The tetraspanin CD63 (also known as LAMP-3) has been implicated in phagocytic and intracellular lysosome-phagosome fusion events. It is also present in eosinophils, with predominant expression on crystalloid granule membrane. However, its role in eosinophil function is obscure. We hypothesized that CD63 is associated with intracellular events involved in eosinophil activation and mediator release. We used a combination of confocal immunofluorescence microscopy, flow cytometry, and secretion assays, including β-hexosaminidase, eosinophil peroxidase, and RANTES, to examine CD63 expression, intracellular localization, and its association with cell activation and mediator release. In resting eosinophils, CD63 immunoreactivity was localized to plasma and crystalloid granule membranes. In interferon-γ (IFN-γ)– or C5a/CB–stimulated cells (10 minutes), intracellular CD63 appeared to shift to the cell periphery and plasma membrane, while stimulation with a cocktail of interleukin-3 (IL-3)/IL-5/granulocyte-macrophage colony-stimulating factor induced the appearance of discrete intracellular clusters of CD63 immunoreactivity. IFN-γ induced mobilization of CD63 to the cell periphery, which coincided with selective mobilization of RANTES prior to its release, implying CD63 association with piecemeal degranulation. Agonist-induced CD63 mobilization and cell surface up-regulation was associated with β-hexosaminidase, eosinophil peroxidase, and RANTES release. Dexamethasone as well as genistein (a broad-spectrum inhibitor of tyrosine kinases) inhibited agonist-induced intracellular mobilization of CD63 and RANTES together with cell surface up-regulation of CD63 and mediator release. This is the first report of an association between CD63 mobilization and agonist-induced selective mediator release, which may imply the involvement of CD63 in eosinophil activation and piecemeal degranulation.
Introduction
Eosinophils are major effector cells in allergic inflammation and asthma.1-3 They synthesize, store, and release a wide range of proinflammatory mediators, including at least 4 cationic proteins1,2 and up to 23 cytokines and growth factors.4,5 Eosinophils contain different populations of mediator-storage organelles, including small secretory vesicles as well as crystalloid granules. The latter secretory granules are the site of storage of cytotoxic cationic proteins as well as a number of cytokines, chemokines, and growth factors.6,7 The membrane-bound crystalloid granule comprises 2 compartments: an electron-dense crystalline core (internum) where major basic protein (MBP)8,9 is stored and an electron-lucent matrix6 where 3 cationic proteins—namely, eosinophil cationic protein, eosinophil peroxidase (EPO), and eosinophil-derived neurotoxin1,8—together with a number of other cytokines, including interleukin-6 (IL-6)10 and RANTES (regulated on activation normal T cells expressed and secreted),11 are stored. Among eosinophil-derived mediators, β-hexosaminidase (β-hex) has been shown to localize to both small secretory vesicles and crystalloid granules.11 12
In eosinophils, 3 mediator release mechanisms have been described: cytolysis or necrotic release, compound exocytosis, and piecemeal degranulation (PMD).13 In cytolysis, the cell membrane loses its integrity and crystalloid granules are released to extracellular space. In compound exocytosis, a number of granules fuse intracellularly to form a large degranulation chamber or cavity, which in turn fuses with the cell membrane before discharging its contents to extracellular space. In physiologic conditions, a more commonly seen mode of exocytotic mediator release in eosinophils is PMD, whereby stored mediators are selectively released from an intragranular pool, leaving portions or all of the granules empty in the intact cell.13,14 Various stimuli, including cross-linking of different subclasses of immunoglobulin receptors, are known to induce selective mediator release from eosinophils.15,16 Our own previous studies have demonstrated that interferon-γ (IFN-γ) induces a rapid and selective intracellular mobilization and release of RANTES as early as 10 minutes after stimulation, while it does not affect MBP translocation.11 The mechanisms and molecules associated with cell activation and selective mediator release in eosinophils remain to be fully elucidated. In fact, intracellular intermediates linking agonist stimulation with cell activation and selective mediator release and associated cell surface markers are not yet identified.
CD63, also known as lysosome-associated membrane protein-3,17,18 is a member of the transmembrane-4 superfamily whose membership has grown to 20 proteins since its first discovery in 1990. Tetraspanins are membrane-associated molecules that span the membrane 4 times (TM1-TM4) with 2 extracellular domains (EC1-EC2). As cell surface proteins, tetraspanins appear to act as “molecular facilitators” by increasing the formation and stability of functional signaling complexes.19 The tetraspanin CD63 is proposed to be involved in a number of cellular functions such as cell activation20 and mediator release.21CD63 is a well-established component of the late endosomal and lysosomal membranes.22 CD63 is also present in “secretory lysosomes,”23 the secretory granules of cells derived from the hemopoietic lineage that are related to lysosomes. These organelles include azurophilic granules of neutrophils24 and α-granules of platelets.25 Thus, CD63 appears to be shared by conventional late endosomes/lysosomes and by specialized, perhaps related secretory organelles.
Present only in myeloperoxidase-containing granules of neutrophils, CD63 has been described as a marker for azurophilic granule fusion, with the plasma membrane suggesting a potential role for this molecule in membrane fusion events.24,26 In the rat basophilic leukemia cell line, an antibody against CD63 (AD1) inhibited immunoglobulin E (IgE)–mediated histamine release, suggesting a role for CD63 in events associated with mediator release.27
A number of tetraspanins, including CD9, CD37, CD53, and CD63, are expressed in peripheral blood eosinophils.28,29Cross-linking of some surface transmembrane-4 superfamily molecules induces significant eosinophil homotypic aggregation, up-regulation of CD11b expression, or CD62L shedding, consistent with cellular activation.29 Yet, no function has been attributed to CD63 in human eosinophils, and very little is known about its possible role. We hypothesized that CD63 is associated with intracellular events involved in eosinophil activation and mediator release. We have used a combination of double immunofluorescent staining and confocal laser scanning microscopy, flow cytometry, β-hex release assay, EPO release assay, and RANTES enzyme-linked immunosorbent assay to determine the expression, localization, and potential association of CD63 with cell activation and mediator release in peripheral blood eosinophils.
Materials and methods
Preparation of eosinophils
Eosinophils were purified from peripheral blood of healthy control subjects and documented mild atopic asthmatic subjects as previously described.10,11 30 Briefly, samples of peripheral blood (100 mL) were collected in heparin-containing tubes. Erythrocytes were sedimented for 45 minutes at room temperature with 5% dextran (100 000-200 000 kd; Sigma, Oakville, ON, Canada). The upper phase of the leukocyte-rich plasma was then layered onto a 15-mL Ficoll (Pharmacia Biotech, Uppsala, Sweden) and centrifuged for 25 minutes at 1000g. After removal of excess plasma, mononuclear layer, and Ficoll, the contaminating erythrocytes were removed by hypotonic lysis on ice in 1 mL sterile H2O for 5 seconds, and the resulting granulocyte pellet was resuspended in 2 mL RPMI 1640 (BioWhittaker, Walkersville, MD). Contaminating erythrocytes were removed by hypotonic lysis on ice in 1 mL sterial H2O for 5 seconds. The resultant pellet was incubated with a mixture of 12 μL anti-CD16, 10 μL anti-CD3, and 10 μL anti-CD14–coated immunomagnetic beads (MACS beads; Miltenyi Biotec, Bergisch-Gladbach, Germany) for 45 minutes at 4°C to remove contaminating neutrophils, lymphocytes, and monocytes, respectively, by negative selection on a magnetic column. The resulting eosinophil purity was more than 99%.
The study was approved by the Health Research Ethics Board of the Faculty of Medicine and Dentistry and University of Alberta Hospital. Informed consent was provided according to the Declaration of Helsinki.
Granule purification
Purified eosinophils (4 × 107) were washed in 10 mL ice-cold buffer A (10 mM HEPES, 0.25 M sucrose, 1 mM ethyleneglycotetraacetic acid, pH 7.4) and resuspended in ice-cold homogenization buffer (buffer B) (HEPES-buffered sucrose supplemented with 2 mM/L MgCl2 and 1 mM adenosine triphosphate and 5 μg/mL each of leupeptin, aprotinin, and TAME, pH 7.4) to optimal subfractionation density (between 10 × 106 and 15 × 106/mL). Cells then were subjected to 15 to 20 passes through a ball-bearing homogenizer (EMBL, Heidelberg, Germany) possessing 12 μm clearance. To obtain the postnuclear supernatant the homogenate was centrifuged at 400g for 10 minutes. The resulting postnuclear supernatant was subjected to density centrifugation at 10 000g for 15 minutes at 4°C. Supernatant containing light membrane was discarded, and the pellet containing crystalloid granules was resuspended in 50 μL buffer B and used in experimental procedures immediately after.
Flow cytometry
Eosinophils (5 × 105 cells per test) incubated in the presence or absence of IFN-γ (20 ng/mL; R&D Systems, Minneapolis, MN); a combination of C5a (800 nM) and cytochalasin B (CB) (10 ng/mL; Sigma); and a cocktail of IL-3 (10 ng/mL), IL-5 (5 ng/mL), and granulocyte-macrophage colony-stimulating factor (GM-CSF) (10 ng/mL) (Genzyme, Cambridge, MA) (10 minutes) were fixed in 5% formalin (10 minutes at 4°C) and blocked in 5% milk in flow buffer (phosphate-buffered saline + 1% bovine serum albumin + 0.1% sodium azide) overnight at 4°C. Following blocking, cells were washed (× 3) in flow buffer and incubated with 5 μg/mL of one of the following antibodies: mouse monoclonal anti-CD63 (IgG1; Pharmingen, San Diego, CA) or mouse IgG1 isotype control (R&D Systems) for 60 minutes on ice. After 3 washing steps, cells were incubated with goat F(ab′)2 antimouse IgG conjugated to phycoerythrin (5 μg/mL) (Cedarlane Laboratories, Hornby, ON, Canada) for 30 minutes at 4°C. The cells were subsequently washed 3 times and resuspended in flow buffer to a final density of 1 × 106/mL and analyzed on a FACScan instrument using CellQuest software (Becton Dickinson, San Jose, CA). Eosinophil crystalloid granules obtained from purified eosinophils were subjected to the same procedure described above and further examined using FACS analysis. To examine the intracellular or intragranular pool of CD63, eosinophils or their purified granules were permeabilized by adding 0.1% saponin to the blocking reagent.
Immunofluorescent labeling
Cytospins of resting as well as IFN-γ (20 ng/mL), a combination of C5a (800 nM) and CB (10 ng/mL), and IL-3 (10 ng/mL)/IL-5 (5 ng/mL)/GM-CSF (10 ng/mL)–stimulated (10 minutes) eosinophils were prepared by sedimenting 3 × 104 cells (suspended in 100 μL 20% fetal calf serum in RPMI 1640) in a Cytospin 2 Centrifuge (Shandon, Runcorn, United Kingdom) at 800 rpm for 2 minutes. To prepare cytospins of crystalloid granules, the same procedure was carried out on highly purified crystalloid granules. Slides were then foil-wrapped and stored at −20°C until used. Slides of resting and agonist-stimulated eosinophils were fixed for 8 minutes in 2% paraformaldehyde in phosphate-buffered saline (room temperature) and washed (× 5) in Tris-buffered saline (TBS, pH 7.4). Following fixation, cytospins were blocked using 3% fetal calf serum in a humidified container (30 minutes). After a second washing step, slides were incubated overnight with TBS containing 1% mouse monoclonal antihuman CD63 (5 μg/mL) at 4°C. BODIPY-FL–conjugated goat antimouse secondary antibody (20 μg/mL) (Molecular Probes, Eugene, OR) was used to detect immunoreactivity of CD63 (2 hours, room temperature). Following another washing step, slides were blocked again for 2 hours using goat antimouse IgG Fab fragment (Jackson ImmunoResearch Laboratories, West Grove, PA) (50 μg/mL) and double-labeled with either mouse monoclonal antihuman MBP (1%) (BMK-13, generated in-house31) or mouse monoclonal antihuman RANTES (5 μg/mL) (R&D Systems) at 4°C. Immunoreactivity against MBP and RANTES was detected by incubating slides with 15 μg/mL rhodamine (TRITC)–labeled goat antimouse secondary antibody (Jackson ImmunoResearch Laboratories) for 2 hours. Mouse IgG1 (R&D Systems) at equivalent concentrations was used as the isotype control. After a final washing step, 10 μL of the antibleaching agent, 0.4% n-propyl gallate (Sigma) in 3:1 glycerol 10 × TBS, was applied to the slides before coverslip attachment.
Confocal laser scanning microscopy
Immunofluorescent stainings of resting and stimulated eosinophils as well as purified crystalloid granules were examined using a Zeiss laser scanning confocal microscope (LSM 510) mounted on a Zeiss Axiovert M100 inverted microscope with a × 63 plan-apochromatic lens (Zeiss, Toronto, Canada). The 488-nm laser line (generated from a 25-mW argon laser) and 543-nm laser line (generated from 1-mW HeNe laser) were used to image BODIPY-FL (green) and TRITC (red), used in the experiments. A bandpass filter (505-550 nm) was used to collect emission from BODIPY-FL, and a longpass filter (560 nm) was used to collect signals from TRITC. To avoid spillover of the fluorochromes, sequential scanning mode of the machine was used to collect images from double-stained samples. Image acquisition was optimized using the required pinhole setting, photomultiplier gain, and offset. Higher spatial resolution was achieved by using the appropriate zoom on the computer. Images were further analyzed and developed using LSMIB 4.0 software.
Measurement of β-hex, EPO, and RANTES release
To measure β-hex, freshly purified eosinophils (2 × 105 cells) were stimulated using IFN-γ, C5a/CB, or IL-3/IL-5/GM-CSF for 10 minutes. Cell-free supernatants were collected, and pellets were lysed using 0.5% Triton X-100 (Sigma) in color-free RPMI. Samples of cell-free supernatants (50 μL) and lysed pellets (50 μL) were mixed with 50 μL substrate solution (1 mM 4-methylumbelliferyl N-acetyl-β-d-glucosaminide in 0.2 M citrate buffer, pH 4.5, and 0.1% Triton X-100) in a 96-well microplate and incubated for 60 minutes at 37°C. The reaction was terminated by the addition of 150 μL ice-cold 0.2 M Tris, and the fluorescence (excitation 360 nm, emission 460 nm) was measured in a Millipore CytoFluor 2350 plate reader (Millipore, Nepean, ON, Canada) as described previously.10 11 For EPO measurement, freshly purified eosinophils (2 × 105 cells) were stimulated using IFN-γ (20 ng/mL). EPO activity in the samples of cell-free supernatants as well as the cell pellets was assayed using TMB (3,3′, 5,5′-tetramethylbenzidine liquid substrate system) solution by combining 50 μL samples with 150 μL substrate solutions in a 96-well microplate and incubating at room temperature for 30 minutes. The reaction was terminated by adding 50 μL of 1 M sulfuric acid, and absorbance was read at 450 nm in a spectrophotometric microplate reader (Vmax Kinetic Microplate Reader, Molecular Devices, Sunnyvale, CA). RANTES immunoreactivity in supernatant of IFN-γ–stimulated cells (10 minutes) was measured using a Quantikine enzyme-linked immunosorbent assay kit (R&D Systems) with a detection sensitivity of 31.2 pg/mL according to manufacturer's instructions.
Statistical analysis
Experiments were done in triplicates. Values were averaged, and their SEM was calculated. Results were analyzed for significance using a Student t test. A P value of less than .01 was considered significant.
Results
CD63 protein expression and localization in human peripheral blood eosinophils
The subcellular distribution of CD63 was examined using a combination of immunofluorescent staining and confocal laser scanning microscopy on cytospins of purified peripheral blood eosinophils. Cytospins of freshly purified eosinophils were prepared and immunostained using a monoclonal anti-CD63 antibody. CD63 was expressed in resting peripheral blood eosinophils and appeared predominantly localized to the membrane of all crystalloid granules (Figure1A). The association of CD63 immunoreactivity with crystalloid granule membrane was further confirmed by immunofluorescent staining of a dispersed population of eosinophil crystalloid granules (Figure 1B).
Confocal laser scanning microscopy images of immunostained peripheral blood eosinophils of asthmatic subjects.
Representative images of human eosinophils stained with TRITC-conjugated secondary antibody (red) to detect the MBP as well as RANTES immunoreactivity, or BODIPY-FL–conjugated secondary antibody (green) to detect immunoreactivity against CD63. (A) Resting peripheral blood eosinophils labeled with BODIPY-FL indicating CD63 immunoreactivity. (B) Highly purified crystalloid granules immunostained with anti-CD63. (C) Higher magnification of a combined image of eosinophil crystalloid granules immunostained for MBP (TRITC) indicating core-associated immunoreactivity and CD63 indicating matrix-associated CD63 immunoreactivity. (D) Combined image of double immunofluorescence (MBP and CD63) staining of resting eosinophils; red (TRITC) represents immunoreactivity against MBP, and green (BODIPY-FL) is indicative of CD63 immunoreactivity. (E) Combined image of double immunofluorescence (RANTES and CD63) staining of resting eosinophils; TRITC detected RANTES, and BODIPY-FL detected CD63. (F-H) Double immunofluorescence (MBP and CD63) staining of IFN-γ–stimulated eosinophils (time = 10 minutes): MBP immunostaining (F), CD63 immunostaining (G), and combined image (H). (I-K) Double immunofluorescence (MBP and CD63) staining of C5a/CB-stimulated eosinophils (time = 10 minutes): MBP immunostaining (I), CD63 immunostaining (J), and combined image (K). (L) Combined image of isotype control for CD63 and MBP immunostaining. (M-O) Double immunofluorescence (MBP and CD63) staining of IL-3/IL-5/GM-CSF–stimulated eosinophils (time = 10 minutes): MBP immunostaining (M), CD63 immunostaining (N), and combined image (O). (P) Combined image of double immunofluorescence (MBP and CD63) staining of IFN-γ–stimulated eosinophils preincubated with genistein (10−6 M), which inhibited the IFN-γ–induced translocation of CD63. (Q-T) Double immunofluorescence (RANTES and CD63) staining of IFN-γ–stimulated eosinophils: RANTES immunostaining (Q), CD63 immunostaining (R), combined image (S), and DIC image of the same cell (T). Original magnification is × 630 for all images.
Confocal laser scanning microscopy images of immunostained peripheral blood eosinophils of asthmatic subjects.
Representative images of human eosinophils stained with TRITC-conjugated secondary antibody (red) to detect the MBP as well as RANTES immunoreactivity, or BODIPY-FL–conjugated secondary antibody (green) to detect immunoreactivity against CD63. (A) Resting peripheral blood eosinophils labeled with BODIPY-FL indicating CD63 immunoreactivity. (B) Highly purified crystalloid granules immunostained with anti-CD63. (C) Higher magnification of a combined image of eosinophil crystalloid granules immunostained for MBP (TRITC) indicating core-associated immunoreactivity and CD63 indicating matrix-associated CD63 immunoreactivity. (D) Combined image of double immunofluorescence (MBP and CD63) staining of resting eosinophils; red (TRITC) represents immunoreactivity against MBP, and green (BODIPY-FL) is indicative of CD63 immunoreactivity. (E) Combined image of double immunofluorescence (RANTES and CD63) staining of resting eosinophils; TRITC detected RANTES, and BODIPY-FL detected CD63. (F-H) Double immunofluorescence (MBP and CD63) staining of IFN-γ–stimulated eosinophils (time = 10 minutes): MBP immunostaining (F), CD63 immunostaining (G), and combined image (H). (I-K) Double immunofluorescence (MBP and CD63) staining of C5a/CB-stimulated eosinophils (time = 10 minutes): MBP immunostaining (I), CD63 immunostaining (J), and combined image (K). (L) Combined image of isotype control for CD63 and MBP immunostaining. (M-O) Double immunofluorescence (MBP and CD63) staining of IL-3/IL-5/GM-CSF–stimulated eosinophils (time = 10 minutes): MBP immunostaining (M), CD63 immunostaining (N), and combined image (O). (P) Combined image of double immunofluorescence (MBP and CD63) staining of IFN-γ–stimulated eosinophils preincubated with genistein (10−6 M), which inhibited the IFN-γ–induced translocation of CD63. (Q-T) Double immunofluorescence (RANTES and CD63) staining of IFN-γ–stimulated eosinophils: RANTES immunostaining (Q), CD63 immunostaining (R), combined image (S), and DIC image of the same cell (T). Original magnification is × 630 for all images.
To determine the intracellular localization site of CD63 relative to MBP (marker for the eosinophil granule crystalline core) and RANTES (marker for the crystalloid granule matrix region), we carried out a double immunofluorescent staining procedure on a population of isolated granules as well as freshly purified eosinophils. While immunoreactivity against MBP predominantly localized to the core region (Figure 1C-D) and did not colocalize with CD63, the immunoreactivity against RANTES and CD63 showed relative colocalization to the peripheral compartment of the crystalloid granules highlighted by the yellow color in the merged image (Figure 1E).
Mobilization of CD63 in stimulated eosinophils
To examine the intracellular localization and kinetics of CD63 mobilization relative to MBP in agonist-stimulated cells, freshly isolated eosinophils were stimulated (10 minutes) with either of IFN-γ (20 ng/mL), C5a (800 nM)/CB (10 ng/mL), or a cocktail of IL-3 (10 ng/mL)/IL-5 (5 ng/mL) and GM-CSF (10 ng/mL). Cytospins of stimulated eosinophils were prepared, double-immunostained for CD63 and MBP, and examined using confocal fluorescent microscopy. As early as 10 minutes after IFN-γ (Figure 1F-H) or C5a/CB stimulation (Figure 1I-K), CD63 immunostaining confined to the regions adjacent to the cell membrane, while MBP immunoreactivity except for some intensification at the cell periphery remained relatively unaltered. In contrast to IFN-γ and C5a/CB, stimulation with IL-3/IL-5/GM-CSF induced the appearance of discrete clusters of CD63 that colocalized predominantly with eosinophil MBP (Figure 1M-O). Interestingly, none of these cytokines on their own at the same doses affected the CD63 localization. Translocation of CD63 during stimulation by IFN-γ, C5a/CB, or IL-3/IL-5/GM-CSF was inhibited by the tyrosine kinase inhibitor, genistein (10−6 M) (Figure 1P) (n = 5).
Agonist-induced cotranslocation of CD63 and RANTES
We have previously shown that IFN-γ stimulation of eosinophils induces the rapid mobilization of RANTES to the cell periphery prior to its release to extracellular space as an in vitro example of PMD.11 To examine the association of CD63 and RANTES translocation to cell periphery and selective release, double immunofluorescent staining with specific antibodies to RANTES and CD63 was conducted on IFN-γ–stimulated eosinophils. Following IFN-γ stimulation (10 minutes) of eosinophils, immunoreactivity against RANTES colocalized with that of CD63, with both signals translocating to the periphery of the cells (Figure 1Q-S). The continued presence of CD63− and RANTES− crystalloid granules in the cytoplasm of IFN-γ–stimulated eosinophils was further evident by differential interference contrast (DIC) imaging (Figure 1T). Mouse IgG1 at equivalent concentrations was used instead of anti-CD63 and anti-RANTES as isotype control (Figure 1L). In double immunostaining procedures, appropriate isotype controls were run concurrently (combination of anti-CD63/BODIPY-FL and mouse IgG1/TRITC or a combination of mouse IgG1/BODIPY-FL and anti-MBP/TRITC); nonspecific binding was not observed (Figure2M-P).
The effects of dexamethasone on agonist-induced CD63 translocation.
Representative images of human eosinophils single-stained with BODIPY-FL–conjugated secondary antibody (green) to detect immunoreactivity against CD63. (A,B) Immunofluorescence (CD63) staining of IFN-γ–stimulated eosinophils and DIC image of the same cell (time = 10 minutes). (C,D) Immunofluorescence staining of eosinophils preincubated with dexamethasone (10−6 M) prior to IFN-γ stimulation; the DIC image of the same cell. (E,F) Immunofluorescence staining of C5a/CB-stimulated eosinophils; DIC image of the same cell. (G,H) Immunofluorescence staining of eosinophils preincubated with dexamethasone (10−6 M) prior to C5a/CB stimulation; the DIC image of the same cell. (I,J) Immunofluorescence staining of IL-3/IL-5/GM-CSF–stimulated eosinophils; DIC image of the same cell. (K,L) Immunofluorescence staining of eosinophils preincubated with dexamethasone (10−6 M) prior to IL-3/IL-5/GM-CSF stimulation; the DIC image of the same cell. (M,P) Combined images of immunofluorescence staining of eosinophils with a combination of anti-CD63/BODIPY-FL and mouse IgG1/TRITC; the DIC image of the same cell (M,N). A combination of mouse IgG1/BODIPY-FL and anti-MBP/TRITC; the DIC image of the same cell (O,P). Original magnification is × 630 for all images.
The effects of dexamethasone on agonist-induced CD63 translocation.
Representative images of human eosinophils single-stained with BODIPY-FL–conjugated secondary antibody (green) to detect immunoreactivity against CD63. (A,B) Immunofluorescence (CD63) staining of IFN-γ–stimulated eosinophils and DIC image of the same cell (time = 10 minutes). (C,D) Immunofluorescence staining of eosinophils preincubated with dexamethasone (10−6 M) prior to IFN-γ stimulation; the DIC image of the same cell. (E,F) Immunofluorescence staining of C5a/CB-stimulated eosinophils; DIC image of the same cell. (G,H) Immunofluorescence staining of eosinophils preincubated with dexamethasone (10−6 M) prior to C5a/CB stimulation; the DIC image of the same cell. (I,J) Immunofluorescence staining of IL-3/IL-5/GM-CSF–stimulated eosinophils; DIC image of the same cell. (K,L) Immunofluorescence staining of eosinophils preincubated with dexamethasone (10−6 M) prior to IL-3/IL-5/GM-CSF stimulation; the DIC image of the same cell. (M,P) Combined images of immunofluorescence staining of eosinophils with a combination of anti-CD63/BODIPY-FL and mouse IgG1/TRITC; the DIC image of the same cell (M,N). A combination of mouse IgG1/BODIPY-FL and anti-MBP/TRITC; the DIC image of the same cell (O,P). Original magnification is × 630 for all images.
Dexamethasone's effect on agonist-induced CD63 translocation
To examine the effect of dexamethasone on agonist-induced intracellular translocation of CD63, freshly purified eosinophils were incubated in the presence of dexamethasone 10−6 for 60 minutes prior to agonist stimulation. Interestingly, dexamethasone inhibited the IFN-γ– (Figure 2A-D), C5a/CB– (Figure 2E-H), or IL-3/IL-5/GM-CSF– (Figure 2I-L) induced intracellular mobilization of CD63 (n = 6).
CD63 surface expression in resting and stimulated eosinophils
The surface expression of CD63 in freshly isolated eosinophils was examined by flow cytometry. Our results indicated that CD63 is expressed on the surface of resting eosinophils. Saponin-permeabilized cells showed a significant shift (50-fold) in mean fluorescent index (MFI), indicating a larger intracellular pool of CD63 (Figure 3) (n = 4). Total CD63 expression was similar in resting and agonist-stimulated cells, yet CD63 surface expression was enhanced after agonist stimulation (10 minutes), with C5a/CB inducing maximum up-regulation in contrast to the combination of IL-3/IL-5/GM-CSF, which induced a smaller degree of increased surface expression (Figure 4) (n = 4). The surface up-regulation of CD63 coincided with intracellular translocation of CD63 and RANTES upon agonist stimulation.
Analysis of CD63 surface expression.
CD63 expression on the surface of purified peripheral blood eosinophils was examined using flow cytometry analysis. (A) Isotype control for nonpermeabilized cells, (B) isotype control for (0.1%) saponin-permeabilized cells, (C) CD63 expression in nonpermeabilized eosinophils, and (D) CD63 expression in (0.1%) saponin-permeabilized cells.
Analysis of CD63 surface expression.
CD63 expression on the surface of purified peripheral blood eosinophils was examined using flow cytometry analysis. (A) Isotype control for nonpermeabilized cells, (B) isotype control for (0.1%) saponin-permeabilized cells, (C) CD63 expression in nonpermeabilized eosinophils, and (D) CD63 expression in (0.1%) saponin-permeabilized cells.
Analysis of CD63 surface expression in agonist-stimulated eosinophils.
CD63 surface expression in agonist-stimulated purified peripheral blood eosinophils was examined using flow cytometry analysis. (A) CD63 surface expression on resting eosinophils, (B) CD63 surface expression on IFN-γ–stimulated eosinophils, (C) CD63 surface expression on C5a/CB-stimulated eosinophils, and (D) CD63 surface expression on IL-3/IL-5/GM-CSF–stimulated eosinophils.
Analysis of CD63 surface expression in agonist-stimulated eosinophils.
CD63 surface expression in agonist-stimulated purified peripheral blood eosinophils was examined using flow cytometry analysis. (A) CD63 surface expression on resting eosinophils, (B) CD63 surface expression on IFN-γ–stimulated eosinophils, (C) CD63 surface expression on C5a/CB-stimulated eosinophils, and (D) CD63 surface expression on IL-3/IL-5/GM-CSF–stimulated eosinophils.
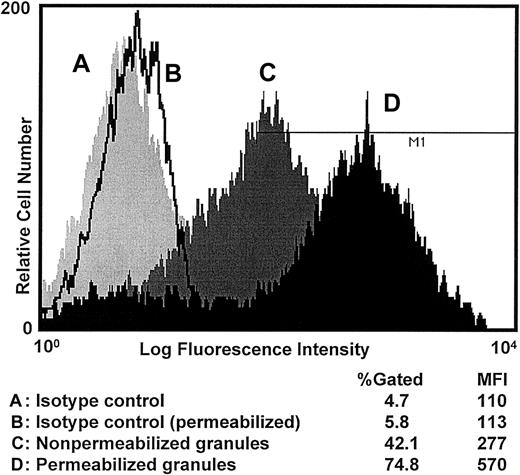
CD63 expression on enriched populations of crystalloid granules
To understand the pattern of membrane fusion and enhanced CD63 expression on the eosinophil surface membrane, we studied the expression of CD63 on the surface of isolated crystalloid granules. We immunostained both a permeabilized and a nonpermeabilized population of dispersed and highly purified eosinophil crystalloid granules. The results indicate that CD63 was expressed on the surface of crystalloid granules. Interestingly, the MFI of permeabilized granules was increased approximately 2-fold in saponin-permeabilized granules, while the MFI for the isotype controls of permeabilized and nonpermeabilized populations of granules was the same (Figure5) (n = 7). To ensure that granules were intact after isolation, granule preparations were immunostained with antibodies against MBP or RANTES, and no immunostaining was detected by flow cytometry against either of the 2 mediators in nonpermeabilized granule preparation. Only the permeabilized granules exhibited MBP and RANTES immunoreactivity (data not shown).
Analysis of CD63 expression on the surface of purified crystalloid granules.
Highly purified crystalloid granules were immunostained and analyzed using flow cytometry technique. (A) Isotype control, (B) isotype control for saponin-permeabilized granules, (C) CD63 expression on the surface of nonpermeabilized crystalloid granules, and (D) CD63 expression in saponin-permeabilized granules. The MFI of permeabilized crystalloid granules was approximately 2-fold increased when compared with nonpermeabilized granules.
Analysis of CD63 expression on the surface of purified crystalloid granules.
Highly purified crystalloid granules were immunostained and analyzed using flow cytometry technique. (A) Isotype control, (B) isotype control for saponin-permeabilized granules, (C) CD63 expression on the surface of nonpermeabilized crystalloid granules, and (D) CD63 expression in saponin-permeabilized granules. The MFI of permeabilized crystalloid granules was approximately 2-fold increased when compared with nonpermeabilized granules.
The association of CD63 translocation and enhanced surface expression with mediator release
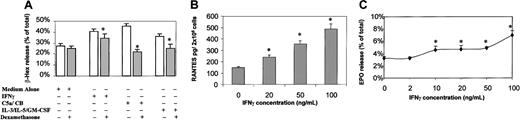
We examined the association between intracellular translocation and surface up-regulation of CD63 with mediator release upon agonist stimulation. We measured β-hex release in the supernatant of eosinophils stimulated with IFN-γ, C5a/CB, or IL-3/IL-5/GM-CSF (10 minutes). Our results indicate that agonist stimulation of eosinophils (10 minutes) induced β-hex release. C5a/CB induced maximum release in contrast to the combination of IL-3/IL-5/GM-CSF, which induced minimum release (n = 6). Release of β-hex occurred concurrently with CD63 translocation to the cell periphery and the cell surface. Indeed, β-hex release triggered by various stimuli was associated with agonist-induced enhancement of CD63 surface expression measured by flow cytometry (Figures 6A and 4). In particular, C5a/CB induced more β-hex release in parallel with its stronger effect on CD63 surface expression (Figures 6A and 4). We also measured RANTES and EPO in the supernatant of IFN-γ–stimulated eosinophils. Our data indicate that IFN-γ stimulation of eosinophils (10 minutes) also induced the release of RANTES and EPO in a dose-dependant fashion (Figures 6B and 6C, respectively). We examined the effect of other agonists on CD63 translocation and surface membrane up-regulation and tested tumor necrosis factor (10 ng/mL), phorbol myristate acetate (10 ng/mL), and each of IL-3 (10 ng/mL), IL-5 (5 ng/mL), and GM-CSF (10 ng/mL) alone. The following combinations were also tested: IL-3 and IL-5, IL-3 and GM-CSF, and IL-5 and GM-CSF. Interestingly, none of the individual or combinations of agonists listed induced CD63 translocation, surface membrane up-regulation, and mediator release (data not shown).
Mediator (β-hex) release in agonist-stimulated eosinophils and effects of dexamethasone.
(A) Freshly purified eosinophils (2 × 105 cells) were stimulated using IFN-γ, C5a/CB, and IL-3/IL-5/GM-CSF for 10 minutes, and β-hex was measured in cell-free supernatants. The effect of dexamethasone on agonist-induced mediator release was examined by preincubating cells with dexamethasone (10−6) for 60 minutes. A similar trend of release was observed in 6 separate donors. The first column shows spontaneous release of β-hex from eosinophils, the second and third columns show IFN-γ–induced release and dexamethasone effect, the fourth and fifth columns represent the C5a/CB-induced release and dexamethasone effect, and the sixth and seventh columns show the IL-3/IL-5/GM-CSF–induced release and dexamethasone effect. (B) Freshly purified eosinophils (2 × 106 cells) were stimulated using a different concentration of IFN-γ for 10 minutes, and RANTES was measured in cell-free supernatants. A similar trend of release was observed in 4 separate donors. (C) Freshly purified eosinophils (2 × 106 cells) were stimulated using a different concentration of IFN-γ for 10 minutes, and EPO was measured in cell-free supernatants. A similar trend of release was observed in 4 separate donors. The Student t test was used to analyze the results. In all experiments, given values represent averages of triplicate measurements. *The statistical significance of dexamethasone inhibition of mediator release; error bars represent the mean and SEM of measurements (*P < .01).
Mediator (β-hex) release in agonist-stimulated eosinophils and effects of dexamethasone.
(A) Freshly purified eosinophils (2 × 105 cells) were stimulated using IFN-γ, C5a/CB, and IL-3/IL-5/GM-CSF for 10 minutes, and β-hex was measured in cell-free supernatants. The effect of dexamethasone on agonist-induced mediator release was examined by preincubating cells with dexamethasone (10−6) for 60 minutes. A similar trend of release was observed in 6 separate donors. The first column shows spontaneous release of β-hex from eosinophils, the second and third columns show IFN-γ–induced release and dexamethasone effect, the fourth and fifth columns represent the C5a/CB-induced release and dexamethasone effect, and the sixth and seventh columns show the IL-3/IL-5/GM-CSF–induced release and dexamethasone effect. (B) Freshly purified eosinophils (2 × 106 cells) were stimulated using a different concentration of IFN-γ for 10 minutes, and RANTES was measured in cell-free supernatants. A similar trend of release was observed in 4 separate donors. (C) Freshly purified eosinophils (2 × 106 cells) were stimulated using a different concentration of IFN-γ for 10 minutes, and EPO was measured in cell-free supernatants. A similar trend of release was observed in 4 separate donors. The Student t test was used to analyze the results. In all experiments, given values represent averages of triplicate measurements. *The statistical significance of dexamethasone inhibition of mediator release; error bars represent the mean and SEM of measurements (*P < .01).
The effect of glucocorticoids and tyrosine kinase inhibitor, genistein, on CD63 surface membrane up-regulation and mediator release
The clinical efficacy of glucocorticoids in allergic inflammation is well documented. It is thought that steroids may partially mediate inhibition of the elaboration of proinflammatory and eosinophil-active cytokines.32 We therefore examined the potential inhibitory effects of glucocorticoids on β-hex, EPO, and RANTES release and correlated this with CD63 translocation and surface membrane up-regulation. Treatment of eosinophils with dexamethasone (60 minutes) prior to agonist activation down-regulated β-hex release significantly (Figure 6A) and also inhibited agonist-induced CD63 membrane up-regulation (data not shown). Dexamethasone, in the absence of agonist stimulation, did not alter the β-hex spontaneous release (Figure 6A). In addition, dexamethasone inhibited the IFN-γ–induced EPO and RANTES release. Dexamethasone had no effect on eosinophil survival determined by trypan blue exclusion test.
To examine the potential involvement of tyrosine kinase activity in CD63 translocation and cell surface up-regulation following agonist stimulation, eosinophils were preincubated with genistein, a broad-spectrum inhibitor of tyrosine kinases (n = 4). Genistein pretreatment of eosinophils down-regulated the IFN-γ–induced β-hex release (33%) and also inhibited agonist-induced CD63 translocation (Figure 1P) and membrane up-regulation (data not shown).
Discussion
In this study, we have investigated the expression and subcellular localization of CD63 in resting and activated human peripheral blood eosinophils. We have further examined the association of CD63 intracellular localization and surface expression with selective mediator mobilization (RANTES) and release (β-hex, EPO, and RANTES) in agonist-activated eosinophils.
Piecemeal degranulation is a well-documented mode of mediator release in human eosinophils. In studies of individuals with asthma,33-35 allergic rhinitis,36,37 and nasal polyposis,13,38,39 approximately 67% of all airway mucosal eosinophils were shown to exhibit intact but lucent granules indicative of PMD. In allergen-exposed nasal airways, virtually all viable eosinophils in mucosal tissue showed signs of PMD under active disease conditions.36 Eosinophil granule alterations reflecting PMD have also been described in guinea pig models of asthma.40 41
In a previous study, our laboratory showed that selective release of RANTES in eosinophils upon IFN-γ stimulation was initiated by a rapid mobilization to the cell periphery followed by secretion to the extracellular space.11 RANTES trafficking as well as release was suggested to be associated with a pool of rapidly mobilizable small mediator–containing vesicles. These vesicles are thought to come directly from the Golgi compartment or bud from crystalloid granules, fuse with plasma membrane, and selectively and rapidly evacuate stored granule contents to the extracellular space.42,43 These studies provided for the first time an in vitro model for eosinophil PMD; however, no molecular and cell surface marker for this gradual and selective mediator mobilization and release have been identified. To date, researchers have relied only on ultrastructural evidence to determine the percentage of altered granules and the selective mediator release in tissue eosinophils.13 Based on our findings in this study, we propose CD63 as a molecular marker for PMD in eosinophils because it is associated with intracellular mediator mobilization to the cell periphery putatively en route to extracellular space. In support of this proposal, we have demonstrated that in resting eosinophils CD63 is expressed on the membrane of all MBP immunoreactive crystalloid granules in close association with RANTES, previously shown to localize to the matrix of crystalloid granules.11 Upon IFN-γ stimulation, CD63 immunoreactivity together with that of RANTES appeared to localize only to the membrane of granules adjacent to the plasma membrane. This initial mobilization of CD63 and RANTES was associated with a parallel CD63 up-regulation on the surface membrane and the release of RANTES to the extracellular space. No reagents or specific inhibitors of CD63 are available to fully characterize the mechanisms of CD63 translocation in association with selective mediator mobilization and release. We therefore can only speculate that small secretory vesicles, previously reported to bud off the crystalloid granules and rapidly shuttle mediators from crystalloid granules to the plasma membrane,42 43 may also be responsible for the translocation of CD63 to the cell periphery and ultimately the plasma membrane.
It is highly unlikely that the loss of CD63 immunoreactivity in the internal population of crystalloid granules of stimulated eosinophils is due to proteolytic degradation of CD63. First, unlike neutrophils or mast cells, the eosinophil lacks potent proteases. Second, CD63 has been shown to be resistant to protease activity due to its heavy glycosylation on its extracellular-2 domain.44 45 Third, while CD63 immunoreactivity was confined to the cell periphery after IFN-γ and C5a/CB stimulation, CD63 expression increased in the center of the cell following stimulation with the cytokine cocktail (IL-3/IL-5/GM-CSF). Fourth, there is no significant difference in the total cell MFI of CD63 in permeabilized cells before and after stimulation, which may suggest translocation rather than degradation of this protein following stimulation. Finally, we used 2 antibodies raised against CD63 that recognize 2 different epitopes of CD63; both antibodies exhibited a similar immunostaining pattern in resting and activated cells.
Enhanced surface expression of CD63 in agonist-stimulated eosinophils appeared to coincide with β-hex, EPO, and RANTES release, further supporting the potential association of CD63 with cell activation and mediator release. Indeed, agonists that induced the higher degree up-regulation of surface membrane expression for CD63 equally induced high β-hex release (Figures 4 and 6). Eosinophil surface up-regulation of CD63 following cell stimulation and subsequent mediator release is in agreement with previous observations in neutrophils.25 CD63 mobilization to the plasma membrane was reported to involve the fusion of azurophilic granules with plasma membrane following neutrophil stimulation.26
Surface up-regulation of CD63 in activated eosinophils appears to occur in vivo as well. Surface expression of CD63 was observed to be up-regulated in eosinophils recovered from bronchoalveolar lavage of allergic asthmatic patients.46 Our own studies showed that CD63 surface expression is enhanced in peripheral blood eosinophils from asthmatics (n = 5) compared with nonasthmatic individuals (n = 5) (data not shown).
In localization studies, we provided evidence that CD63 is expressed on the membrane of purified crystalloid granules. Following permeabilization, granules showed 2-fold increase in immunoreactivity against CD63, potentially caused either by intragranular storage of CD63 or by the presence of granule membrane–associated CD63 on the inner leaflet of the granule membrane. However, it is unlikely that there will be an intragranular pool of CD63 approximately equal to the membrane-associated pool. Furthermore, a previous ultrastructural study of eosinophils localized CD63 to the membrane and not to the matrix of crystalloid granules.28 We therefore speculate that this 2-fold increase in immunoreactivity of permeabilized granules may be associated with the orientation of the epitopes whereby CD63 faces both directions, with half CD63 facing the inner leaflet of the granules. Potentially, this may be caused by the dynamic fusion of small (secretory and/or endocytic) vesicles with crystalloid granules. Indeed, a previous study showed that CD63 cycles between endocytic and secretory compartments in human endothelial cells.17Another study also illustrated that members of the tetraspanin superfamily such as CD37, CD53, CD63, CD81, and CD82 are concentrated on the internal membrane of an MHC class II–enriched compartment,47 indicating that the “extracellular loops” of these tetraspanins may face the inner comportment of an intracellular organelle.
Unlike IFN-γ and C5a/CB, stimulation with IL-3/IL-5/GM-CSF induced the appearance of discrete clusters of CD63, which predominantly colocalized to large intracellular (central) stores of MBP+granules. The formation of such large intracellular pools of MBP may be associated with intracellular fusion of secondary granules likely to lead to compound exocytosis. The colocalization of CD63 with the intracellular stores of MBP may indicate the association of CD63 with eosinophil secretory processes and compound exocytosis induced by eosinophil-active cytokines.
Tyrosine kinase activity has been implicated in eosinophil activation and mediator release.48-52 In our experiments, genistein fully inhibited agonist-induced (IFN-γ, C5a/CB, and IL-3/IL-5/GM-CSF) intracellular translocation and mobilization of CD63 and RANTES as well as cell surface up-regulation of CD63 and β-hex release. The inhibitory effects of genistein on intracellular movement of CD63 indicated a functional involvement of protein kinases in these processes. Nevertheless, the precise signal transduction pathways that link agonist stimulation with CD63 translocation and mediator release are not clear and are currently being pursued.
A number of studies have suggested a direct effect of glucocorticoids on the survival, activation, adhesion, apoptosis, and degranulation of eosinophils.53-57 In our hands, dexamethasone inhibited agonist-induced intracellular translocation, mobilization, and cell surface up-regulation of CD63. It also down-regulated agonist-induced β-hex release. Interdependence between inhibitory effects of dexamethasone on mediator release and CD63 mobilization as well as surface up-regulation is plausible but requires further studies to explore this potential association, particularly the rapidity of the effect.
Members of the SNARE fusion complex have been proposed to play a central role in exocytosis.58 Our recent studies have shown that human peripheral blood eosinophils express members of the SNARE fusion complex, including VAMP-2,59syntaxin-4,60 and SNAP-23.61 In addition to SNAREs, members of Rho-related GTPases, including Rac-2, have been identified in eosinophils and shown to be critical in the assembly of NADPH oxidase complex prior to the generation of superoxide.62 Yet, the potential interdependence between different elements of eosinophil exocytosis remains the subject of speculation. We are intrigued by the likely interplay between the CD63 and members of SNARE fusion complex and/or Rho-related GTPases and are currently pursuing studies to define the mode of these putative interactions.
In conclusion, our novel observations on eosinophil CD63 have important implications for the expansion of current knowledge on the processes underlying eosinophil activation and exocytosis. Our data point to a new association of CD63 with agonist-induced eosinophil activation and mediator release, particularly PMD. These findings may ultimately lead to novel therapeutic strategies in the treatment of eosinophil-related allergic inflammatory diseases, particularly asthma.
The authors thank Drs Paige Lacy and Paul Forsythe, Department of Medicine, University of Alberta, for their helpful comments on the manuscript and Dr Harissios Vliagoftis for helpful discussion. We acknowledge Dorothy Putkowski, Department of Medical Microbiology and Immunology, University of Alberta, for her expert assistance in flow cytometry.
Supported by the Canadian Institutes for Health Research and the Alberta Heritage Foundation for Medical Research. R.M. is an Alberta Heritage Senior Medical Scholar. G.P.D. is the recipient of a Canada Research Chair in Respiration and is the current holder of the R. Fraser Elliott Chair in transplantation research from the Toronto General Hospital.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Redwan Moqbel, Director, Pulmonary Research Group, 550A Heritage Medical Research Center, University of Alberta, Edmonton, Alberta, T6G 2S2, Canada; e-mail: redwan.moqbel@ualberta.ca.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal