A national prospective study was designed to collect all cases of lymphoma appearing in patients with rheumatoid arthritis (RA) treated with methotrexate (MTX) throughout France over a period of 3 years. A total of 25 cases of lymphoma were recorded, 18 cases of non-Hodgkin lymphoma (NHL), 3 of which were associated with the presence of Epstein-Barr virus (EBV) in lymphoma cells, and 7 cases of Hodgkin disease (HD), 5 of them associated with EBV. Among the 8 patients who were treated by MTX withdrawal alone, 3 underwent remission, but 2 of them had a relapse, the third patient with clonal EBV-associated large granular lymphocytes T-cell NHL remaining alive in complete remission. The estimated annual incidence rate of NHL in RA patients treated with MTX was 33.3.10−5 (0-80.5) among men and 16.7.10−5 (0-33.3) among women. There was no significant excess with the French population as a comparison: the standardized mortality ratio (SMR) adjusted for age and sex was 1.07 (0.6-1.7). The estimated annual incidence rate of HD among men and women was, respectively, 27.8.10−5 (0-70.1) and 2.8.10−5(0- 9.6). The incidence of HD was significantly increased compared with the French incidence, with an SMR adjusted for age and sex of 7.4 (3.0-15.3; P < .001). Thus, this 3-year prospective study indicated that, whereas the risk of NHL was not significantly increased in RA patients treated with MTX, the incidence of HD appeared to be higher in these patients compared to the French population.
Introduction
Several studies have suggested an increased risk of lymphoma in patients with rheumatoid arthritis (RA).1-3Recently, it was shown that RA patients treated with methotrexate (MTX) can develop lymphoproliferative disorders that share characteristics with the lymphomas occurring in immunosuppressed patients; the Epstein-Barr virus (EBV) genome was present in lymphoma cells and spontaneous regression was possible after withdrawal of the drug alone.4-8 MTX, now the most common disease-modifying antirheumatic drug (DMARD) used in RA, was, however, introduced 20 years ago and no excessive risk of lymphoma has been observed in one retrospective study9 and in several longitudinal studies of patients receiving MTX, even after long-term follow-up.10-13 However, these studies included only several hundred patients, which might not be enough to detect an excessive risk of a rare event, which is the occurrence of lymphoma. A truly longitudinal study of thousands of patients treated with MTX during a long period was impossible. Therefore, we decided to design a national prospective study, through an organization linking the 61 French departments of rheumatology, to collect all new cases of lymphoma appearing in French patients with RA treated with MTX over a period of 3 years. The objectives were to characterize these lymphomas, to assess their outcome after several years, and to estimate their incidence as compared to the general population.
Patients, materials, and methods
Study design
A prospective national study was initiated in 1995 by the Club Rhumatismes et Inflammation (CRI), which is a subcommittee of the French Society of Rheumatology. The objective was to collect all new cases of lymphoma appearing in French RA patients treated with MTX over a period of 3 years (1996, 1997, and 1998). The 61 French rheumatology departments were contacted and all agreed to participate. During the study, each of these departments consulted every 6 months the local oncology and hematology departments where they normally refer patients. All the centers were contacted by mail every 6 months during both the 3-year study and the follow-up of patients with lymphoma. A diagnosis of RA was made according to the American College of Rheumatology criteria.14 All cases were reviewed by 2 senior rheumatologists (X.M. and J.S.) to confirm the diagnosis of RA, and the patients with lymphoma were followed for a mean duration of 34 months.
Histology
Histologic analyses.
A paraffin-embedded fixed specimen from each case was collected and reviewed by one pathologist (D.C.-H.). The aims of this review were to confirm the diagnosis of lymphoma and to classify non-Hodgkin lymphoma (NHL) according to the Revised European and American Lymphoma (REAL) classification15 and Hodgkin disease (HD) according to the Rye system.16 Histologic samples had been fixed in formalin and were analyzed after hematoxylin and eosin, Giemsa, and periodic-acid-Schiff staining and Gordon-Sweet impregnation. In all cases, the immunohistochemistry was determined by means of a 3-step indirect immunoperoxidase method followed by the diaminobenzidine (DAB) reaction, using a panel of primary antibodies against CD3, CD20/L26, κ and λ immunoglobulin, CD30 (BerH2), and CD15 (LeuM1).
Detection of EBV.
The presence of the EBV genome was systematically investigated by conventional RNA in situ hybridization methods17 using fluorescein-conjugated EBV oligonucleotides (EBER1 and EBER2, Dako, Glostrup, Denmark), and by immunohistochemistry against LMP-1 (EBV latent membrane protein, clone CS1-4, Dako). In one case of leukemic T-cell lymphoma, polymerase chain reaction (PCR) amplification of the EBNA1 region was used to detect EBV.
Detection of clonality.
Clonality was assessed by immunohistochemistry against κ and λ immunoglobulin light chains and by conventional RNA in situ hybridization methods. In a case of leukemic T-cell lymphoma, PCR of the Vγ rearrangement of the T-cell receptor (TCR) gene was performed.
Epidemiology and statistical analyses
Comparison of patients with NHL and HD.
Differences in clinical characteristics between patients with NHL and patients with HD were assessed by the Wilcoxon rank sum test (for continuous variables) or the χ2 test corrected by Yates or Fisher exact test (for categorical variables). Overall survival was calculated by the Kaplan-Meier method. The statistical significance of differences in outcome between patients with NHL and patients with HD was assessed by the log-rank test and all tests of significance were 2-tailed. A hazard ratio was estimated with its 95% CI.
Comparison of NHL and HD incidence rates with the French national incidence rates.
We could assume to have collected most if not all the cases of lymphoma occurring in RA patients treated with MTX in France over the study period. However, no direct data were available for the total number of RA patients treated by MTX in France during the study period. We used several sources of data to estimate this number.
A first estimation was provided from surveys conducted among rheumatologists. Two independent surveys based on cases identified by rheumatologists in 2 different French areas18,19 led to an estimation of 0.17% for the prevalence of RA in France in persons over 18 years of age in the 1990s. This represented a total of 78 000 RA patients in France, because the French population aged over 18 years was 46 million in 1998 (Bilan démographique 1998. Bulletin mensuel de statistiques, INSEE, 1999). Two surveys of consecutive cases seen by rheumatologists in private practice showed a similar result for the percentage of RA patients treated with MTX in France: 35%21 and 38%.22 This first calculation led to a total of 78 000 × 0.38 = 29 640 cases of RA patients treated by MTX. The mean age of RA patients treated with MTX in France was 57 years with a female-male ratio of 4:1.22
These surveys, conducted among rheumatologists, probably underestimate the RA prevalence and overestimate the percentage of RA patients treated with MTX because MTX is prescribed almost exclusively by rheumatologists. Then, a second estimation was provided from a recent telephone cross-sectional survey of the adult general population of Brittany, a large region in western France. After adjustment for the French population, the RA prevalence was 0.50%, that is, a total of 230 000 RA patients in France. However, the proportion of RA patients treated with MTX was only 12% in this population (A. Saraux, unpublished data, April 2001), which is explained by the fact that MTX is prescribed for RA almost exclusively by rheumatologists. The total obtained from this population-based survey then was 230 000 × 0.12 = 27 600 RA patients treated with MTX, very close to the estimation obtained from rheumatologist sources.
The consistency of estimates obtained by independent surveys in various target populations was a strong argument for their validity. We then estimated incidence rate of NHL and HD with 95% CIs, using for the number of person-times the lowest and the highest estimation of RA patients treated by MTX, that is, 27 000 and 30 000. The MTX-treated RA population during the 3 years of our study was likely to be stable because MTX was not the favorite first-line treatment in early RA at this period (between 1996 and 1998). Some new patients should have been treated during this period but others should have stopped because of failure or of adverse events.
Morbidity excesses and deficits were expressed as standardized mortality ratios (SMRs). The SMR is the number of observed NHL or HD in our study divided by the number of NHL or HD that would be expected if the incidence rate of the French population were in effect. This expected number is adjusted for age and sex of the RA population treated with MTX obtained from the national survey among rheumatologists with a ratio of 1:4 for men to women.22The 5-year age class annual NHL and HD incidence rates were published from French registries.23 The 95% CIs for each SMR were calculated and its deviation from 1.00 was tested assuming a Poisson distribution for the observed NHL and HD.24
Results
Characteristics of the patients
During the 3 years of the study, 25 new cases of lymphoma were recorded among RA patients treated with MTX in France. At the time of lymphoma diagnosis, the mean age of the patients was 63 years (range, 39-82 years), the mean duration of RA was 16.2 years (range, 25-49 years) and the mean duration of MTX treatment was 5.2 years (range, 1.4-13 years) with a mean cumulative dose of 2.2 g (range, 0.5-5.2 g). RA was erosive and rheumatoid factor positive in 24 of the 25 patients, whereas secondary Sjögren syndrome was present in only 2. There was no concomitant DMARD except in 4 cases (cyclosporine, azathioprine, hydroxychloroquine, and tiopronine, respectively). No patient had hepatitis C or human immunodeficiency virus infection (tested in all patients). As seen in Table1, extranodal locations of lymphoma were frequent and observed in 13 patients (52%; 12 of 18 NHL and 1 of 7 HD). A comparison between patients with NHL and patients with HD is given in Table 2.
Characteristics of the 25 non-Hodgkin and Hodgkin lymphomas associated with low-dose methotrexate in RA patients
| Patient . | Sex . | Age . | Lymphoma classification . | Extranodal location . | EBV . | Clonality . | Duration of RA (y) . | Duration of MTX (mo) . | Total dose of MTX (mg) . | Secondary Sjögren . | Associated CS or IS drugs . | Previous DMARD . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 75 | NHL T pleomorphic | − | − | UN | 6 | 24 | 1325 | − | CS | 0 |
| 2 | F | 67 | NHL large B cell | − | + | + | 10 | 63 | 3260 | − | Aza + CS | 3 |
| 3 | F | 71 | NHL large B cell | − | − | − | 22 | 120 | 5200 | − | CS | 4 |
| 4 | F | 74 | NHL MZ B cell | Stomach | − | UN | 27 | 84 | 2730 | − | CS | 1 |
| 5 | M | 67 | NHL large B cell | − | − | + | 15 | 144 | 4560 | + | CS | 3 |
| 6 | F | 64 | NHL large B cell | Bone | − | + | 10.5 | 69 | 3085 | − | CS | 6 |
| 7 | F | 44 | NHL large B cell | Orbit | − | UN | 8 | 63 | 2000 | − | HCQ | 1 |
| 8 | F | 70 | NHL large B cell | Stomach | + | UN | 29 | 31 | 1500 | − | Cyclo + CS | 5 |
| 9 | M | 58 | NHL T LGL | Bone marrow | + | + | 2.5 | 6 | 300 | + | CS | 1 |
| 10 | F | 51 | NHL large B cell | Pleura | − | + | 7 | 40 | 1700 | − | − | 0 |
| 11 | F | 73 | NHL MZ B cell | Lung | − | + | 49 | 16 | 865 | − | CS | 2 |
| 12 | M | 67 | NHL large B cell | − | − | UN | 25 | 84 | 2000 | − | − | 3 |
| 13 | F | 69 | NHL large B cell | Skin | − | + | 22 | 156 | 5100 | − | CS | 6 |
| 14 | M | 68 | NHL B lymphocytic | − | − | + | 7 | 21 | 944 | − | CS | 6 |
| 15 | F | 66 | NHL MZ B cell | Skin Orbit | − | + | 3.9 | 16 | 517 | − | CS | 1 |
| 16 | F | 69 | NHL large B cell | Bone | − | UN | 21 | 60 | 1630 | − | CS | 2 |
| 17 | F | 64 | NHL large B cell | Lung | − | + | 24 | 69 | 2250 | − | − | 2 |
| 18 | M | 52 | NHL large B cell | Thyroid | − | UN | 11 | 96 | 4800 | − | CS | 2 |
| 19 | M | 65 | HD (II, type 2) | − | − | 16 | 60 | 2600 | − | − | 3 | |
| 20 | M | 55 | HD (III, type 3) | − | − | 24 | 72 | 4600 | − | CS | 3 | |
| 21 | M | 46 | HD (III, type 3) | − | + | 12 | 42 | 1900 | − | CS | 2 | |
| 22 | F | 82 | HD (III, type 3) | − | + | 11 | 40 | 1115 | − | CS | 2 | |
| 23 | F | 55 | HD (III, type 2) | − | + | 29 | 72 | 2500 | − | CS | 4 | |
| 24 | M | 39 | HD (III, type 3) | − | + | 5 | 16 | 585 | − | Tiop | 2 | |
| 25 | M | 43 | HD (IV) | Liver | + | 10 | 38 | 1800 | − | CS | 3 |
| Patient . | Sex . | Age . | Lymphoma classification . | Extranodal location . | EBV . | Clonality . | Duration of RA (y) . | Duration of MTX (mo) . | Total dose of MTX (mg) . | Secondary Sjögren . | Associated CS or IS drugs . | Previous DMARD . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 75 | NHL T pleomorphic | − | − | UN | 6 | 24 | 1325 | − | CS | 0 |
| 2 | F | 67 | NHL large B cell | − | + | + | 10 | 63 | 3260 | − | Aza + CS | 3 |
| 3 | F | 71 | NHL large B cell | − | − | − | 22 | 120 | 5200 | − | CS | 4 |
| 4 | F | 74 | NHL MZ B cell | Stomach | − | UN | 27 | 84 | 2730 | − | CS | 1 |
| 5 | M | 67 | NHL large B cell | − | − | + | 15 | 144 | 4560 | + | CS | 3 |
| 6 | F | 64 | NHL large B cell | Bone | − | + | 10.5 | 69 | 3085 | − | CS | 6 |
| 7 | F | 44 | NHL large B cell | Orbit | − | UN | 8 | 63 | 2000 | − | HCQ | 1 |
| 8 | F | 70 | NHL large B cell | Stomach | + | UN | 29 | 31 | 1500 | − | Cyclo + CS | 5 |
| 9 | M | 58 | NHL T LGL | Bone marrow | + | + | 2.5 | 6 | 300 | + | CS | 1 |
| 10 | F | 51 | NHL large B cell | Pleura | − | + | 7 | 40 | 1700 | − | − | 0 |
| 11 | F | 73 | NHL MZ B cell | Lung | − | + | 49 | 16 | 865 | − | CS | 2 |
| 12 | M | 67 | NHL large B cell | − | − | UN | 25 | 84 | 2000 | − | − | 3 |
| 13 | F | 69 | NHL large B cell | Skin | − | + | 22 | 156 | 5100 | − | CS | 6 |
| 14 | M | 68 | NHL B lymphocytic | − | − | + | 7 | 21 | 944 | − | CS | 6 |
| 15 | F | 66 | NHL MZ B cell | Skin Orbit | − | + | 3.9 | 16 | 517 | − | CS | 1 |
| 16 | F | 69 | NHL large B cell | Bone | − | UN | 21 | 60 | 1630 | − | CS | 2 |
| 17 | F | 64 | NHL large B cell | Lung | − | + | 24 | 69 | 2250 | − | − | 2 |
| 18 | M | 52 | NHL large B cell | Thyroid | − | UN | 11 | 96 | 4800 | − | CS | 2 |
| 19 | M | 65 | HD (II, type 2) | − | − | 16 | 60 | 2600 | − | − | 3 | |
| 20 | M | 55 | HD (III, type 3) | − | − | 24 | 72 | 4600 | − | CS | 3 | |
| 21 | M | 46 | HD (III, type 3) | − | + | 12 | 42 | 1900 | − | CS | 2 | |
| 22 | F | 82 | HD (III, type 3) | − | + | 11 | 40 | 1115 | − | CS | 2 | |
| 23 | F | 55 | HD (III, type 2) | − | + | 29 | 72 | 2500 | − | CS | 4 | |
| 24 | M | 39 | HD (III, type 3) | − | + | 5 | 16 | 585 | − | Tiop | 2 | |
| 25 | M | 43 | HD (IV) | Liver | + | 10 | 38 | 1800 | − | CS | 3 |
CS indicates corticosteroids; IS, immunosuppressive drugs; +, present; −, absent; UN, unknown; Aza, azathioprine; HCQ, hydroxychloroquine; Cyclo, cyclosporine; Tiop, tiopronine.
Comparison of clinical characteristics between patients with NHL and patients with HD
| . | NHL . | HD . | . |
|---|---|---|---|
| No. of patients | 18 | 7 | |
| Age (y) | 65 | 55 | P = .04 |
| % male | 33 | 71 | P = .20 |
| Duration RA (y) | 17 | 15 | NS |
| Dose MTX (g) | 2.4 | 2.2 | NS |
| Duration MTX (mo) | 65 | 49 | NS |
| EBV | 3/18 | 5/7 | |
| Median overall survival (mo) | Not reached | 27 | NS |
| Remission after MTX WD alone | 3/5 | 0/3 | |
| Long-term remission after MTX WD alone | 1/5 | 0/3 |
| . | NHL . | HD . | . |
|---|---|---|---|
| No. of patients | 18 | 7 | |
| Age (y) | 65 | 55 | P = .04 |
| % male | 33 | 71 | P = .20 |
| Duration RA (y) | 17 | 15 | NS |
| Dose MTX (g) | 2.4 | 2.2 | NS |
| Duration MTX (mo) | 65 | 49 | NS |
| EBV | 3/18 | 5/7 | |
| Median overall survival (mo) | Not reached | 27 | NS |
| Remission after MTX WD alone | 3/5 | 0/3 | |
| Long-term remission after MTX WD alone | 1/5 | 0/3 |
NS indicates not significant; WD, withdrawal.
Pathology
The 25 new cases of lymphoma comprised 18 NHL and 7 HD (Table 1). The 18 NHL cases consisted of 2 T-cell NHL (one pleomorphic small and medium T-cell lymphoma and one large granular lymphocyte [LGL] T-cell lymphoma) and 16 B-cell NHL (12 diffuse large B-cell lymphomas, 3 marginal zone B-cell lymphomas [MZLs] and one lymphocytic B-cell lymphoma).
Clonality, assessed in 8 of the 16 B-cell NHL by immunohistochemistry or in situ hybridization, revealed in all studied cases a restrictive expression of κ or λ chains in the tumoral lymphoid cells. Lastly, the LGL T-cell lymphoma was monoclonal by PCR analysis of the Vγ-Jγ region.
In NHL, EBV was detected by immunohistochemistry with anti-LMP antibodies and in situ hybridization with the EBER probes in 2 of the 12 diffuse large B-cell NHLs, whereas no low-grade B-cell NHL expressed EBV. PCR amplification of the EBNA1 gene of EBV was strongly positive in peripheral blood lymphocytes of the patient with LGL T-cell lymphoma.
The 7 cases of HD were subclassified as follows: 2 of the nodular sclerosis type, 4 of the mixed cellularity type, and 1 unclassified case due to extranodal involvement (liver). Hodgkin cells expressed CD30 in all cases, CD15 in 6 of 7 cases, and CD20 in 1 case, whereas EBV was detected in 5 of the 7 patients.
Outcome
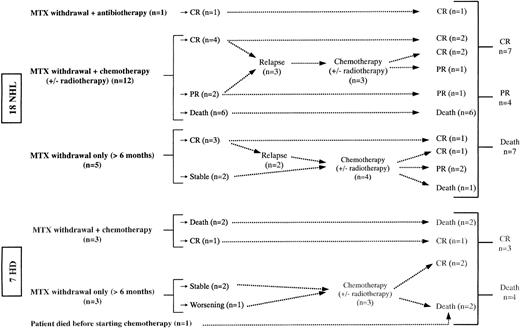
The outcome for the patients is shown in Figure1. MTX therapy was stopped in all patients but one. In 8 patients (5 with NHL, 3 with HD), MTX was withdrawn without any specific treatment of the lymphoma for at least 6 months. Four cases remained stable (2 HD, 1 large B-cell NHL, and 1 lymphocytic lymphoma), 1 patient with HD worsened, and all 5 subsequently received chemotherapy. Among 3 patients who achieved initial spontaneous remission, 2 had relapse at 12 and 14 months and were given further chemotherapy (1 large B-cell NHL and 1 MZL, respectively). The third patient with LGL T-cell NHL is still alive in complete remission (CR) at 41 months of follow-up. Two of these 3 lymphomas were EBV+ (1 large B-cell NHL and the LGL T-cell NHL), and all of them were monoclonal.
Follow-up and outcome of the 25 MTX-associated lymphomas in RA patients.
Mean 34 months (25-54). CR indicates complete remission; PR, partial remission; NHL, non-Hodgkin lymphoma; HD, Hodgkin disease.
Follow-up and outcome of the 25 MTX-associated lymphomas in RA patients.
Mean 34 months (25-54). CR indicates complete remission; PR, partial remission; NHL, non-Hodgkin lymphoma; HD, Hodgkin disease.
One patient with mediastinal HD died before starting chemotherapy. One with Helicobacter pylori–associated gastric mucosa-associated lymphoid tissue (MALT) lymphoma (extranodal MZL) was successfully treated with antibiotics and is still alive in CR. The remaining patients received chemotherapy or radiotherapy or both.
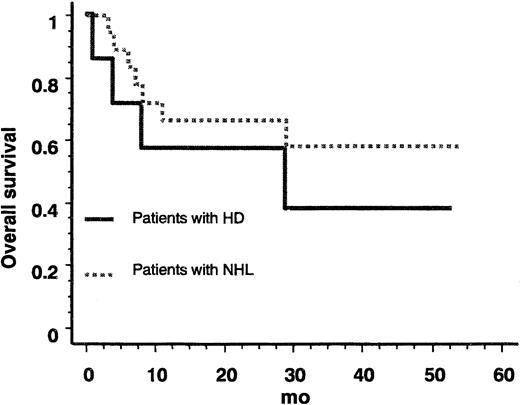
The median follow-up of surviving patients was 34 months (range, 25-54 months). The death-free survival rate was plotted for NHL and HD (Figure 2). The 2 survival curves did not significantly differ (P = .42, log-rank test) with an estimated hazard ratio of 1.6 (95% CI, 0.5-5.6). In patients with HD, the median survival was 27 months. Eleven of the 25 patients died (4 of 7 HD, 1 of 2 T-cell NHL, 0 of 4 low-grade malignancy B-cell NHL, and 6 of 12 large B-cell NHL). Among the 14 patients still alive, 10 are in CR and 4 in partial remission (PR).
Overall survival of patients with MTX-associated lymphoma.
NHL indicates non-Hodgkin lymphoma; HD, Hodgkin disease.
Overall survival of patients with MTX-associated lymphoma.
NHL indicates non-Hodgkin lymphoma; HD, Hodgkin disease.
Analysis of relapses
During the mean follow-up of 34 months, 5 relapses were observed after an initial remission: 2 in patients treated by withdrawal of MTX alone and 3 in patients given chemotherapy. In all cases, the histologic pattern was similar at relapse and diagnosis: 4 cases of large B-cell NHL and 1 case of MZL B-cell NHL. Clonality was demonstrated in the 5 B-cell NHLs at both diagnosis and relapse, while in the patient with EBV+ large B-cell NHL who relapsed, EBV was also detected at relapse.
Epidemiology
In the present study, we observed 18 new cases of NHL in 3 years (6 every year): 12 in women and 6 in men. Thus, the observed annual incidence of NHL in RA women and men treated with MTX was 4/24 000 = 16.7 × 10−5 (95% CI, 0-33.3) and 2/6000 = 33.3 × 10−5 (95% CI, 0-80.5), respectively, using the highest estimation of RA patients treated with MTX (n = 30 000; Table 3). This observed annual incidence of NHL did not significantly exceed the annual incidence of NHL in France23 after adjustment for age and sex: SMR = 1.07 (0.6-1.7).
Estimation of NHL and HD incidence rates and SMRs with French population as a comparison, adjusted for age and sex
| . | Lowest estimation of number of RA patients treated by MTX3-150 (N = 27 000) . | Highest estimation of number of RA patients treated by MTX3-151 (N = 30 000) . | ||
|---|---|---|---|---|
| . | 95% CI . | . | 95% CI . | |
| NHL | ||||
| Incidence rate (per 100 000 person-years) | ||||
| Among men | 37.0 | (0 -89.4) | 33.3 | (0 -80.5) |
| Among women | 18.5 | (0 -37.0) | 16.7 | (0 -33.3) |
| Total | 22.2 | (4.1-40.4) | 20.0 | (3.7-36.3) |
| Comparison with the French national incidence | ||||
| Observed (expected) | 18 (15.1) | 18 (16.7) | ||
| SMR, standardized for age and sex | 1.2 | (0.7 -1.9) | 1.07 | (0.6 -1.7) |
| HD | ||||
| Incidence rate per 100 000 person-years) | ||||
| Among men | 30.9 | (0 -78.7) | 27.8 | (0 -70.8) |
| Among women | 3.1 | (0 -10.6) | 2.8 | (0 -9.6) |
| Total | 8.6 | (0 -20.0) | 7.8 | (0 -18.0) |
| Comparison with the French national incidence | ||||
| Observed (expected) | 7 (0.8) | 7 (0.9) | ||
| SMR, standardized for age and sex (95% CI) | 3-1508.2 | (3.3-17.0) | 3-1507.4 | (3.0-15.3) |
| . | Lowest estimation of number of RA patients treated by MTX3-150 (N = 27 000) . | Highest estimation of number of RA patients treated by MTX3-151 (N = 30 000) . | ||
|---|---|---|---|---|
| . | 95% CI . | . | 95% CI . | |
| NHL | ||||
| Incidence rate (per 100 000 person-years) | ||||
| Among men | 37.0 | (0 -89.4) | 33.3 | (0 -80.5) |
| Among women | 18.5 | (0 -37.0) | 16.7 | (0 -33.3) |
| Total | 22.2 | (4.1-40.4) | 20.0 | (3.7-36.3) |
| Comparison with the French national incidence | ||||
| Observed (expected) | 18 (15.1) | 18 (16.7) | ||
| SMR, standardized for age and sex | 1.2 | (0.7 -1.9) | 1.07 | (0.6 -1.7) |
| HD | ||||
| Incidence rate per 100 000 person-years) | ||||
| Among men | 30.9 | (0 -78.7) | 27.8 | (0 -70.8) |
| Among women | 3.1 | (0 -10.6) | 2.8 | (0 -9.6) |
| Total | 8.6 | (0 -20.0) | 7.8 | (0 -18.0) |
| Comparison with the French national incidence | ||||
| Observed (expected) | 7 (0.8) | 7 (0.9) | ||
| SMR, standardized for age and sex (95% CI) | 3-1508.2 | (3.3-17.0) | 3-1507.4 | (3.0-15.3) |
On the other hand, we observed 7 new cases of HD in 3 years (2 in 1996, 3 in 1997, and 2 in 1998), 2 in women and 5 in men. Thus, the observed annual incidence of HD in RA women and men treated with MTX was 0.67/24 000 = 2.8 × 10−5 (95% CI, 0-9.6) and 1.67/6000 = 27.8 × 10−5 (95% CI, 0-70.8), respectively. This annual incidence of HD in RA patients treated with MTX was significantly higher than the French annual incidence after adjustment for age and sex: SMR = 7.4 (95% CI, 3.0-15.32;P < .001).
Using a male-female ratio of 1:12 among RA patients treated with MTX instead of 1:4 did not change conclusions with SMRs, adjusted for age and sex, equal to 1.0 (95% CI, 0.6-1.5) and 6.5 (95% CI, 2.6-13.4) for NHL and HD, respectively. Using the lowest estimation of the number of RA patients (n = 27 000) also led to similar conclusions (Table 3).
Discussion
This 3-year prospective study is the first prospective study of the literature recording all the cases of lymphomas occurring in RA patients treated with MTX in a whole country. This enabled us to define the characteristics of the 25 collected lymphomas, their outcome, and, because we could assume to have collected most if not all the cases in France over the study period, to estimate the incidence of this complication in RA patients treated with MTX. In addition, the 3-year follow-up of the patients gives precious indications on the long-term prognosis of such lymphomas.
The histologic patterns of the observed lymphomas were heterogeneous: 18 NHL (16 B-cell NHL and 2 T-cell NHL) and 7 HD. All the NHL that could be studied were monoclonal (tested in 9 of 18). Extranodal locations of NHL were frequent (12 of 18, 67%) and several marginal zone-type NHLs were observed (3 cases), as in the lymphomas occurring in patients with Sjögren syndrome25 or chronic hepatitis C virus infection.26 Interestingly, in both these diseases, a chronic autoantigenic stimulation has been suspected to play a role in the etiology of lymphoma.27 However, the frequency of secondary Sjögren syndrome was not increased in the present RA patients with lymphoma and this complication was found in none of the 3 patients with MALT lymphoma. Thus, whether treated or not with MTX, RA could represent another disease in which a chronic autoantigenic stimulation might lead to an increased risk of lymphoma.
It has been known since 1993 that some lymphoproliferative disorders appearing in patients treated with MTX were associated with EBV and might spontaneously resolve after withdrawal of the drug, like the lymphoproliferations occurring in immunosuppressed patients.4 These results are particularly interesting, because EBV is frequently found in the synovium of RA patients28 and these patients could have impaired immunologic control of EBV.29 As compared to populations with no immunodeficiency, an association with EBV could be slightly more frequent in our patients: EBV was detected in 5 of 7 HD patients, in 2 of 12 with diffuse large B-cell NHL, and in 1 with LGL T-cell NHL. This latter patient is of particular interest because LGL T-cell NHL, which can be associated with untreated RA, has recently been described in immunosuppressed renal allograft patients,30 but has only very rarely been associated with EBV.30-32Moreover, this patient with monoclonal LGL T-cell NHL is the only patient in stable remission more than 3 years after MTX withdrawal without other treatment. Two other patients with monoclonal B-cell NHL experienced remission after MTX withdrawal alone, but had a relapse 12 to 14 months later. Among our patients with NHL, EBV was detected in lymphoma cells in 2 of 3 patients who experienced remission after MTX withdrawal alone, but in only 1 of the 15 others. Hence, like others,4-8 we suggest an association between the presence of EBV in lymphoma cells and spontaneous regression after withdrawal of the drug. Therefore, among lymphomas occurring in RA patients treated with MTX, there is probably a minor subset that can spontaneously resolve after withdrawal of the drug. Close monitoring of these patients is, however, mandatory because recurrence is frequent during follow-up. Overall, our findings are in accordance with a recent 6-year case-control study by Kamel et al (49 patients with NHL and RA), who found that the majority of NHL occurring in RA patients did not share characteristics with the lymphomas of immunosuppressed patients.33
Whether or not RA patients treated with MTX have an increased risk of developing lymphoma remains a matter of controversy. No excessive risk of lymphoma has been observed in several groups of patients receiving MTX, even after long-term follow-up.9-12 In a recent survey13 and in another smaller study,34 it was suggested that treatment with MTX did not have any effect on the occurrence of lymphoma, which was associated with a persistent inflammatory activity of the disease. In addition, it was recently demonstrated that a response to MTX was related to reduced mortality in patients with severe RA.35Because we could assume we had collected most of the cases of lymphoma appearing in RA patients treated with MTX in France, we estimated the annual incidence of NHL in these patients (16.7 × 10−5in women and 33.3 × 10−5 in men). No significant increased risk of NHL with the French NHL incidence was observed,23 with an SMR close to 1, after adjustment for sex and age. Unfortunately, no comparison was possible with RA patients not receiving MTX, because many such patients are followed in private practice in France without being referred to rheumatology departments. If the activity of the disease was one of the most important risk factors for lymphoma in RA patients,13 34 the lack of effect of MTX on this risk could result from 2 opposing factors: a reduction of the risk as MTX is one of the most efficient drugs to reduce the activity of RA, together with a slight increase of the risk of NHL due to immunosuppression.
Lastly, our study points to a high incidence of HD in RA patients treated with MTX. Interestingly, 5 of 7 patients had HD associated with EBV, whereas this association is usually present in only 40% of those with HD.36 These were typical cases of HD with a CD30+CD15+ phenotype and not lymphoproliferative disorders resembling HD, as previously described in some RA patients treated with MTX.37 Typical HD has, however, also been described in RA patients receiving MTX.37-40 It is noteworthy that in 2 cases in the literature the lymphoproliferation, which decreased after MTX withdrawal perhaps only because steroids were increased to control the activity of RA, recurred several months later.39 40 Among our 3 patients with HD who were treated with MTX withdrawal alone without any chemotherapy for at least 6 months, none of them experienced spontaneous remission.
In our study, the annual incidences of HD in RA patients treated with MTX were estimated to be 2.8 × 10−5 (95% CI, 0-9.6) in women and 27.8 × 10− (95% CI, 0-70.8) in men. The number of HD significantly exceeded the expected number based on the French national population, after adjustment for age and sex, with SMRs equal to 7.4 or 8.2, according to the estimation of RA patients treated with MTX (Table 3). These values were higher than the highest 5-year age class annual incidence in France (1.6-2.7 × 10−5 in women aged 50 to 64 years and 3.8-4.2 × 10−5 in men aged 50-64 years),23or in the United States (1.6-2.7 × 10−5 in women aged 50-64 years and 3.8-4.2 × 10−5 in men aged 50-64 years).41,42 It is now accepted that the occurrence of HD, most often in association with EBV, is more frequent in immunosuppressed patients, such as patients infected with human immunodeficiency virus.43 Cases of HD have been reported in RA patients treated with low-dose MTX, but this study is, to our knowledge, the first indicating that the frequency of HD could be increased in such patients. Moreover, the clinical characteristics of these patients could be different from those of RA with NHL. Our HD patients were younger, 55 years versus 65 years, P = .04, there was a higher proportion of men (71% versus 33%,P = .20), and the prognosis was poor, as in HD occurring in immunosuppressed patients, because the median overall survival was only 27 months. Indeed, the nonsignificant log-rank test between survival of HD and NHL was not informative because the power of the survey to demonstrate a hazard ratio of 2 is only 14%. Therefore, we cannot rule out the possibility that overall survival differs between the HD and NHL patients.
Several reasons argue for the consistency of our conclusions, concerning comparisons of NHL and HD incidence with French incidences. (1) The numbers of new cases of NHL and HD were not likely to have been overestimated because all cases were reviewed by the same pathologist. Moreover, the number of annual new cases were similar during the 3-year study. (2) Although the number of RA patients treated with MTX was not directly available, very similar estimations of number of persons-years (between 27 000 and 30 000) were obtained from multiple and independent sources. (3) The conclusions were similar by using the lowest and the highest of these estimations. (4) The conclusions were similar by using a male-female ratio of 1:4 women corresponding to the sex ratio of RA patients treated with MTX in France22 as well as using a male-female ratio of 1:12 corresponding to the lowest sex ratio of RA in the literature.
In conclusion, this 3-year prospective study in France indicates that the overall risk of NHL is not increased in RA patients treated with MTX. However, a minor subset of NHL occurring in these patients is probably linked to MTX treatment and may resolve after withdrawal of the drug alone. When possible, it might therefore be advisable to wait for 2 to 3 months after MTX withdrawal before starting chemotherapy. Close monitoring of these patients is nevertheless mandatory because recurrence is frequent during short-term follow-up. Finally, EBV-associated HD not resolving after withdrawal of MTX and requiring classical chemoradiotherapy could be more common in RA patients treated with MTX.
We are indebted to Prof J. P. Daurès for providing the sex and age distribution of French RA patients treated with MTX, to Prof A. Saraux, Pr J. Sany, and Dr B. Decot for valuable advice and comments in epidemiology and statistics, to Prof F. Stoll-Keller and Dr C. Pallier for EBV PCR, to Dr E. Guérin for PCR to detect T-cell clonality, and to S. Vo for technical assistance.
The practitioners of the CRI who referred patients were J. M. Berthelot (Nantes), P. Bertin (Limoges), M. Blanc (Chambéry), A. Cantagrel (Toulouse), D. Clerc (Le Kremlin-Bicêtre), B. Combe (Montpellier), M. Dougados (Paris), J. P. Eschard (Reims), J. P. Larbre (Lyon), P. Le Blay (Lorient), P. Le Goff (Brest), J. L. Le Quintrec (Paris), V. Lucas (La Roche sur Yon), P. Miossec (Lyon), X. Puéchal (Le Mans), H. Roux (Marseille), A. Saraux (Brest), J. Tebib (Lyon), E. Veillard (Rennes), and J. M. Ziza (Paris).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Xavier Mariette, Service de Rhumatologie, Hôpital de Bicêtre, 78 rue du Général Leclerc, 94275 Le Kremlin Bicêtre, France; e-mail:xavier.mariette@bct.ap-hop-paris.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal