Platelets can bind and phagocytose infectious microorganisms and so enable their transport for a prolonged time. To investigate the subcellular events of these interactions, platelets were incubated either with Staphylococcus aureus or with HIV and analyzed by electron microscopy (EM) and immuno-EM. HIV and bacteria internalization occurred exclusively within platelets showing morphological evidence of activation. Platelet activation enhanced the degree of bacterial internalization. Immunolabeling revealed that the engulfing vacuoles and the open canalicular system (OCS) were composed of distinct antigens. The engulfing vacuoles eventually became the site of prominent α-granule release. In platelets incubated with HIV, characteristic endocytic vacuoles were identified close to the plasma membrane, tightly surrounding 1 or 2 HIV particles. Virus particles were also located within the OCS. Immunogold labeling for the viral core protein p24 confirmed the presence of HIV within platelets. Finally, examination of platelets from a patient with acquired immunodeficiency syndrome and high viremia suggested that HIV endocytosis may also occur in vivo.
Introduction
Although the critical role of blood platelets in hemostasis and thrombosis is clearly recognized, their capacity to function during host defense against infection has received much less attention. Thrombocytopenia is often a severe complication during or after bacterial and viral infections. The mechanisms responsible appear to be multiple: increased platelet destruction, due either to the nonspecific deposition of circulating immune complexes on platelets or to the presence of specific antiplatelet antibodies, and decreased platelet production.1,2 More specifically, several studies have indicated that during infection with HIV, there is a direct interaction of HIV with megakaryocytes and platelets: mature megakaryocytic cells express CD4 along with HIV coreceptors CXCR1, 2, and 4 and CCR3 and 5 on their surface3-6 and are thus susceptible to HIV infection; productive infection of megakaryocytes by X4 and R5 HIV-1 isolates has also been shown7; and megakaryocytes from seropositive subjects have been found to contain viral RNA and antigens by in situ hybridization.3,8 These are arguments for decreased platelet production. HIV-1 RNA has also been detected in platelet preparations by reverse-transcriptase–polymerase chain reaction, and platelets that are washed, but depleted of leukocytes, appear to retain high concentration of tightly associated HIV messenger RNA.9 A direct interaction between platelets and HIV has also been shown.10 Moreover, platelets also express the HIV coreceptors (CXCR1, 2, and 4 and CCR 1, 3, and 4) on their surface.4 11
It is also conceivable that during bacterial infection, direct interaction between platelets and bacteria may contribute to the observed thrombocytopenia. Several studies performed in vitro on the interaction of platelets with Staphylococcus aureusindicated that the bacteria induce the platelet-release reaction and rapid irreversible platelet aggregation in the presence of normal plasma; in these experiments, internalization of S aureus by individual platelets occasionally occurred.12-15 It has also been shown that α-toxin, the major cytolysin of S aureus, promotes blood coagulation by its attack on human platelets.16 17 Thus, infectious thrombocytopenias are secondary to multiple and combined mechanisms, but each parameter deserves to be isolated and carefully studied individually.
Therefore, the aim of this study was to use electron microscopy (EM) and immuno-EM to fully elucidate the mechanism of direct platelet-microorganism interactions, in particular those associated with S aureus and HIV internalization by platelets. We also examined the potential role of platelet activation on the ability of platelets to internalize infectious microorganisms and documented the subcellular distribution of platelet antigens during the interaction of platelets with bacteria. The interaction of platelets with HIV was also investigated with specific immunogold labeling of internalized HIV, either in vitro or in vivo, within platelets of patients with AIDS and thrombocytopenia.
Materials and methods
Cells
Platelets were obtained from healthy donors by venipuncture into plastic tubes containing EDTA or acid-citrate-dextrose (ACD)–C buffer. The platelet-rich plasma was obtained by centrifugation for 15 minutes at 180g and 22°C. Isolated platelets were obtained by centrifugation of platelet-rich plasma for 5 minutes at 1100g and were washed 3 times with Tyrode buffer (360 mM citric acid, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 103 mM glucose, pH 7.4) containing 0.35% bovine serum albumin (Sigma Chemical, St Louis, MO). Washed platelets were resuspended in Hanks balanced salt solution (HBSS).
Platelets of a patient with AIDS, high plasma viremia, and thrombocytopenia were obtained by venipuncture into plastic tubes containing ACD-C, fixed in whole blood in 1.5% glutaraldehyde for 1 hour, and washed 3 times in 0.1 M phosphate buffer, pH 7.4.
Bacteria
Suspensions of S aureus were prepared from overnight broth cultures, centrifuged for 5 minutes at 1500g, and washed 2 times with HBSS. Bacteria were resuspended in HBSS and sonicated to disrupt bacterial clusters.
Platelet-bacteria interaction
Washed platelets (2 × 109/mL) were incubated withS aureus suspension with an excess of bacteria (1010/mL) at 37°C for 1 hour. The effect of platelet activation was also studied by preactivating the platelets with 10 μM adenosine 5′-diphosphate (ADP) for 30 minutes or 0.1 U/mL thrombin for 5 minutes, with appropriate controls. Cells were then fixed by the addition of glutaraldehyde up to a final concentration of 1% in 0.1 M phosphate buffer.
Human immunodeficiency virus
The HIV-1 particles were obtained from the supernatant of peripheral blood mononuclear cell (PBMC) culture of HIV-1–infected patients by centrifugation at 25 000g for 15 minutes and were resuspended in HBSS.
Platelet-HIV interaction
Washed platelets (2 × 109/mL) were directly incubated with a viral pellet with reverse-transcriptase activity of approximately 10 000 cpm.
Antibodies
A pool of 3 monoclonal antibodies against the core protein p24 HIV-1 was a generous gift from Dr H. R. Gelderblom (Berlin, Germany).18 Antihuman polyclonal rabbit antibodies anti-αIIbβ3,19antiglycocalicin (anti–glycoprotein Ib [anti-GPIb]),20 and anti–P-selectin (CD62p)21 were kindly provided by Dr Michael Berndt (Prahran, Victoria, Australia). Antifibrinogen22was purchased from Dako (Glostrup, Denmark). These antibodies were used at 10 μg/mL. Goat anti–rabbit and goat anti–mouse immunoglobulin-G fractions coupled to 10 nm gold particles (GAR-G10, GAM-G10) were purchased from British Biocell International (Cardiff, United Kingdom) and used at 1/30 dilution.
Electron microscopy
Samples were fixed in 1.5% glutaraldehyde for 1 hour and washed 3 times in 0.1 M phosphate buffer, pH 7.4. For morphologic examination, fixed platelets were postfixed in 1% osmic acid, dehydrated in ethanol, and embedded in Epon by standard methods.
Immunolabeling
For immunolabeling experiments, fixed platelets were embedded in sucrose (for cryosections)23 or glycolmethacrylate (for ultrathin sections), and the immunochemical reactions were performed on thin sections collected on nickel grids.24Briefly, we labeled sections first by incubating them with the primary antibodies for 2 hours at 22°C in a humidified atmosphere and then washing them thoroughly in Tris-buffered saline. This was followed by incubation with GAR-G10 or GAM-G10 for 1 hour at 22°C. Samples were counterstained and were observed on a Philips CM 10 electron microscope (Eindhoven, The Netherlands).
Results
Platelet and bacteria interaction
Activated platelets are able to internalize bacteria (S aureus).
The ultrastructural examination of washed platelets incubated withS aureus demonstrated that the internalization of bacteria within platelets was rare. Nevertheless, in the few platelets where bacteria internalization appeared to have occurred, the platelets presented morphological signs of activation with characteristic spherical shape, bristled surface with a few pseudopods, dilation of the open canalicular system (OCS), and rare cytoplasmic granules.
Platelet activation increases bacteria internalization.
To elucidate whether platelet activation preceded and facilitated bacteria internalization or whether bacteria uptake induced platelet activation, washed platelets were activated by ADP or thrombin and incubated with bacteria. Examination of platelet samples incubated withS aureus in the absence of an exogenous platelet activator demonstrated that platelets remained discoid and apparently inactivated, and bacteria were retrieved in the vicinity of platelets without being internalized (Figure 1A). In contrast, agonist-activated platelets exhibited frequent images of bacteria internalization (Figure 1B). This observation led to the conclusion that platelet activation considerably increases bacteria internalization.
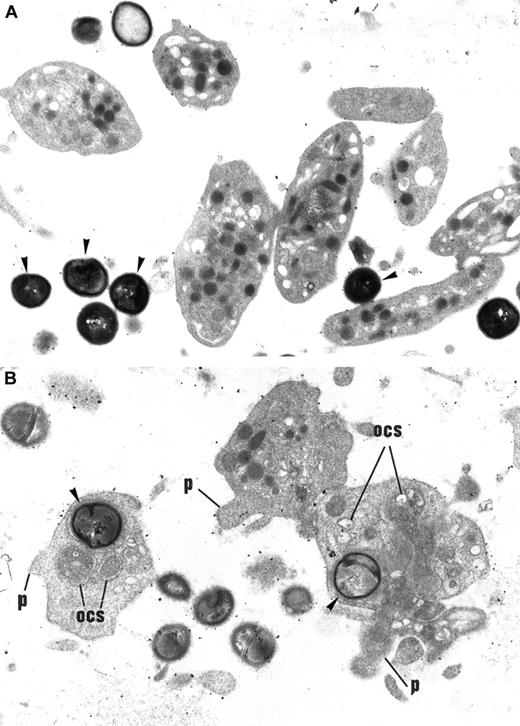
Effect of ADP on S aureus internalization by platelets.
(A) Washed control platelets incubated with a S aureus suspension. Platelets remain discoid and fully granulated, displaying no morphological signs of activation. Bacteria (arrowheads) remain in the extracellular space and are not internalized within platelets. Original magnification, × 14 040. (B) Washed platelets stimulated with 10 μM ADP and incubated with S aureus. Some bacteria (arrowheads) have been internalized by platelets. These platelets present signs of morphological activation: loss of discoid shape, extension of small pseudopods (p), dilated OCS, disappearance of cytoplasmic granules. Original magnification, × 14 040.
Effect of ADP on S aureus internalization by platelets.
(A) Washed control platelets incubated with a S aureus suspension. Platelets remain discoid and fully granulated, displaying no morphological signs of activation. Bacteria (arrowheads) remain in the extracellular space and are not internalized within platelets. Original magnification, × 14 040. (B) Washed platelets stimulated with 10 μM ADP and incubated with S aureus. Some bacteria (arrowheads) have been internalized by platelets. These platelets present signs of morphological activation: loss of discoid shape, extension of small pseudopods (p), dilated OCS, disappearance of cytoplasmic granules. Original magnification, × 14 040.
Bacteria engulfment occurs in a specific subcellular compartment.
To document the antigenic composition of the limiting membrane of vacuoles that surround internalized bacteria (engulfing vacuoles), immunolabeling experiments were performed on thin sections of thrombin-activated platelets incubated with S aureus, by using antibodies against several platelet glycoproteins (αIIbβ3, P-selectin, GPIb, and fibrinogen). Within resting platelets, αIIbβ3 is present along the plasma membrane, the membrane of the OCS, and the α-granule membrane (Figure 2A, inset); P-selectin is restricted to the α-granule membrane (Figure 2B, inset); GPIb is located mainly on plasma membrane (Figure 2C, inset). During platelet activation, antigens redistribute in the following way: αIIbβ3 plasma membrane expression significantly increased and P-selectin appeared on the plasma membrane; OCS and GPIb have cleared from the plasma membrane and are internalized into the OCS membrane. In our samples, upon platelet activation, OCS membrane expressed the 3 antigens αIIbβ3, P-selectin, and GPIb.25 The limiting membrane of the engulfing vacuoles also expressed αIIbβ3 and P-selectin (Figure 2A-B), but GPIb was consistently absent from this compartment (Figure 2C). Thus, GPIb, which is expressed mainly in OCS after platelet activation, is apparently not expressed in engulfing vacuoles. These results suggest that the engulfing vacuoles exhibit a different antigenic composition from the OCS.
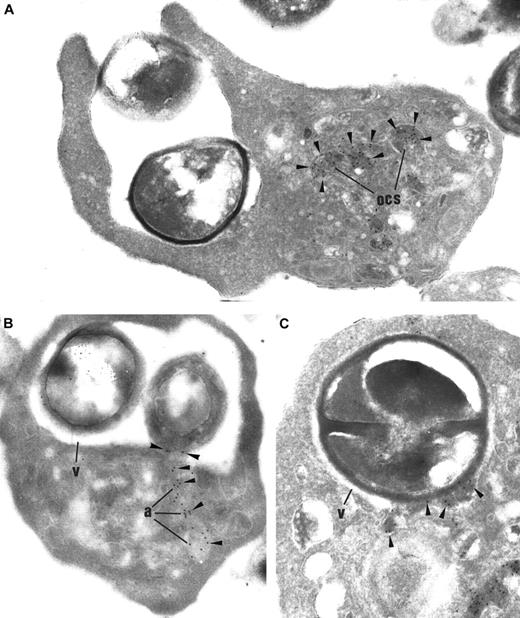
Immunogold labeling of thrombin-activated platelets incubated with
S aureus. (A) In platelets containing bacteria (B), αIIbβ3 labeling (arrowheads) is prominent along the plasma membrane (pm) and the OCS membrane. Like OCS, the limiting membrane of the engulfing vacuoles (v) is heavily labeled. Original magnification, × 23 250. Inset: In control platelets, αIIbβ3 is immunodetected along the plasma membrane and the α-granule membrane. Inset original magnification, × 34 100. (B) P-selectin (arrowheads) is consistently detected along the plasma membrane (pm) and the OCS, as expected, following platelet activation. The membrane of the engulfing vacuoles surrounding the bacteria is also labeled for P-selectin. Original magnification, × 23 250. Inset: In control platelets, P-selectin is restricted to the α-granular membrane and is absent from plasma membrane. Inset original magnification, × 34 100. (C) GPIb (arrowheads), which is immunodetected on the OCS membrane, is not found within the membrane of the engulfing vacuoles, showing that this compartment is distinct from the OCS. Noteworthy, GPIb is absent from the plasma membrane (pm), showing its redistribution into the OCS, a distribution pattern that is well established in activated platelets. Original magnification, × 23 250. Inset: In control platelets, GPIb is located mainly on the plasma membrane. Inset original magnification, × 34 100.
Immunogold labeling of thrombin-activated platelets incubated with
S aureus. (A) In platelets containing bacteria (B), αIIbβ3 labeling (arrowheads) is prominent along the plasma membrane (pm) and the OCS membrane. Like OCS, the limiting membrane of the engulfing vacuoles (v) is heavily labeled. Original magnification, × 23 250. Inset: In control platelets, αIIbβ3 is immunodetected along the plasma membrane and the α-granule membrane. Inset original magnification, × 34 100. (B) P-selectin (arrowheads) is consistently detected along the plasma membrane (pm) and the OCS, as expected, following platelet activation. The membrane of the engulfing vacuoles surrounding the bacteria is also labeled for P-selectin. Original magnification, × 23 250. Inset: In control platelets, P-selectin is restricted to the α-granular membrane and is absent from plasma membrane. Inset original magnification, × 34 100. (C) GPIb (arrowheads), which is immunodetected on the OCS membrane, is not found within the membrane of the engulfing vacuoles, showing that this compartment is distinct from the OCS. Noteworthy, GPIb is absent from the plasma membrane (pm), showing its redistribution into the OCS, a distribution pattern that is well established in activated platelets. Original magnification, × 23 250. Inset: In control platelets, GPIb is located mainly on the plasma membrane. Inset original magnification, × 34 100.
In these samples, some platelets appeared to extend pseudopods that wrapped around the bacteria. The platelet pseudopods surrounded the particle by the process of circumferential adherence until they fully enclosed the particle in a vacuole consisting of internalized extracellular space surrounded by plasma membrane, in a manner that closely resembles phagocytes engulfing bacteria.
This observation suggests that an active process leads to bacteria internalization (Figure 3A) and that passive, amorphous passage of bacteria into the OCS is unlikely.
Thrombin-activated platelets incubated with S aureus, immunolabeled for fibrinogen (arrowheads).
(A) Platelet pseudopods surround the particle by a process of circumferential adherence. Fibrinogen release in OCS indicates platelet activation. Original magnification, × 37 200. (B) Several α-granules (a) fuse together and their fibrinogen content is released into the engulfing vacuole (v). Original magnification, × 27 900. (C) The α-granule fibrinogen is detected within the engulfing vacuole, in close contact with the bacterium. Original magnification, × 44 950.
Thrombin-activated platelets incubated with S aureus, immunolabeled for fibrinogen (arrowheads).
(A) Platelet pseudopods surround the particle by a process of circumferential adherence. Fibrinogen release in OCS indicates platelet activation. Original magnification, × 37 200. (B) Several α-granules (a) fuse together and their fibrinogen content is released into the engulfing vacuole (v). Original magnification, × 27 900. (C) The α-granule fibrinogen is detected within the engulfing vacuole, in close contact with the bacterium. Original magnification, × 44 950.
Finally, in platelets with 1 or 2 large vacuoles containing bacteria, immunolabeling for fibrinogen provided evidence that secretion of the granule contents also occurs around internalized bacteria. Also apparent were some images demonstrating fusion of α-granules either among themselves or with the endocytic vacuole (Figure 3B-C). Finally, fusion of the engulfing vacuoles with the OCS-containing fibrinogen also occurred (not shown). These observations indicate that, at the final step of internalization, bacteria appear to be in contact with granule secretory products.
Platelets and HIV interaction
HIV internalization by platelets occurs in endosomelike structures.
The ultrastructural examination of washed platelets incubated with the supernatant of HIV-infected cultured cells showed typical images of virus internalization. In the early stage of internalization, HIV particles were found in small vacuoles tightly surrounding 1, 2, or 3 particles, were located close to the plasma membrane (Figure4A), and resembled endosomal structures. Virus particles were also found in the dilated channels of OCS, possibly following their fusion with the engulfing vacuoles (Figure 4B-C). This process resembled what had been observed with bacteria.
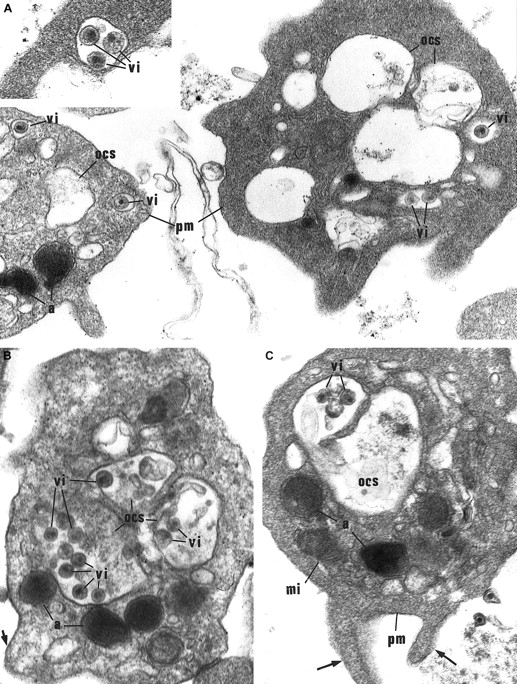
Morphological study of washed platelets incubated with HIV particles.
(A) Internalization of HIV particles in endosomelike structures. In the early stage of internalization, HIV particles are found in some characteristic small vacuoles as endosomelike structures tightly surrounding 1 or 2 particles (vi), and located close to the plasma membrane (pm). a indicates α-granules. Original magnification, × 36 800; inset original magnification, × 55 200. (B) (C) HIV particles (vi) are further retrieved in the OCS. These platelets show morphological activation signs: spherical shape, bristled surface with pseudopods (arrows), dilated OCS. mi indicates mitochondria. Original magnification, × 41 850.
Morphological study of washed platelets incubated with HIV particles.
(A) Internalization of HIV particles in endosomelike structures. In the early stage of internalization, HIV particles are found in some characteristic small vacuoles as endosomelike structures tightly surrounding 1 or 2 particles (vi), and located close to the plasma membrane (pm). a indicates α-granules. Original magnification, × 36 800; inset original magnification, × 55 200. (B) (C) HIV particles (vi) are further retrieved in the OCS. These platelets show morphological activation signs: spherical shape, bristled surface with pseudopods (arrows), dilated OCS. mi indicates mitochondria. Original magnification, × 41 850.
HIV internalization occurs preferentially in activated platelets.
Interestingly, viral particles were observed exclusively in platelets undergoing secretion and displaying morphological activation signs: uneven surface, extension of pseudopods, degranulation, and dilated OCS (Figure 4A-C).
Specific uptake of HIV by platelets.
HIV identification was also performed by immunogold labeling on ultrathin cryosections of platelets incubated with HIV particles, with the use of a pool of 3 monoclonal antibodies directed against the core protein p24. This technique was sensitive enough to specifically detect a single viral particle within the whole platelet cytoplasmic area (Figure 5A) without any unspecific labeling.
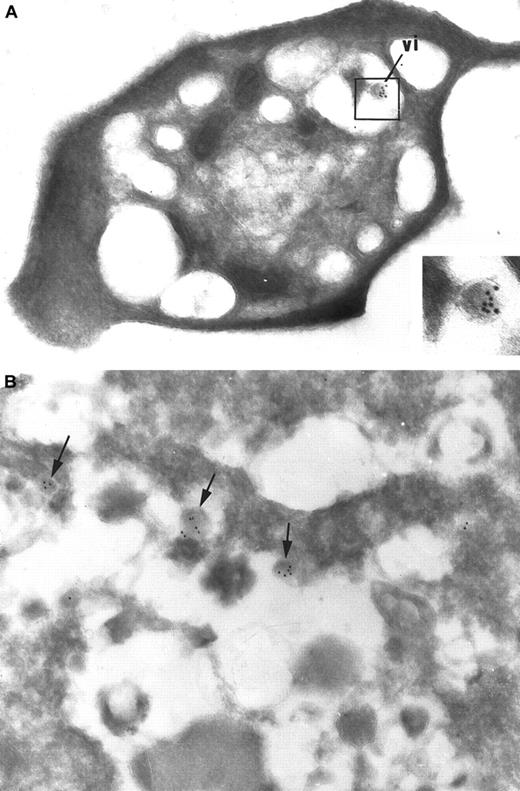
Immunogold detection of HIV on ultrathin cryosections.
(A) In platelets incubated with the supernatant of HIV-infected cultured PBMCs, immunogold labeling for the HIV core protein p24 can specifically and sensitively identify a virionlike particle (vi) that has been internalized in the platelet. This allows its definite identification as an HIV particle. Virtually no background labeling is observed. Original magnification, × 40 250; inset original magnification, × 86 250. (B) Immunogold labeling for p24 was applied on the supernatant of HIV-infected cultured PBMCs: viral particles (arrows) are rare and are scattered among abundant cellular debris. Original magnification, × 46 200.
Immunogold detection of HIV on ultrathin cryosections.
(A) In platelets incubated with the supernatant of HIV-infected cultured PBMCs, immunogold labeling for the HIV core protein p24 can specifically and sensitively identify a virionlike particle (vi) that has been internalized in the platelet. This allows its definite identification as an HIV particle. Virtually no background labeling is observed. Original magnification, × 40 250; inset original magnification, × 86 250. (B) Immunogold labeling for p24 was applied on the supernatant of HIV-infected cultured PBMCs: viral particles (arrows) are rare and are scattered among abundant cellular debris. Original magnification, × 46 200.
This technique was also performed on the supernatant of infected PBMCs used for platelet incubation. It showed that the proportion of viral particles in the culture supernatant was quite low in terms of cellular debris (Figure 5B). This tends to demonstrate that platelets selectively take up HIV particles, preferentially to cellular debris.
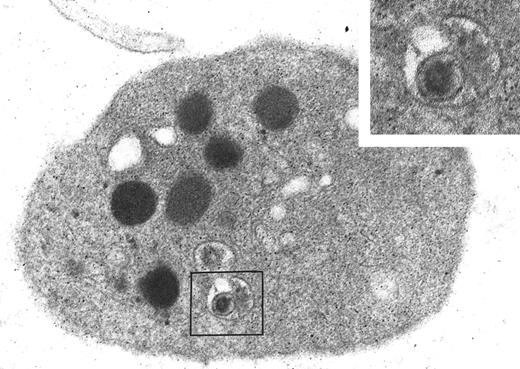
Ultrastructural aspect of platelet-HIV interaction in vivo.
An ultrastructural study of isolated platelets from a patient with AIDS, high viremia level, and thrombocytopenia was performed. In this sample, we observed a viral particle tightly enclosed within a small, typical endocytic vacuole (Figure 6). The particle exhibited the size, shape, and dense core characteristic of HIV. The results obtained in vivo tend to confirm the in vitro data and indicate that HIV endocytosis into platelets may occur during HIV infection.
Ultrastructural study of platelets from a patient with AIDS and thrombocytopenia.
A foreign particle with the size, shape, and dense core characteristic of HIV is detected within an endocytic vacuole in a circulating platelet of this patient. Original magnification, × 48 050; inset original magnification, × 96 100.
Ultrastructural study of platelets from a patient with AIDS and thrombocytopenia.
A foreign particle with the size, shape, and dense core characteristic of HIV is detected within an endocytic vacuole in a circulating platelet of this patient. Original magnification, × 48 050; inset original magnification, × 96 100.
Discussion
The mechanism by which platelets interact with infectious microorganisms is poorly understood. In this study, we have focused on the interaction of washed normal platelets with either bacteria(S aureus) or viral particles (HIV). The susceptibility of bacteria internalization was initially studied under conditions that prevent platelet aggregation (washed platelets and washed bacteria). Although engulfment of bacteria by resting platelets was rare, the few platelets in which internalization was evident displayed the characteristics of platelet activation. However, under these experimental conditions, no major platelet activation was induced by the bacteria.
To determine whether platelet activation triggered or enhanced bacterial uptake, platelet agonists were added in the incubation medium. These experiments showed that platelet activation greatly increases bacteria internalization.
The first step of phagocytosis is bacterial adherence to the cell surface, which usually requires the participation of a receptor and a ligand. Recently, it has been shown that the receptor for the complement component C1q (C1qR/p33) is recognized by S aureus protein26; this indicates that C1qR could be a potential binding site for S aureus. It is noteworthy that C1qR/p33 is expressed on platelet surface after platelet activation.27 In addition, thrombospondin, which has been described as an S aureus–adherence mediator, is also secreted from the α-granules upon activation.28Therefore, these studies provide additional evidence that platelet activation can facilitate S aureus internalization.
To elucidate the mechanisms of S aureus interaction with platelets, we proceeded to study the subcellular distribution of receptors in platelets engulfing bacteria. Immunolabeling experiments for αIIbβ3, GPIb, P-selectin, and fibrinogen were performed. These experiments confirmed that the platelets that had engulfed bacteria displayed classical activation signs (GPIb internalization, P-selectin surface expression, and fibrinogen release).
Examination of the antigenic composition of the engulfing vacuoles suggested that they express a distinct composition from the OCS since GPIb, which is present in OCS, was not detected within the engulfing vacuoles. In fact, the antigenic composition of the engulfing vacuoles is the same as the plasma membrane after activation (expression of αIIbβ3 and P-selectin, but not GPIb).25 This indicates that the engulfing vacuoles are probably formed by plasma membrane invagination (endocytosis) after platelet activation. It has been shown that GPIb is associated with cytoskeletal actin and that it is responsible for maintenance of platelet discoid form. The clearance of GPIb from the platelet plasma membrane upon activation accompanies classical shape changes.29 In contrast, αIIbβ3becomes associated with cytoplasmic actin filaments upon activation.30 Thus, the interaction of the 2 surface receptors with cytoskeletal components could be implicated in the contractile events necessary for bacteria internalization.
In platelets with large vacuoles containing bacteria, fibrinogen labeling suggests that α-granular fusion with the engulfing vacuoles occurs. This results in granular secretion around bacteria, thus allowing interaction of both bacteria and α-granule proteins.
Examination of platelets incubated with the supernatant of HIV-infected PBMC culture shows intracellular HIV particles with their characteristic size, shape, and eccentric dense core.31 At the early step of internalization, HIV particles were tightly surrounded by endosomelike structures and located close to the plasma membrane. These images are very similar to those associated with HIV transcytosis in epithelial cells. Transcytosis is a mechanism that involves translocation of HIV particles through epithelial cells by endocytosis without infecting the epithelium itself.32 33 Transcytosis in platelets differs from epithelial cells because platelets are isolated, secretory cells. In addition, endosomelike structures containing HIV particles in platelets would be expected to fuse with the OCS as observed. Remarkably, platelets that contained intracellular viruses also displayed activation signs. Granular components would then also be secreted on HIV particles, in a manner similar to the process that occurs in bacteria internalization by platelets.
Immuno-EM using a pool of monoclonal antibodies against the core protein p24 was demonstrated to be a specific and sensitive technique since we could detect a single viral particle in the whole platelet surface. It also showed that HIV particles were rare in the supernatant of infected platelets, suggesting that the cells could selectively internalize HIV virions preferentially over cellular debris. Finally, examination of HIV viremia in various plasma samples more or less contaminated with platelets showed an increase of HIV RNA copy number per unit of plasma volume parallel to the intensity of platelet contamination. Finally, examination of platelets of a patient with AIDS allowed us to validate the results we obtained in vitro. Our observations suggest that this phenomenon can occur in HIV-infected patients and may therefore be a cause of platelet destruction and thrombocytopenia.
In analogy with the phagocytic process, engulfing vacuoles can be compared to phagosomes, and the fusion of these vacuoles with granules can be compared to the phagosome-lysosome fusion, which permits the release of granular materials onto the microorganisms. The granular components exhibit microbicidal and antiviral activity. Indeed, previous studies have shown the existence of 2 microbicidal proteins from human blood platelets called thrombocidins34and have shown that platelets are a source of RANTES (regulate upon activation normal T-cell–expressed and secreted) chemokines,35-37 which have been known for their suppressive effects on HIV infection,38 even before the discovery of HIV CCR5 coreceptor. These proteins, stored in the α-granules, are released in the endocytic compartment in contact with internalized particles. Platelet α-granules also contain β-thromboglobulin and platelet factor–4, which have been shown to be platelet-derived CXC chemokines and to contribute to coordinate recruitment and activation of neutrophils in response to acute tissue injury.39 These data show the potential antimicrobial role of platelets and the objective of microorganism internalization by platelets. We have also shown that microorganism internalization specifically occurs in activated platelets that express P-selectin on their surface, enabling their attachment to phagocytes, such as polymorphonuclear leukocytes, monocytes, and macrophages,40 that bear P-selectin glycoprotein ligand–1. This could also contribute to the thrombocytopenia. Therefore, this study suggests that platelets may assist phagocytes in the clearance of microorganisms. However, neither killing nor digestion of microorganisms within platelets has so far been demonstrated. Thus, we have avoided using the term phagocytosis and have used “engulfment” and “internalization” instead.
An alternative hypothesis would be that endocytosis of HIV by platelets would be a pathway that HIV could use to evade either the immune system or anti-HIV treatment. Therefore, further studies need to be performed to elucidate the incidence of platelet/microorganism interaction and its effect on the general capacity of the human host to defend against infection.
In conclusion, our results demonstrate that (1) platelet activation greatly facilitates microorganism uptake; (2) internalization occurs in endosomelike structures that are different from the OCS, in a probably selective and active manner; and (3) platelets may play an essential role in host defense.
Prepublished online as Blood First Edition Paper, April 30, 2002; DOI 10.1182/blood-2001-12-0191.
Supported by a fellowship from L'Association Nationale pour la Recherche contre le SIDA (T.Y.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Elisabeth Cramer, INSERM U 474, Maternité5ème étage, Hôpital de Port-Royal, 123 Boulevard Port-Royal, 75014, Paris, France; e-mail:elisabeth.cramer@cochin.inserm.fr.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal