Falciparum malaria is a major cause of disease and death in African children and pregnant women, primarily due to severe anemia. We studied anemia in vaccinated Aotus monkeys during a second infection where the animals were considered to be semi-immune. Most animals had extremely low or undetectable levels of parasitemia; in some, anemia did not develop and reticulocytemia remained unchanged; in others, moderate to severe anemia developed with inappropriately low reticulocytemia indicating bone marrow dysfunction. Bone marrow rapidly responded after parasite clearance. The rapid drop in hematocrit despite extremely low to undetectable parasitemia indicated massive removal of uninfected red blood cells from the circulation that, in the presence of bone marrow dysfunction, led to severe anemia—the problem that occurs in African children. We demonstrate that Aotusmonkeys are a nonhuman primate model to gain insight into the pathogenesis of severe anemia in African children.
Introduction
Malaria is a major cause of severe anemia in Africa, with children younger than 2 years of age and pregnant women the most affected groups.1-4 Most persons living in holo-endemic regions have low-level malaria parasitemia; many of them are anemic.5 The relationship between anemia and malaria is demonstrated by increased levels of hospitalization for anemia in times of peak malaria transmission and decreased levels of anemia after antimalarial therapy.1,6-8 The relationship between parasitemia and anemia differs according to endemic setting. In areas of low malarial endemicity, such as Thailand, the level of anemia correlates with high parasitemia.9 In hyperendemic areas, such as the Gambia, severe anemia is usually associated with lower levels of parasitemia.10 Parasitemia preceding hospital admission, however, is unknown. Vaccination and malaria parasite exposure induce a state of semi-immunity in Aotusmonkeys11; here we present evidence that semi-immuneAotus monkeys can serve as a nonhuman primate model for investigating the mechanisms of malaria-induced anemia.
Study design
Antigens
Vaccination
Monkeys received 200 μg recombinant protein per vaccination, 4 vaccinations per monkey, each 3 weeks apart. The first vaccination was an emulsion of antigen (in 200 μL phosphate-buffered saline [PBS]) with 200 μL complete Freund adjuvant given subcutaneously at 4 sites on the back, the next 2 vaccinations were emulsions in incomplete Freund adjuvant given as before, and the fourth was given intramuscularly in PBS (200 μL total volume) in one site. Monkeys were vaccinated before or after an initial malaria parasite exposure.
Infection
All 31 Aotus monkeys (29 A nancymai and 2A vociferans) had experienced one malarial parasite challenge infection and so experienced a second infection in this study. A donor monkey was infected intravenously with 106freshly thawed Plasmodium falciparum parasites of the FVO strain from a frozen sample. When a 1.5% parasitemia level was reached, blood was collected, washed twice in RPMI, and diluted in RPMI. Each monkey was challenged with 105 parasitized red blood cells (RBCs).
Monitoring parasitemia and anemia
Parasitemia was monitored daily with Giemsa-stained thin blood smears, anemia was monitored biweekly with hematocrits, and reticulocyte level was monitored weekly with methylene blue-stained thin blood smears. Blood was collected by puncture of superficial veins in the dorsum of the calf. After antimalaria drug cure, blood smears were taken daily until there was no detectable parasitemia for 3 consecutive days, and then they were taken once weekly. Hematocrits were taken daily when levels were lower than 30%. Drug cure, 50 mg mefloquine by mouth, was applied when the parasitemia level reached 5% or more or hematocrits decreased below 25%. All untreated monkeys were given chemotherapy on day 29. Parasitemia was calculated based on examination of 2000 RBCs, and reticulocyte level was calculated based on examination of 1000 RBCs.
Polymerase chain reaction
Polymerase chain reaction (PCR) of genomic DNA was carried out on blood samples collected from hematocrit tubes of infected monkeys and a malaria parasite–naive A nancymai. Packed RBCs (20 μL) were resuspended in 4 vol buffer solution (50 mM NaAc, pH 5.2, 100 mM NaCl, 1 mM EDTA) and were lysed with 3% sodium dodecyl sulfate. After 2 cycles of extraction and precipitation, DNA samples were resuspended in 20 μL diethyl pyrocarbonate (DEPC) water. Genomic DNA was used as template for amplification of a fragment of the P falciparumStevor multicopy gene family.13 Primers and PCR were performed as described previously.14
Results and discussion
Anemia experienced in this trial did not appear to be antigen specific because vaccination with either MSP-119 or GST resulted in anemia (Table 1). Whether monkeys were vaccinated before the first infection or between the first and second infections also seemed to have no effect on the development of anemia (Table 1). Anemia did not result from vaccination with yeast-produced recombinant proteins or from CFA, as attested by the vaccination of Aotus monkeys with DNA without adjuvant or yeast-produced antigens in a different adjuvant, which also resulted in partial protection and anemia.15
Relationship between parasitemia, anemia, and reticulocyte count
| Response to infection . | Aotusno. . | Peak parasitemia % (day)† . | Day of treatment‡ . | Reticulocyte %*(Hematocrit %) . | ||||
|---|---|---|---|---|---|---|---|---|
| Day 7 (8) . | Day 14 (15) . | Day 21 (22) . | Day 29 (29) . | Day 36 (36) . | ||||
| Group 1: No microscopically detectable parasitemia; no anemia | 5231-153 | 0 | 2.8 (58) | 7.8 (53) | 1.5 (49) | 6.5 (56) | 4.9 (63) | |
| 5301-153 | 0 | 6.8 (64) | 5.9 (60) | 3.4 (64) | 2.3 (66) | 4.9 (68) | ||
| 5591-153,1-154 | 0 | 4.0 (58) | 3.5 (49) | 3.3 (46) | 6.3 (57) | 2.6 (61) | ||
| 6031-153 | 0 | 3.8 (44) | 1.5 (43) | 1.6 (44) | 2.3 (41) | 3.8 (40) | ||
| 7321-153 | 0 | 3.3 (62) | 4.2 (57) | 2.4 (50) | 12.7 (43) | 5.6 (52) | ||
| 546 | 0 | 5.1 (60) | 2.4 (56) | 5.2 (61) | 1.7 (66) | 2.5 (71) | ||
| 602 | 0 | 3.1 (65) | 2.7 (60) | 1.7 (54) | 3.0 (62) | 3.6 (68) | ||
| 680 | 0 | 2.4 (58) | 3.3 (57) | 4.8 (59) | 5.7 (62) | 3.0 (65) | ||
| 692 | 0 | 2.1 (59) | 5.5 (57) | 1.4 (60) | 1.3 (59) | 2.1 (57) | ||
| 747 | 0 | 2.8 (57) | 3.7 (51) | 3.4 (51)1-164 | 2.2 (50) | 7.6 (40) | ||
| 749 | 0 | 1.9 (53)1-160 | 3.0 (53) | 2.0 (50) | 1.9 (59) | 2.4 (53) | ||
| Group 2: No microscopically detectable parasitemia; positive by PCR; severe anemia (hematocrit lower than 25%) | 5671-153 | 0 | 22 | 2.9 (60) | 2.4 (52) | 2.1 (22)1-161 | 14.8 (28) | 10.8 (49) |
| 6571-153 | 0 | 15 | 4.4 (43) | 2.3 (20) | 16.4 (29) | 14.9 (37) | 11.1 (44) | |
| 6761-153 | 0 | 22 | 2.5 (56) | 1.5 (45) | 0.1 (19)1-161 | 20.8 (27) | 7.1 (48) | |
| 26111-153,1-159 | 0 | 21 | 9.6 (60) | 4.5 (45) | 0.0 (16)1-161 | 6.3 (43) | 1.5 (53) | |
| 557 | 0 | 17 | 3.5 (52) | 1.5 (29) | 17.9 (26) | 8.4 (18) | 4.9 (25) | |
| 7481-154 | 0 | 17 | 3.2 (55) | 2.9 (30) | 6.9 (22) | 7.9 (32) | 6.2 (50) | |
| Group 3: Parasitemia level lower than 5%; moderate anemia | 5131-153,1-154 | 3.61 (20) | 5.8 (57) | 2.1 (56) | 4.0 (34) | 11.3 (36) | 4.9 (47) | |
| 5601-153 | 0.41 (21) | 4.8 (62) | 3.5 (60) | 0.4 (48) | 24.5 (33) | 10.8 (55) | ||
| 5771-153 | 0.73 (21) | 5.0 (54) | 5.5 (53) | 1.9 (34) | 9.6 (49) | 3.4 (62) | ||
| 6271-153 | 0.14 (17) | 3.4 (57) | 1.4 (39) | 3.7 (27)1-164 | 11.6 (38) | 9.9 (46) | ||
| 738 | 0.36 (12) | 4.6 (62) | 3.8 (39) | 8.0 (37) | 3.5 (49) | 3.9 (52) | ||
| 5781-153 | 0 | 3.7 (44) | 3.8 (34) | 6.5 (39) | 7.8 (41) | 5.9 (57) | ||
| 6311-153 | 0 | 2.8 (59) | 4.5 (52) | 6.1 (39) | 6.0 (45) | 5.8 (54) | ||
| Group 4: Parasitemia level lower than 5%; severe anemia | 5951-153,1-154 | 0.31 (18) | 22 | 3.0 (56) | 1.3 (51) | 2.2 (19)1-161 | 6.4 (27) | 6.5 (49) |
| 6001-153 | 3.10 (21) | 22 | 4.1 (54) | 3.7 (52) | 3.9 (24)1-161 | 18.4 (34) | 7.1 (50) | |
| 6061-153 | 0.14 (19) | 22 | 3.5 (60) | 3.3 (49) | 1.8 (20)1-161 | dead | dead | |
| 26121-153,1-159 | 0.35 (21) | 24 | 2.9 (52) | 2.7 (45) | 4.4 (29) | 7.1 (29) | 6.6 (41) | |
| 5851-154 | 0.35 (12) | 16 | 6.2 (57) | 2.6 (30) | 8.6 (20) | 8.8 (36) | 10.4 (46) | |
| 604 | 0.20 (16) | 19 | 3.9 (57) | 2.5 (41) | 9.4 (23) | 7.4 (48) | 4.7 (54) | |
| Group 5: Parasitemia level equal to or greater than 5%; severe anemia | 6781-154 | 9.70 (14) | 14 | 5.3 (58) | 7.1 (22)1-161 | 11.2 (40) | 10.1 (59) | 5.6 (63) |
| Response to infection . | Aotusno. . | Peak parasitemia % (day)† . | Day of treatment‡ . | Reticulocyte %*(Hematocrit %) . | ||||
|---|---|---|---|---|---|---|---|---|
| Day 7 (8) . | Day 14 (15) . | Day 21 (22) . | Day 29 (29) . | Day 36 (36) . | ||||
| Group 1: No microscopically detectable parasitemia; no anemia | 5231-153 | 0 | 2.8 (58) | 7.8 (53) | 1.5 (49) | 6.5 (56) | 4.9 (63) | |
| 5301-153 | 0 | 6.8 (64) | 5.9 (60) | 3.4 (64) | 2.3 (66) | 4.9 (68) | ||
| 5591-153,1-154 | 0 | 4.0 (58) | 3.5 (49) | 3.3 (46) | 6.3 (57) | 2.6 (61) | ||
| 6031-153 | 0 | 3.8 (44) | 1.5 (43) | 1.6 (44) | 2.3 (41) | 3.8 (40) | ||
| 7321-153 | 0 | 3.3 (62) | 4.2 (57) | 2.4 (50) | 12.7 (43) | 5.6 (52) | ||
| 546 | 0 | 5.1 (60) | 2.4 (56) | 5.2 (61) | 1.7 (66) | 2.5 (71) | ||
| 602 | 0 | 3.1 (65) | 2.7 (60) | 1.7 (54) | 3.0 (62) | 3.6 (68) | ||
| 680 | 0 | 2.4 (58) | 3.3 (57) | 4.8 (59) | 5.7 (62) | 3.0 (65) | ||
| 692 | 0 | 2.1 (59) | 5.5 (57) | 1.4 (60) | 1.3 (59) | 2.1 (57) | ||
| 747 | 0 | 2.8 (57) | 3.7 (51) | 3.4 (51)1-164 | 2.2 (50) | 7.6 (40) | ||
| 749 | 0 | 1.9 (53)1-160 | 3.0 (53) | 2.0 (50) | 1.9 (59) | 2.4 (53) | ||
| Group 2: No microscopically detectable parasitemia; positive by PCR; severe anemia (hematocrit lower than 25%) | 5671-153 | 0 | 22 | 2.9 (60) | 2.4 (52) | 2.1 (22)1-161 | 14.8 (28) | 10.8 (49) |
| 6571-153 | 0 | 15 | 4.4 (43) | 2.3 (20) | 16.4 (29) | 14.9 (37) | 11.1 (44) | |
| 6761-153 | 0 | 22 | 2.5 (56) | 1.5 (45) | 0.1 (19)1-161 | 20.8 (27) | 7.1 (48) | |
| 26111-153,1-159 | 0 | 21 | 9.6 (60) | 4.5 (45) | 0.0 (16)1-161 | 6.3 (43) | 1.5 (53) | |
| 557 | 0 | 17 | 3.5 (52) | 1.5 (29) | 17.9 (26) | 8.4 (18) | 4.9 (25) | |
| 7481-154 | 0 | 17 | 3.2 (55) | 2.9 (30) | 6.9 (22) | 7.9 (32) | 6.2 (50) | |
| Group 3: Parasitemia level lower than 5%; moderate anemia | 5131-153,1-154 | 3.61 (20) | 5.8 (57) | 2.1 (56) | 4.0 (34) | 11.3 (36) | 4.9 (47) | |
| 5601-153 | 0.41 (21) | 4.8 (62) | 3.5 (60) | 0.4 (48) | 24.5 (33) | 10.8 (55) | ||
| 5771-153 | 0.73 (21) | 5.0 (54) | 5.5 (53) | 1.9 (34) | 9.6 (49) | 3.4 (62) | ||
| 6271-153 | 0.14 (17) | 3.4 (57) | 1.4 (39) | 3.7 (27)1-164 | 11.6 (38) | 9.9 (46) | ||
| 738 | 0.36 (12) | 4.6 (62) | 3.8 (39) | 8.0 (37) | 3.5 (49) | 3.9 (52) | ||
| 5781-153 | 0 | 3.7 (44) | 3.8 (34) | 6.5 (39) | 7.8 (41) | 5.9 (57) | ||
| 6311-153 | 0 | 2.8 (59) | 4.5 (52) | 6.1 (39) | 6.0 (45) | 5.8 (54) | ||
| Group 4: Parasitemia level lower than 5%; severe anemia | 5951-153,1-154 | 0.31 (18) | 22 | 3.0 (56) | 1.3 (51) | 2.2 (19)1-161 | 6.4 (27) | 6.5 (49) |
| 6001-153 | 3.10 (21) | 22 | 4.1 (54) | 3.7 (52) | 3.9 (24)1-161 | 18.4 (34) | 7.1 (50) | |
| 6061-153 | 0.14 (19) | 22 | 3.5 (60) | 3.3 (49) | 1.8 (20)1-161 | dead | dead | |
| 26121-153,1-159 | 0.35 (21) | 24 | 2.9 (52) | 2.7 (45) | 4.4 (29) | 7.1 (29) | 6.6 (41) | |
| 5851-154 | 0.35 (12) | 16 | 6.2 (57) | 2.6 (30) | 8.6 (20) | 8.8 (36) | 10.4 (46) | |
| 604 | 0.20 (16) | 19 | 3.9 (57) | 2.5 (41) | 9.4 (23) | 7.4 (48) | 4.7 (54) | |
| Group 5: Parasitemia level equal to or greater than 5%; severe anemia | 6781-154 | 9.70 (14) | 14 | 5.3 (58) | 7.1 (22)1-161 | 11.2 (40) | 10.1 (59) | 5.6 (63) |
Treatment was given for hematocrit lower than 25% or parasitemia equal to or greater than 5%.
Normal reticulocyte count for Aotus monkeys: 2.9% ± 2.2% (arithmetic mean ± 2 SD deduced from 34 monkeys never exposed to malaria or vaccination).
0 = not microscopically detectable or only 1 or 2 parasites seen in 29 days.
All animals not previously treated were treated on day 29.
Immunized before first infection; all others immunized between first and second infections.
Vaccinated with control antigen GST; all others vaccinated with MSP-119.
A vociferans monkeys; all others were A nancymai.
Hematocrit % on day 5.
Hematocrit % on day 23.
Reticulocyte % determined on day of treatment.
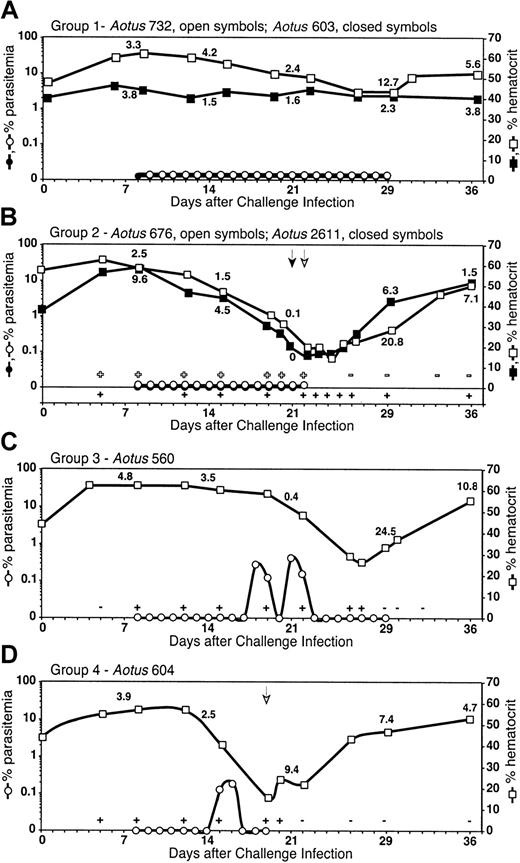
There was a wide spectrum of outcomes in parasitemia and anemia in the semi-immune Aotus monkeys in response to the second infection. Outcomes were categorized into 5 groups (Table 1). PCR carried out on blood samples collected from monkeys in groups 1, 2, and 3, which had no microscopically detectable parasitemia during the trial, confirmed that they were infected with malaria parasites. Eleven of 31 monkeys (group 1) did not develop detectable parasitemia or anemia, and the reticulocyte count remained steady (eg,Aotus 603, Figure 1A), with some exceptions (eg, Aotus 732, Figure 1A). Seven of 31 monkeys developed low-level (lower than 5%) to undetectable levels of parasitemia and became moderately anemic but were able to self-resolve the infection and the anemia (group 3; eg, Aotus 560, Figure1C). Twelve of 31 monkeys were able to control parasitemia to microscopically undetectable (6 monkeys; group 2; eg, Aotus676 and 2611, Figure 1B) or low (6 monkeys; group 4; eg,Aotus 604, Figure 1D) parasitemia levels, became severely anemic, and were treated with antimalarial drugs because of concern for the survival of the monkeys. One monkey had a high parasitemia level and became severely anemic (group 5).
Parasitemia, hematocrit, and reticulocyte percentage of
P falciparum–infected Aotus monkeys over the course of infection. (A) Group 1: monkeys did not develop a microscopically detectable parasitemia, did not become anemic, and reticulocyte production remained low and steady in most cases (eg, Aotus 603), with some exceptions (eg, Aotus 732). (B) Group 2: monkeys did not develop a microscopically detectable parasitemia but were positive by PCR, developed severe anemia (hematocrit less than 25%), and reticulocyte production stopped. Reticulocyte production and hematocrit level greatly increased after parasites were cleared by drug cure (eg, Aotus 676 and 2611). (C) Group 3: monkeys developed low-level (below 5%) to undetectable parasitemia positive by PCR, became moderately anemic, and reticulocyte production greatly decreased. Monkeys were able to self-resolve the infection and the anemia with increased reticulocyte production once parasites were cleared (eg, Aotus 560). (D) Group 4: monkeys developed low-level parasitemia, became severely anemic, and reticulocyte production decreased. Reticulocyte production and hematocrit level greatly increased after parasites were cleared by drug cure (eg,Aotus 604). Individual examples shown here are representative of the group. ± indicates PCR positive/negative forP falciparum DNA; arrow, antimalaria drug cure administered to monkey. All untreated monkeys received antimalarial drug cure on day 29, the last day of the trial. Numbers in graphs, reticulocyte percentage, taken on days 7, 14, 21, 29, and 36.
Parasitemia, hematocrit, and reticulocyte percentage of
P falciparum–infected Aotus monkeys over the course of infection. (A) Group 1: monkeys did not develop a microscopically detectable parasitemia, did not become anemic, and reticulocyte production remained low and steady in most cases (eg, Aotus 603), with some exceptions (eg, Aotus 732). (B) Group 2: monkeys did not develop a microscopically detectable parasitemia but were positive by PCR, developed severe anemia (hematocrit less than 25%), and reticulocyte production stopped. Reticulocyte production and hematocrit level greatly increased after parasites were cleared by drug cure (eg, Aotus 676 and 2611). (C) Group 3: monkeys developed low-level (below 5%) to undetectable parasitemia positive by PCR, became moderately anemic, and reticulocyte production greatly decreased. Monkeys were able to self-resolve the infection and the anemia with increased reticulocyte production once parasites were cleared (eg, Aotus 560). (D) Group 4: monkeys developed low-level parasitemia, became severely anemic, and reticulocyte production decreased. Reticulocyte production and hematocrit level greatly increased after parasites were cleared by drug cure (eg,Aotus 604). Individual examples shown here are representative of the group. ± indicates PCR positive/negative forP falciparum DNA; arrow, antimalaria drug cure administered to monkey. All untreated monkeys received antimalarial drug cure on day 29, the last day of the trial. Numbers in graphs, reticulocyte percentage, taken on days 7, 14, 21, 29, and 36.
In most monkeys that developed moderate or severe anemia, hematocrit decreased rapidly over 7 days or less. Reticulocyte counts were low despite the anemia, and the monkeys only began to undergo reticulocytosis after parasite clearance (either by antimalaria drug treatment or self-cure) as determined by PCR. Some monkeys with chronic, low-level parasitemia (lower than 5%) (groups 3 and 4) or with microscopically undetectable but PCR-positive parasitemia (groups 2 and 3) had marked decreases in hematocrit, but reticulocyte levels remained low and steady or decreased despite the anemia. Some monkeys in group 4, and all the monkeys in group 2, that required drug cure did not have microscopically detectable parasites in the peripheral blood on the day of cure but were PCR positive. This bone marrow dysfunction was clearly related to the malarial infection because the reticulocyte level markedly increased a few days after parasite clearance, either through self-resolution by the monkey (as determined by PCR) (group 3) or through antimalarial drug cure (groups 2 and 4). Hematocrit recovered to prechallenge levels within 1 to 2 weeks except in one monkey (monkey 557), which remained anemic despite marked reticulocytosis.
Direct destruction of parasitized RBCs cannot account for the anemia experienced by the monkeys that had low-level or microscopically undetectable parasitemia. Evidence that the anemia was caused by malaria was based on the observation that the hematocrit usually rose rapidly after treatment or self-cure. Possible causes for this severe anemia in monkeys with extremely low parasitemia include inhibition of erythropoiesis, destruction of uninfected RBCs, and sequestration of uninfected RBCs. It has been suggested that the inhibition of erythropoiesis may result from hematopoiesis-suppressive cytokine tumor necrosis factor-α and interferon-γ release in the bone marrow of anemic children because of their immune response to the malaria parasite.16 The rapid decrease in hematocrit can be explained by RBC removal in monkeys with bone marrow dysfunction. It has been hypothesized that the premature removal of uninfected RBCs results from changes of the RBC surface, such as loss of complement-regulatory RBC surface proteins, leading to complement-mediated lysis of uninfected RBCs17 or to antibody attachment to parasite antigens coating nonparasitized RBCs, leading to erythrophagocytosis.18-20 Despite the seriousness of this common complication of malaria, the mechanisms of malarial anemia are poorly understood, and a laboratory model, ideally a nonhuman primate model, is needed for a mechanistic approach to investigating malarial anemia. The semi-immune Aotus model system results in a variety of outcomes of infection, from protection to severe anemia, and it would provide the opportunity to qualitatively compare, identify, and evaluate the relative importance of the mechanisms of anemia and to investigate the detailed pathways involved in producing anemia.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Andrea F. Egan, Fogarty International Center, Rm 31/B2C39, MSC 2220, National Institutes of Health, 31 Center Dr, Bethesda, MD 20892; e-mail: egana@mail.nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal