines released by HUVECs could contribute to the transendothelial migration of PMNs promoted by iTCC was first explored by evaluating the ability of iTCC to stimulate the secretion of IL-8 and MCP-1 by ECs. To this end, the cells were incubated overnight with the complex, and ELISA was used to measure the amounts of the 2 chemokines released in the culture supernatant. The data presented in Figure 8A show that HUVECs treated with iTCC were able to secrete levels of IL-8 and MCP-1 significantly higher than those released by unstimulated cells, though they were lower than the amount produced by TNF-α–stimulated ECs. To confirm this observation, we analyzed the levels of mRNA for IL-8 and MCP-1, expressed by HUVECs treated with iTCC or TNF-α, as a positive control. As shown in Figure 8B, low amounts of the 2 transcripts were already detected in unstimulated HUVECs, but their levels increased substantially in cells stimulated by iTCC or TNF-α.
Production of IL-8 and MCP-1 by HUVECs exposed to iTCC.
(A) Levels of chemokines secreted by HUVECs in the culture supernatant after an overnight incubation with iTCC (5 nM), TNF-α (20 U/mL) or medium alone measured by ELISA. Values are means ± SD of triplicate determinations of 3 separate experiments. *P < .01 versus control. (B) Reverse transcription-PCR of IL-8 and MCP-1 mRNA expression of HUVECs unstimulated or treated with iTCC (5 nM) or TNF-α (200 U/mL).
Production of IL-8 and MCP-1 by HUVECs exposed to iTCC.
(A) Levels of chemokines secreted by HUVECs in the culture supernatant after an overnight incubation with iTCC (5 nM), TNF-α (20 U/mL) or medium alone measured by ELISA. Values are means ± SD of triplicate determinations of 3 separate experiments. *P < .01 versus control. (B) Reverse transcription-PCR of IL-8 and MCP-1 mRNA expression of HUVECs unstimulated or treated with iTCC (5 nM) or TNF-α (200 U/mL).
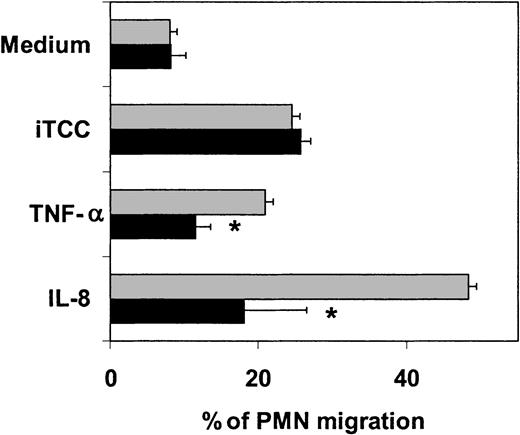
These results suggest but do not prove that the 2 chemokines released by iTCC-activated ECs are actually involved in the transendothelial migration of PMNs. To address specifically this question, we stimulated diapedesis of PMNs across HUVECs with the complex in the presence of a neutralizing antibody directed against IL-8. The data shown in Figure9 clearly indicate that this antibody is effective in inhibiting PMN migration induced by IL-8 and TNF-α, but not that stimulated by iTCC.
Effect of anti–IL-8 antibodies on PMN migration across HUVECs induced by iTCC.
Cells were incubated with iTCC (5 nM), TNF-α (1.5 × 103U/mL), or IL-8 (10−8 M) either alone (░) or mixed with affinity-purified rabbit antibodies to IL-8 (10 μg/mL) (▪). PMNs were introduced into the upper chamber, and the percentage of migrated PMNs was evaluated after an incubation of 30 minutes. Values are means ± SD of triplicate determinations of 4 separate experiments. *P < .01 versus response of HUVECs to the various stimuli in the absence of anti–IL-8.
Effect of anti–IL-8 antibodies on PMN migration across HUVECs induced by iTCC.
Cells were incubated with iTCC (5 nM), TNF-α (1.5 × 103U/mL), or IL-8 (10−8 M) either alone (░) or mixed with affinity-purified rabbit antibodies to IL-8 (10 μg/mL) (▪). PMNs were introduced into the upper chamber, and the percentage of migrated PMNs was evaluated after an incubation of 30 minutes. Values are means ± SD of triplicate determinations of 4 separate experiments. *P < .01 versus response of HUVECs to the various stimuli in the absence of anti–IL-8.
Contribution of PECAM-1 to the iTCC-dependent transendothelial migration of PMN
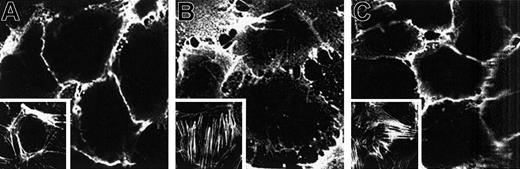
PECAM-1 molecules are constitutively expressed on endothelial cells, and their localization at the borders of adjacent cells makes them a good candidate for playing an active role in the process of cell adhesion and migration. To examine whether the transmigration of PMNs across HUVECs caused by iTCC was associated with changes in the distribution of PECAM-1 at the interendothelial junctions, confluent HUVEC monolayers were incubated with either iTCC or TNF-α or control medium, and the distribution of PECAM-1 stained with a specific mAb was analyzed by confocal scanning microscopy. As shown in Figure10, unstimulated confluent cells were brightly stained for PECAM-1 molecules that showed a linear and homogeneous distribution at the cell borders (Figure 10A). Conversely, staining for PECAM-1 was less intense in TNF-stimulated HUVECs, and these molecules appeared to be redistributed on the cell surface from a compact junctional to a punctate, speckled pattern (Figure 10B). HUVECs stimulated with iTCC exhibited a pattern similar to that of the control cells, with no overt changes in the staining and distribution of PECAM-1 molecules (Figure 10C). However, iTCC-treated ECs differed from unstimulated cells in the distribution of F-actin filaments stained with rhodamine-labeled phalloidin. In particular, the latter cells were characterized by a peripheral rim of fluorescent staining and by a lack of prominent filament bundles in the central area, whereas a proportion of HUVECs activated by either iTCC or TNF-α showed a less distinct peripheral staining rim and a reorganization of F-actin into thick cables running parallel to the long axis of the cells as stress fibers (Figure 10 insets).
Localization of PECAM-1 on HUVECs treated with iTCC.
HUVECs were grown to confluence on a glass coverslip and were incubated for 2 hours at 37°C with medium alone (A) or in the presence of either 200 U/mL TNF-α (B) or iTCC (5 nM) (C). Cells were then stained with anti–PECAM-1 (M89D3) mAb followed by FITC-GAM. Microscopic analysis was carried out in a Bio-Rad MRC 1000 confocal microscope (1 cm = 0.9 μm). Note the decrease of intercellular PECAM-1 staining in the TNF-α–treated HUVECs and the normal distribution of PECAM-1 in iTCC-treated HUVECs. Insets show fluorescence micrographs of F-actin distribution in HUVECs incubated with control medium (A), TNF-α (B), or iTCC (C).
Localization of PECAM-1 on HUVECs treated with iTCC.
HUVECs were grown to confluence on a glass coverslip and were incubated for 2 hours at 37°C with medium alone (A) or in the presence of either 200 U/mL TNF-α (B) or iTCC (5 nM) (C). Cells were then stained with anti–PECAM-1 (M89D3) mAb followed by FITC-GAM. Microscopic analysis was carried out in a Bio-Rad MRC 1000 confocal microscope (1 cm = 0.9 μm). Note the decrease of intercellular PECAM-1 staining in the TNF-α–treated HUVECs and the normal distribution of PECAM-1 in iTCC-treated HUVECs. Insets show fluorescence micrographs of F-actin distribution in HUVECs incubated with control medium (A), TNF-α (B), or iTCC (C).
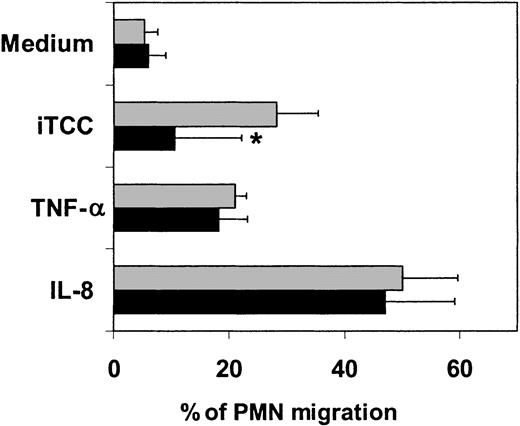
To further investigate the involvement of PECAM-1 in the transmigration of PMNs, we sought to determine whether this process could be controlled by the presence of mAbs directed against the junctional molecule in the assay system. Prior incubation of ECs with a mAb to PECAM-1 resulted in a significantly reduced number of PMNs migrating across HUVECs stimulated by iTCC, though inhibition was not complete, whereas the same mAb had no effect on cell migration induced by either IL-8 or TNF-α (Figure 11).
Effect of mAb to PECAM-1 on PMN transendothelial migration induced by iTCC.
Cells were incubated with iTCC (5 nM), TNFα (1.5 × 103U/mL), or IL-8 (10−8 M) either alone (░) or after exposure to mAb anti–PECAM-1 (10 μg/mL) (▪) for 20 minutes before the addition of PMNs to the upper chamber. Percentage of migrated PMNs was evaluated after an incubation of 30 minutes. Values are means ± SD of duplicate determinations of 3 separate experiments. *P < .05 versus response of HUVECs to the various stimuli in the absence of anti–PECAM-1.
Effect of mAb to PECAM-1 on PMN transendothelial migration induced by iTCC.
Cells were incubated with iTCC (5 nM), TNFα (1.5 × 103U/mL), or IL-8 (10−8 M) either alone (░) or after exposure to mAb anti–PECAM-1 (10 μg/mL) (▪) for 20 minutes before the addition of PMNs to the upper chamber. Percentage of migrated PMNs was evaluated after an incubation of 30 minutes. Values are means ± SD of duplicate determinations of 3 separate experiments. *P < .05 versus response of HUVECs to the various stimuli in the absence of anti–PECAM-1.
Discussion
The C system has long been known to recruit leukocytes from the circulation through the action of the chemotactic fragment C5a, made available in inflamed tissues as a result of C activation by various stimulating agents.31 We now present in vivo and in vitro data indicating that TCC, even in a cytolytically inactive form, can contribute to mobilize leukocytes.
Intravital microscopy, used in this study to analyze the mobilization of leukocytes in response to iTCC, provides a useful tool for observing leukocyte–endothelium interaction in vivo. The finding that leukocytes circulating in the rat mesentery firmly adhered to the endothelium of the postcapillary venules after the topical application of iTCC was not surprising because we have previously shown that this complex stimulates HUVECs to express molecules including endothelial E-selectin, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1, involved in leukocyte adhesion.16 iTCC differs in this regard from sublytic MAC because the latter complex induces the expression of P-selectin3 whereas it is ineffective in up-regulating the cell surface appearance of E-selectin and ICAM-1 unless TNF-α is also present in the assay system.4 The observation that few cells adhered to endothelial cells in control rats treated with saline excludes that the pro-adhesive property acquired by the endothelium as a reaction to the surgical procedure directly contributed to iTCC-dependent cell adhesion and also rules out nonspecific effects of the fluorescent dye injected intravenously. In addition, iTCC did not cause hemodynamic changes that could account for the observed cell trafficking. Our data indicating that C5a is also able to induce leukocyte adherence are in line with the results obtained in rabbits by DiScipio et al,32 though priming with IL-1β was required in their model for C5a to produce the effect.
Both iTCC and C5a proved to be effective in promoting the transmigration of leukocytes across the mesenteric venules into extravascular sites in our in vivo model. Results obtained with C5a are compatible with the known ability of this fragment to induce the expression of adhesion molecules on endothelium33 and to exert a chemotactic attraction on leukocytes,34 and again they confirm similar observations made in rabbits by DiScipio et al.32 An interesting finding of this study was that iTCC was able to induce cell migration to an extent and with a kinetic pattern that did not differ from those seen in C5a-treated rats. The presence of C5a in our iTCC may explain the in vivo biologic effect of the complex. However, the level of C5a detected in the iTCC preparation used in all the experiments was below the amount that was effective in inducing leukocyte mobilization. Alternatively, the inflammatory reaction observed with iTCC might have been caused by contaminating LPS, but a significant contribution of endotoxin to the effect induced by the complex was excluded by the finding that the activity of iTCC was lost after boiling.
The rat model has been extremely useful to ascertain the in vivo efficacy of iTCC in recruiting leukocytes into tissues, but it does not clarify the mechanism responsible for this process. It is possible that the complex elicits cell mobilization through the intermediate action of peritoneal cells, in particular macrophages and mast cells, stimulating these cells to release cytokines that, in turn, activate endothelium and promote PMN chemotaxis. The involvement of macrophages and mast cells in the migration of PMNs into the mouse peritoneal cavity has recently been elucidated in an elegant study by Ajuebor et al.35 Although this possibility cannot be excluded, we favor the hypothesis that ECs may play a relevant role in the mobilization of PMNs induced by iTCC, based on our previous finding of increased expression of surface adhesion molecules on HUVECs treated with this complex.16 To investigate this point more directly, we set up an in vitro model of PMN migration across ECs grown on the insert of a transwell cell culture chamber and found that iTCC promotes PMN passage when added to the lower compartment. These results led us to question whether iTCC was chemotactic for PMNs, but repeated attempts to document this activity failed. This indicates that the fully assembled terminal complex differs from the trimolecular complex C5b67 shown to stimulate chemotaxis of PMNs in vitro and in vivo.36-38 The presence of the trimolecular complex in our iTCC preparation appeared unlikely. iTCC, to be active, must be prepared with C9 in excess; it does not work when C9 is limited. Several groups have reported data demonstrating that transendothelial migration of PMNs can also be promoted by biologic factors that are not necessarily chemotactic for PMNs, including IL-1 and TNF-α.39-41
The 2 cytokines, IL-1 and TNF-α, and iTCC share the ability to support increased adhesiveness of PMNs to activated HUVECs, but the morphologic appearance of the adherent cells is different. PMNs bound to TNF-α–stimulated ECs acquired a flattened and irregular shape, whereas the cells adherent to iTCC-treated HUVECs remained rounded, indicating that they were more loosely attached. This observation is consistent with the lower expression of adhesion molecules on HUVECs activated by iTCC compared with those treated with IL-1 and TNF-α.16
Because C5a and IL-8 have been shown to induce diapedesis of PMNs across ECs,42 and IL-8 is apparently implicated in neutrophil migration across cytokine or LPS-activated ECs,43 we wondered whether these chemotactic factors could contribute to produce the effect caused by iTCC. As mentioned above, direct intervention of C5a can be ruled out by the finding that the iTCC used in these experiments had no chemotactic activity and contained an amount of C5a unable to induce the transendothelial migration of PMNs. One cannot exclude, however, that iTCC-stimulated ECs produce chemokines that are ultimately responsible for the PMN passage. The increased expression of mRNA for IL-8 and MCP-1 and the secretion of these 2 chemokines in the culture supernatant of HUVECs stimulated by iTCC are in line with this hypothesis and confirm similar findings obtained by Kilgore et al5 using sublytic concentrations of MAC to activate HUVECs. However, the amount of chemokines produced by HUVECs at the time (4 hours) iTCC mobilized PMNs was negligible and was, therefore, unlikely to account for the iTCC-dependent leukocyte transmigration. This conclusion is further supported by the failure of the anti–IL-8 antibody to inhibit the migration of PMNs induced by iTCC, whereas it was effective in neutralizing the biologic activity of IL-8. Conversely, anti–IL-8 antibody inhibited to some extent PMN migration through TNF-α–stimulated ECs, confirming the involvement of EC-derived IL-8 in this process.44 45
HUVECs treated with iTCC maintain a regular distribution of PECAM-1 on the intercellular junctions despite the rearrangement of cytoskeletal microfilaments; thus, they behave differently than the cells activated with TNF-α that show, in this and in previous studies,46,47 reduced intercellular staining and more random distribution of this molecule. Persistence of PECAM-1 at cell–cell borders is believed to favor neutrophil adherence to stimulated ECs as a result of homophilic interaction of the molecules expressed on both types of cells and to facilitate the passage of leukocytes across ECs.48 Blocking experiments with a specific mAb provide more direct evidence for the requirement of PECAM-1 in the iTCC-induced transendothelial migration of PMNs and further support previous observations made in vitro and in vivo.49,50 It should be mentioned, however, that the mAb to PECAM-1 did not cause complete inhibition of PMN migration, suggesting the additional implication of junctional molecules other than PECAM-1 in this process. It is also important to emphasize that these data on the contribution of PECAM-1 to iTCC-induced PMN migration must be confirmed in PECAM-1–deficient animals recently used by Thompson et al51 to prove that PECAM-1 is not involved in PMN migration through TNF-α–stimulated ECs, as we have shown in the current study.
In conclusion, the results of the current investigation disclose a yet unrecognized function of the cytolytically inactive TCC in promoting transendothelial migration and egression of PMNs from postcapillary venules. The novelty of our observation is that PMNs responded to the complex applied on the abluminal side of the EC monolayer in the in vitro model and on the external side of the vessel wall in the rat model, suggesting that iTCC present at the tissue level is involved in recruiting leukocytes at inflammatory sites. This possibility is not unlikely because the complex has been detected in various biologic fluids19,20 besides plasma, and recently it has been detected in human lymph (Tedesco et al, unpublished observations, 2001). Local formation of an excessive amount of iTCC may cause an unrestricted inflammatory process that is deleterious to tissue and that should be prevented by an appropriate therapeutic approach, such as the use of specific antibodies.
We thank Paolo Macor and Tito Ubaldini for excellent technical assistance.
Supported by grants from the Italian MIUR, CNR (Target Project on Biotechnology), Ministry of Health (Progetto Finalizzato Cod. B08), and Region Friuli-Venezia Giulia.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Francesco Tedesco, Dipartimento di Fisiologia e Patologia, Università di Trieste, Via Fleming 22, 34127 Trieste, Italy; e-mail: tedesco@univ.trieste.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal