The purpose of the present study was to evaluate the effects of graft-versus-host disease (GVHD) prophylaxis with the Janus kinase 3 (JAK3) inhibitor WHI-P131/JANEX-1 on the graft-versus-leukemic (GVL) function of marrow allografts in mice undergoing bone marrow transplantation (BMT) after being challenged with an otherwise invariably fatal dose of BCL-1 leukemia cells. GVHD prophylaxis using WHI-P131 markedly improved the survival outcome after BMT. The probability of survival at 30 days after BMT was 11% ± 6% for vehicle-treated recipients (median survival time, 25 days) versus 63% ± 12% for recipients treated with WHI-P131 (median survival time, 36 days; P < .0001). Because WHI-P131 is devoid of antileukemic activity against BCL-1 leukemia cells, this marked improvement in survival outcome was due to reduced incidence of GVHD-associated fatalities combined with sustained GVL function of the allografts in the WHI-P131 group. Notably, adoptive transfer experiments demonstrated that the spleens of WHI-P131–treated allograft recipients contained less than 0.001% BCL-1 cells. Notably, GVHD prophylaxis with WHI-P131 plus methotrexate resulted in 100% survival of mice receiving allotransplants challenged with an otherwise invariably fatal dose of BCL-1 leukemia. Taken together, our results provide strong experimental evidence that GVHD prophylaxis using WHI-P131 does not impair the GVL function of the allografts and consequently contributes to an improved post-BMT survival outcome of the recipient mice.
Introduction
Bone marrow transplantation (BMT) has become one of the standard treatment modalities offered to high-risk leukemia patients.1-6 Very intensive “supralethal” myeloablative chemotherapy or radiochemotherapy regimens can be applied in the context of BMT with a curative intent to overcome the drug resistance of residual leukemia cells of certain leukemia patients who are unlikely to be cured by standard chemotherapy.1-6 In addition, leukemia patients undergoing allogeneic BMT may benefit from the graft-versus-leukemia (GVL) effect of the marrow allograft. Graft-versus-host disease (GVHD), a donor T-cell–initiated highly complex pathologic condition that frequently follows allogeneic BMT, especially in the context of a major HLA disparity, is associated with significant morbidity and mortality.1-12 Severe GVHD remains a major obstacle to a more successful outcome of allogeneic BMT using HLA-matched unrelated donors as well as partially HLA-mismatched related donors.6,7 Therefore, GVHD prophylaxis aimed at reducing the risk of severe GVHD is an integral component of all BMT programs.1-7
Contemporary methods for GVHD prophylaxis, including ex vivo T-cell depletion of marrow grafts,13-15 use of positively selected CD34+ hematopoietic precursor cells,16 and systemic immunosuppression2 are associated with an increased risk of relapse in leukemia patients undergoing BMT,17 which has generally been attributed to loss of the GVL function of the marrow allografts. Hence, novel anti-GVHD agents that spare the GVL function of the marrow allografts are urgently needed for effective prevention of GVHD after BMT without facilitating the recurrence of leukemia.
We recently reported the structure-based design of specific inhibitors of Janus kinase 3 (JAK3) as apoptosis-inducing antileukemic agents.18 The lead compound 4-(4′-hydroxyphenyl)-amino-6,7-dimethoxyquinazoline (WHI-P131/JANEX-1) was very well tolerated by mice and monkeys, and plasma concentrations of WHI-P131 that are cytotoxic to human leukemia cells in vitro could be achieved at nontoxic dose levels.19 Our recent studies in an acute GVHD model using BALB/c (H-2d) donor bone marrow/spleen cells and H-2 disparate C57BL/6 (H-2b) recipient mice20demonstrated that targeting JAK3 in alloreactive donor lymphocytes with a chemical inhibitor such as WHI-P131 can attenuate the severity of GVHD after BMT.
The purpose of the current study was to evaluate the effects of GVHD prophylaxis with the JAK3 inhibitor WHI-P131 on the GVL function of marrow allografts in mice undergoing BMT after being challenged with an otherwise invariably fatal dose of BCL-1 leukemia cells. Our results presented herein provide experimental evidence that GVHD prophylaxis with WHI-P131 spares the GVL function of the marrow allografts against BCL-1 leukemia cells and consequently contributes to an improved post-BMT survival outcome of the recipient mice.
Materials and methods
Mice
Female BALB/cJ (H-2d), C57BL/6J (B6) (H-2b), and CB6F1/J (BALB/cJxC57BL/6J) (F1) (H-2d/b) mice were purchased from the Jackson Laboratory (Bar Harbor, ME) at 6 to 8 weeks of age. Mice were housed in a controlled environment (12-hour light/12-hour dark photoperiod, 22° ± 1°C, 60% ± 10% relative humidity). Animal studies were approved by the Parker Hughes Institute Animal Care and Use Committee, and all animal care procedures conformed to the Principles of Laboratory Animal Care (National Institutes of Health publication no. 85-23, revised 1985).
Total body irradiation and allogeneic BMT
For pre-BMT conditioning, recipient F1 mice, positioned in a pie-shaped Lucite holder (Braintree Scientific, Boston, MA) underwent total body irradiation (TBI; 9.5 Gy) 1 day before BMT, which was delivered by a cesium instrument (JL Sheppard Labs, 64.38 rad/min). Recipients were given antibiotic-supplemented water (sulfamethoxazole/trimethoprim, Hi-Tech Pharmacal, Amityville, NY) starting the day before BMT. Donor (B6) bone marrow (BM) was collected into RPMI 1640 medium supplemented with l-glutamine (Cellgro; Mediatech, Herndon, VA) by flushing the shafts of femurs and tibias, as described previously.20 The standard BM/splenocyte (S) inoculum consisted of 25 × 106 BM cells and 25 × 106 splenocytes in 0.5 mL RPMI 1640 and was injected intravenously into the caudal vein.
WHI-P131 treatment
WHI-P131 was synthesized and characterized as previously described in detail.18 WHI-P131 was administered in 200 μL phosphate-buffered saline (PBS) supplemented with 10% dimethyl sulfoxide (DMSO) as the vehicle, as previously reported.20Control mice were treated daily with vehicle (200 μL PBS supplemented with 10% DMSO). WHI-P131 (50 mg/kg per day in 2 divided doses) was administered daily via intraperitoneal injections to BMT recipient mice for prevention of fatal GVHD starting on the day of BMT/BCL-1 injection (day 0) until day 60 or an earlier elective kill date due to the moribund clinical condition of the mouse. BALB/c mice injected with BCL-1 cells were treated daily by 75 mg/kg per day (in 3 divided doses) and 150 mg/kg per day (in 3 divided doses) WHI-P131 starting on the day of the BCL-1 inoculation until the death or elective euthanizing of the mouse. The standard anti-GVHD drug methotrexate (MTX; Immunex, Seattle, WA) was administered once daily on days 1, 3, 6, and 11 after BMT at a dose level of 10 mg/m2 per day.
GVHD monitoring
The BMT recipients were monitored daily for any clinical evidence of GVHD (weight loss, manifestations of skin erythema, alopecia, hunching, diarrhea) and survival during the 60-day observation period. Survival times were measured from the day of BMT (day 0).
BCL-1 leukemia model and syngeneic BMT
Recipient F1 mice received TBI of 9.5 Gy on day −1 (as described above) before inoculation of BCL-1 leukemia cells21-24 and syngeneic BMT. Syngeneic donor (F1) BM (25 × 106) and spleen cells (25 × 106) were prepared as described above and injected intravenously to F1 recipients in parallel with a BCL-1 cell inoculum (5 × 106 cells) on day 0. Survival of mice was monitored by daily observation, and the day of death was recorded as the day the mouse spontaneously died or was killed in moribund condition.
GVL model
The BCL-1 leukemia cells (5 × 106) were injected intravenously into TBI-conditioned (day −1) F1 recipients in parallel with an allogeneic marrow graft (B6 BM/S cells, 25 × 106each) on day 0 of BMT. Survival was monitored daily, and the cause of death for each mouse was determined by postmortem gross and histopathologic examination.
H-2D typing by multiparameter flow cytometry
The engraftment status of WHI-P131–treated BMT recipients was studied by 2-color flow cytometric H-2D typing of splenocytes using phycoerythrin (PE)–labeled anti-H-2Db antibody (clone KH95, Pharmingen, San Diego, CA), which marks C57BL/6-type cells and fluorescein isothiocyanate (FITC)–labeled anti-H-2Ddantibody (clone 34-2-12, Pharmingen), which marks BALB/c-type cells. The leukemic burden was studied by 2-color flow cytometry of splenic lymphocyte subpopulations using the PE-labeled anti-H-2Dbantibody in combination with FITC-labeled B-cell reactive anti-B220 (clone RA3-6B2, Pharmingen) antibody. Immunofluorescent staining of cells and multiparameter flow cytometry were performed using standard procedures and a FACSCaliber instrument (Becton Dickinson, Mountain View, CA).20
Histopathology
Spleen, liver, skin, and gut were harvested from mice at the time of death. Tissues were fixed in 10% buffered formalin, paraffin embedded, cut into 4-μm sections, and stained with hematoxylin and eosin. The presence of leukemic BCL-1 infiltrates in the liver and spleens of mice challenged with a BCL-1 inoculum was histopathologically confirmed, as described previously.23Livers were scored positive for GVHD when there was a periportal infiltrate with acute necrosis, skin was scored positive when there was single-cell necrosis, and colon was scored positive when there was single-cell necrosis or crypt dropout. After initial scoring, all slides were reviewed for GVHD grading, as described.20
Detection of residual BCL-1 cells by adoptive transfer experiments
To determine whether or not residual BCL-1 cells were present in BMT recipients, 5 × 105 or 1 × 106 spleen cells obtained from F1 recipients 11 days, 23 days, or more than 60 days after BMT/BCL-1 inoculation were adoptively transferred to untreated secondary syngeneic (BALB/c) recipients via intravenous injection into the caudal vein. Leukemia-free survival for at least 100 days in secondary syngeneic recipients was indicative of absence of residual leukemia in BMT recipients because as few as 10 BCL-1 cells cause leukemic death within 12 weeks and 100 BCL-1 cells cause leukemic death within 6 weeks.22,23 25
Statistical analysis
Group comparisons of continuous variables were done using Student t tests. The survival data were analyzed by life-table methods. P < .05 (log-rank test) was considered significant.
Results
BCL-1 cells cause fatal leukemia in BALB/c mice and irradiated (BALB/cJxC57BL/6J)F1 mice undergoing syngeneic BMT
An intravenous inoculum containing 1 × 106 BCL-1 cells caused fatal leukemia in 100% of control BALB/c mice (n = 20) with a median survival time (MST) of only 12 days. At dose levels of 75 mg/kg per day (divided in 3 doses; n = 20) or 150 mg/kg per day (divided in 3 doses; n = 20), which are 1.5 to 3 times higher than the effective 50-mg/kg per day anti-GVHD dose,20 WHI-P131 exhibited no antileukemic activity against BCL-1 leukemia cells. The MSTs were 11 days for the 75-mg/kg dose level and 12 days for the 150-mg/kg dose level (Table 1). All 20 control and 40 WHI-P131–treated BALB/c mice had massive splenomegaly at the time of death and histopathologic examination of their liver and spleen showed diffuse effacement of the normal architecture with leukemic cells.
Effects of the JAK3 inhibitor WHI-P131/JANEX-1 on post-BMT survival outcome in murine GVHD and GVL models
| Treatment protocol . | N . | MST . | Cumulative proportion surviving (% ± SEM) . | |
|---|---|---|---|---|
| d . | 30 d . | 60 d . | ||
| A. BCL-1 → vehicle | 20 | 12 | 0 ± 0 | 0 ± 0 |
| B. BCL-1 → WHI-P131, 75 mg/kg/d | 20 | 11 | 0 ± 0 | 0 ± 0 |
| C. BCL-1 → WHI-P131, 150 mg/kg/d | 20 | 12 | 0 ± 0 | 0 ± 0 |
| D. TBI | 5 | 11 | 0 ± 0 | 0 ± 0 |
| E. TBI + syngeneic BMT → vehicle | 5 | > 60 | 100 ± 0 | 100 ± 0 |
| F. TBI + syngeneic BMT + BCL-1 → vehicle | 22 | 14 | 14 ± 7 | 0 ± 0 |
| G. TBI + syngeneic BMT + BCL-1 → WHI-P131 | 13 | 12 | 8 ± 7 | 0 ± 0 |
| H. TBI + allo BMT → vehicle | 23 | 24 | 9 ± 6 | 0 ± 0 |
| I. TBI + allo BMT → WHI-P131 | 17 | 37 | 60 ± 12 | 29 ± 11 |
| J. TBI + allo BMT + BCL-1 → vehicle | 28 | 25 | 11 ± 6 | 4 ± 4 |
| K. TBI + allo BMT + BCL-1 → WHI-P131 | 16 | 36 | 63 ± 12 | 44 ± 12 |
| Treatment protocol . | N . | MST . | Cumulative proportion surviving (% ± SEM) . | |
|---|---|---|---|---|
| d . | 30 d . | 60 d . | ||
| A. BCL-1 → vehicle | 20 | 12 | 0 ± 0 | 0 ± 0 |
| B. BCL-1 → WHI-P131, 75 mg/kg/d | 20 | 11 | 0 ± 0 | 0 ± 0 |
| C. BCL-1 → WHI-P131, 150 mg/kg/d | 20 | 12 | 0 ± 0 | 0 ± 0 |
| D. TBI | 5 | 11 | 0 ± 0 | 0 ± 0 |
| E. TBI + syngeneic BMT → vehicle | 5 | > 60 | 100 ± 0 | 100 ± 0 |
| F. TBI + syngeneic BMT + BCL-1 → vehicle | 22 | 14 | 14 ± 7 | 0 ± 0 |
| G. TBI + syngeneic BMT + BCL-1 → WHI-P131 | 13 | 12 | 8 ± 7 | 0 ± 0 |
| H. TBI + allo BMT → vehicle | 23 | 24 | 9 ± 6 | 0 ± 0 |
| I. TBI + allo BMT → WHI-P131 | 17 | 37 | 60 ± 12 | 29 ± 11 |
| J. TBI + allo BMT + BCL-1 → vehicle | 28 | 25 | 11 ± 6 | 4 ± 4 |
| K. TBI + allo BMT + BCL-1 → WHI-P131 | 16 | 36 | 63 ± 12 | 44 ± 12 |
(BALB/cJxC57BL/6J)F1 (H-2d/b) recipients were lethally irradiated (9.5 Gy) and transplanted with BM/S grafts from syngeneic F1 or allogeneic C57BL/6 (H-2b) donors. In protocols A, B, and C, 1 × 106 BCL-1 cells were injected intravenously. In protocols F, G, J, and K, 5 × 106 BCL-1 were injected intravenously. In all GVHD and GVL groups (G, I, K), the dose of WHI-P131 was 50 mg/kg per day.
P < .0001 between groups J and K, H and I, E and F, D and E; there were no significant differences between groups H and J, F and G, I and K. Statistically significant differences were obtained by life-table analysis (log-rank test).
We next examined the ability of BCL-1 cells to cause fatal leukemia in irradiated F1 recipients undergoing syngeneic BMT. An intravenous inoculum of 5 × 106 BCL-1 cells caused fatal leukemia in 100% of control F1 recipients (n = 22) with an MST of 14 days (Table1). Thus, any microenvironmental changes caused by TBI or BMT or both did not prevent BCL-1 cells from engrafting and expanding in the organs of F1 mice. In accordance with the BALB/c data, WHI-P131 (50 mg/kg per day) did not exhibit any antileukemic activity against BCL-1 cells in irradiated F1 recipients undergoing syngeneic BMT. All 13 WHI-P131–treated F1 recipients died of leukemia with an MST of 12 days (Table 1). All 22 control and 13 WHI-P131–treated F1 mice had massive splenomegaly at the time of death and histopathologic examination of their liver and spleen showed diffuse effacement of the normal architecture with leukemic cells.
Targeting JAK3 with WHI-P131 prevents fatal acute GVHD across the major histocompatibility barrier in (BALB/cJxC57BL/6J)F1 mice
Severe GHVD was induced in lethally irradiated F1 mice (H-2d/b) across the major histocompatibility complex (MHC) barrier by injection of BM/S grafts from C57BL/6 mice (H-2b). In an attempt aimed at preventing the development of fatal GVHD in this model, recipient mice were treated with WHI-P131 (25 mg/kg per dose, twice daily) every day from the day of BMT until the end of the 60-day observation period, whereas control mice were treated with vehicle alone. As shown in Table 1, all 5 lethally irradiated F1 mice that did not receive any BM/S grafts died of acute radiation toxicity with an MST of 11 days, whereas all of the 5 lethally irradiated F1 mice injected with syngeneic BM/S grafts remained alive throughout the 60-day observation period. By comparison, the TBI-conditioned, vehicle-treated control F1 mice (n = 23) receiving BM/S grafts from C57BL/6 mice survived the acute TBI toxicity, but they all developed severe multiorgan GVHD, as evidenced by development of overt diarrhea, hunching, more than 20% weight loss, alopecia, and ruffled fur. One hundred percent of these control mice died with an MST of 24 days (Table 1). Histopathologic examination of multiple organs from randomly picked vehicle-treated mice (n = 6) confirmed the diagnosis of GVHD (Table2). The average GVHD scores were 2.8 ± 0.1 for the liver, 1.8 ± 0.1 for the skin, 0.8 ± 0.1 for the small intestine, and 1.2 ± 0.4 for the large intestine.
Severity of GVHD in BMT recipients
| Group . | Time of analysis (wk) . | N . | GVHD score (mean ± SEM) . | |||
|---|---|---|---|---|---|---|
| Liver . | Skin . | Small intestine . | Large intestine . | |||
| A. Allogeneic BMT → vehicle | 2-4 | 6 | 2.8 ± 0.1 | 1.8 ± 0.1 | 0.8 ± 0.1 | 1.2 ± 0.4 |
| B. Allogeneic BMT → WHI-P131 | > 8 | 4 | 1.0 ± 0.3† | 0.5 ± 0.0‡ | 0.5 ± 0.0 | 0.1 ± 0.1* |
| C. Allogeneic BMT + BCL-1 → vehicle | 2-4 | 13 | 2.6 ± 0.1 | 1.9 ± 0.3 | 0.7 ± 0.1 | 1.2 ± 0.2 |
| D. Allogeneic BMT + BCL-1 → WHI-P131 | 2-4 | 5 | 2.0 ± 0.0* | 1.0 ± 0.3* | 0.2 ± 0.1* | 0.5 ± 0.0* |
| E. Allogeneic BMT + BCL-1 → WHI-P131 | > 8 | 4 | 0.6 ± 0.1‡ | 0.0 ± 0.0† | 0.3 ± 0.1* | 0.1 ± 0.1* |
| F. Syngeneic BMT (control) | 2-4 | 17 | 0.5 ± 0.0 | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.1 |
| G. Syngeneic BMT (control) | > 8 | 5 | 0.5 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.0 ± 0.0 |
| Group . | Time of analysis (wk) . | N . | GVHD score (mean ± SEM) . | |||
|---|---|---|---|---|---|---|
| Liver . | Skin . | Small intestine . | Large intestine . | |||
| A. Allogeneic BMT → vehicle | 2-4 | 6 | 2.8 ± 0.1 | 1.8 ± 0.1 | 0.8 ± 0.1 | 1.2 ± 0.4 |
| B. Allogeneic BMT → WHI-P131 | > 8 | 4 | 1.0 ± 0.3† | 0.5 ± 0.0‡ | 0.5 ± 0.0 | 0.1 ± 0.1* |
| C. Allogeneic BMT + BCL-1 → vehicle | 2-4 | 13 | 2.6 ± 0.1 | 1.9 ± 0.3 | 0.7 ± 0.1 | 1.2 ± 0.2 |
| D. Allogeneic BMT + BCL-1 → WHI-P131 | 2-4 | 5 | 2.0 ± 0.0* | 1.0 ± 0.3* | 0.2 ± 0.1* | 0.5 ± 0.0* |
| E. Allogeneic BMT + BCL-1 → WHI-P131 | > 8 | 4 | 0.6 ± 0.1‡ | 0.0 ± 0.0† | 0.3 ± 0.1* | 0.1 ± 0.1* |
| F. Syngeneic BMT (control) | 2-4 | 17 | 0.5 ± 0.0 | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.1 |
| G. Syngeneic BMT (control) | > 8 | 5 | 0.5 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.0 ± 0.0 |
Scoring was done as described in “Materials and methods.” Statistical analysis of the differences between the groups (B versus A and D versus C and E versus C) was done by Student ttest.
P < .05.
P < .005.
P < .0001.
We have previously reported that WHI-P131 significantly attenuates the severity of GVHD.20 In accordance with our earlier observations,20 GVHD prophylaxis with WHI-P131 (25 mg/kg per dose, twice daily, days 0-60) significantly improved the survival of BMT recipients (Table 1) and prolonged the MST to 37 days. The probability of survival at 30 days after BMT was 9% ± 6% for vehicle-treated control mice (n = 23) and 60% ± 12% for mice treated with WHI-P131 (n = 17; P < .0001; Table 1). The histopathologic examination of the organs from 4 representative WHI-P131–treated long-term (> 8 weeks) survivors revealed evidence for subclinical mild GVHD in the liver, skin, small intestine, and large intestine. According to the scoring system, the GVHD grades were 1.0 ± 0.3 for the liver, 0.5 ± 0.0 for the skin, 0.5 ± 0.0 for the small intestine, and 0.1 ± 0.1 for the large intestine (Table2). Flow cytometric H-2Dd/b–typing of splenocytes obtained from these 4 long-term survivors of WHI-P131–treated group showed 96.3% ± 1.1% H-2Dd−/b+ donor cell engraftment (Table3), indicating that WHI-P131 does not prevent donor cell engraftment under these experimental conditions and the attenuation of GVHD in WHI-P131–treated recipient mice was not due to lack of donor cell engraftment with concomitant autologous recovery.
Donor cell engraftment
| Group . | N . | H-2Dd−/b+ (%) . |
|---|---|---|
| Allogeneic BMT → WHI-P131 | 4 | 96.3 ± 1.1 |
| Allogeneic BMT + BCL-1 → WHI-P131 | 4 | 95.3 ± 0.8 |
| Allogeneic BMT + BCL-1 → MTX | 5 | 93.2 ± 2.6 |
| Allogeneic BMT + BCL-1 → WHI-P131 + MTX | 7 | 95.6 ± 1.0 |
| Syngeneic BMT | 5 | 0.0 ± 0.0 |
| Group . | N . | H-2Dd−/b+ (%) . |
|---|---|---|
| Allogeneic BMT → WHI-P131 | 4 | 96.3 ± 1.1 |
| Allogeneic BMT + BCL-1 → WHI-P131 | 4 | 95.3 ± 0.8 |
| Allogeneic BMT + BCL-1 → MTX | 5 | 93.2 ± 2.6 |
| Allogeneic BMT + BCL-1 → WHI-P131 + MTX | 7 | 95.6 ± 1.0 |
| Syngeneic BMT | 5 | 0.0 ± 0.0 |
Splenocytes of long-term survivors (> 60 days after BMT/BCL-1 injection), WHI-P131-, MTX-, and WHI-P131 + MTX-treated F1 recipients of allogeneic (B6) BM/S grafts and BCL-1 injections were analyzed by flow cytometry for engraftment. Syngeneic (F1 to F1) BM/S recipients are presented as controls. Data are presented as mean ± SEM values for the percentage of H-2Dd−/b+ donor cells.
These findings confirm and extend our previous studies that demonstrated the beneficial effects of WHI-P131 on the post-BMT survival outcome of B6 (H-2b) recipients transplanted with BM/S grafts from allogeneic BALB/c (H-2d) donors.20 Thus, targeting JAK3 in alloreactive donor lymphocytes with a chemical inhibitor such as WHI-P131 can improve survival after BMT across the MHC barrier by decreasing the probability of fatal GVHD.
GVL function of BM/S allografts and its resistance to WHI-P131
We performed a series of experiments to determine if BCL-1 cells are sensitive to the antileukemic activity of donor allografts. To this end, we first examined the effect of donor allografts on in vivo leukemic cell growth in F1 mice within the first 23 days after inoculation of leukemic cells. Specifically, F1 mice (H-2d/b) were first irradiated and then received allogeneic BM/S grafts from C57BL/6 mice (H-2b) or syngeneic BM/S grafts on the same day they received an intravenous inoculum of 5 × 106 BCL-1 leukemia cells. Mice were electively killed either on day 11 or day 23 after inoculation of BCL-1 cells and their leukemia burden was assessed by gross as well as histopathologic examination of their spleen and liver, determination of spleen weight, and immunophenotyping of spleen mononuclear cells. Control F1 mice undergoing syngeneic BMT had a small size spleen (76 ± 3 mg) when they were electively killed on day 11. By contrast, F1 mice undergoing syngeneic BMT and receiving BCL-1 leukemia cells all developed rapidly progressive leukemia and consequently showed massive splenomegaly on day 11 (571 ± 70 mg; P < .001; Table4, group A.2). Histopathologic examination of the spleen showed diffuse effacement of the normal architecture with leukemic cells. Leukemic infiltrates were easily detectable in the liver as well. Unlike F1 mice undergoing syngeneic BMT, none of the F1 mice undergoing allogeneic BMT that were inoculated with the same number of BCL-1 cells developed leukemia (Table 4, group A.5). The average day 11 spleen weights were 176 ± 17 mg for mice undergoing allogeneic BMT without a BCL-1 challenge and 163 ± 11 mg for mice undergoing allogeneic BMT and receiving a BCL-1 inoculum (Table 4, groups A.4 and A.5). Similarly, the average day 23 spleen weights were 111 ± 10 mg for mice undergoing allogeneic BMT without a BCL-1 challenge and 121 ± 8 mg for mice undergoing allogeneic BMT and receiving a BCL-1 inoculum (Table 4). No leukemic infiltrates were found in the day 11 or day 23 spleens or livers of mice receiving allotransplants (Table 4).
Effects of the allogeneic splenocyte dose and target leukemia burden on the GVL effect of the BM/S allografts in BMT recipient mice challenged with BCL-1 leukemia cells
| Groups . | BM/S dose . | BCL-1 dose no. (× 106) . | Days after BMT . | Histopathologic evidence of leukemia in spleen/liver . | Spleen BCL-1 leukemia burden . | |||
|---|---|---|---|---|---|---|---|---|
| BM no. (× 106) . | S no. (× 106) . | Spleen (mg) . | B220+ (%) . | BCL-1 B220+H-2Db− (% of B220+) . | ||||
| A.1 Syngeneic BMT | 25 | 25 | 0 | 11 | 0/6 | 76 ± 3 | 58 ± 3 | 0 ± 0 |
| A.2 Syngeneic BMT + BCL-1 | 25 | 25 | 5 | 11 | 8/8 | 571 ± 70 | 63 ± 8 | 48 ± 13 |
| A.3 Syngeneic BMT + BCL-1 → WHI-P131 | 25 | 25 | 5 | 11 | 8/8 | 591 ± 51 | 44 ± 2 | 40 ± 4 |
| A.4 Allo BMT | 25 | 25 | 0 | 11 | 0/6 | 176 ± 17 | 9 ± 0 | 0 ± 0 |
| 23 | 0/4 | 111 ± 10 | ND | ND | ||||
| A.5 Allo BMT + BCL-1 | 25 | 25 | 5 | 11 | 0/7 | 163 ± 11 | 8 ± 1 | 0 ± 0 |
| 23 | 0/5 | 121 ± 8 | ND | ND | ||||
| A.6 Allo BMT + BCL-1 → WHI-P131 | 25 | 25 | 5 | 11 | 0/7 | 181 ± 11 | 6 ± 1 | 0 ± 0 |
| 23 | 0/6 | 102 ± 4 | ND | ND | ||||
| B.1 Allo BMT + BCL-1 | 25 | 25 | 10 | 15 | 0/3 | 115 ± 18 | 11 ± 1 | 0 ± 0 |
| 30 | 0/3 | 89 ± 12 | 8 ± 1 | 0 ± 0 | ||||
| B.2 Allo BMT + BCL-1 → WHI-P131 | 25 | 25 | 10 | 15 | 0/5 | 120 ± 5 | 11 ± 1 | 0 ± 0 |
| 30 | 0/5 | 98 ± 8 | 13 ± 1 | 0 ± 0 | ||||
| B.3 Allo BMT + BCL-1 | 25 | 25 | 15 | 15 | 0/4 | 124 ± 4 | 10 ± 1 | 0 ± 0 |
| 30 | 0/3 | 70 ± 9 | 13 ± 1 | 0 ± 0 | ||||
| B.4 Allo BMT + BCL-1 → WHI-P131 | 25 | 25 | 15 | 15 | 0/5 | 140 ± 18 | 9 ± 1 | 0 ± 0 |
| 30 | 0/5 | 106 ± 13 | 22 ± 6 | 0 ± 0 | ||||
| B.5 Allo BMT + BCL-1 | 25 | 25 | 20 | 15 | 0/5 | 113 ± 10 | 11 ± 1 | 0 ± 0 |
| 30 | 0/3 | 85 ± 7 | 15 ± 1 | 0 ± 0 | ||||
| B.6 Allo BMT + BCL-1 → WHI-P131 | 25 | 25 | 20 | 15 | 0/3 | 111 ± 27 | 9 ± 1 | 0 ± 0 |
| 30 | 0/3 | 97 ± 1 | 19 ± 5 | 0 ± 0 | ||||
| C.1 Allo BMT + BCL-1 | 25 | 2.5 | 5 | 15 | 0/5 | 148 ± 20 | 12 ± 1 | 0 ± 0 |
| 30 | 0/5 | 63 ± 11 | 25 ± 9 | 0 ± 0 | ||||
| C.2 Allo BMT + BCL-1 → WHI-P131 | 25 | 2.5 | 5 | 15 | 0/5 | 158 ± 9 | 12 ± 2 | 0 ± 0 |
| 30 | 0/5 | 80 ± 5 | 33 ± 3 | 0 ± 0 | ||||
| C.3 Allo BMT + BCL-1 | 25 | 5 | 5 | 15 | 0/5 | 133 ± 9 | 8 ± 1 | 0 ± 0 |
| 30 | 0/5 | 73 ± 5 | 16 ± 3 | 0 ± 0 | ||||
| C.4 Allo BMT + BCL-1 → WHI-P131 | 25 | 5 | 5 | 15 | 0/5 | 130 ± 9 | 7 ± 0 | 0 ± 0 |
| 30 | 0/5 | 81 ± 7 | 19 ± 2 | 0 ± 0 | ||||
| C.5 Allo BMT + BCL-1 | 25 | 10 | 5 | 15 | ND | ND | ND | 0 ± 0 |
| 30 | 0/5 | 85 ± 4 | 14 ± 1 | 0 ± 0 | ||||
| C.6 Allo BMT + BCL-1 → WHI-P131 | 25 | 10 | 5 | 15 | 0/4 | 160 ± 10 | 7 ± 1 | 0 ± 0 |
| 30 | 0/5 | 91 ± 10 | 15 ± 3 | 0 ± 0 | ||||
| C.7 Allo BMT + BCL-1 | 25 | 20 | 5 | 15 | 0/5 | 115 ± 12 | 6 ± 1 | 0 ± 0 |
| 30 | 0/3 | 79 ± 13 | ND | ND | ||||
| C.8 Allo BMT + BCL-1 → WHI-P131 | 25 | 20 | 5 | 15 | 0/4 | 100 ± 18 | 6 ± 1 | 0 ± 0 |
| 30 | 0/4 | 85 ± 3 | 17 ± 3 | 0 ± 0 | ||||
| Groups . | BM/S dose . | BCL-1 dose no. (× 106) . | Days after BMT . | Histopathologic evidence of leukemia in spleen/liver . | Spleen BCL-1 leukemia burden . | |||
|---|---|---|---|---|---|---|---|---|
| BM no. (× 106) . | S no. (× 106) . | Spleen (mg) . | B220+ (%) . | BCL-1 B220+H-2Db− (% of B220+) . | ||||
| A.1 Syngeneic BMT | 25 | 25 | 0 | 11 | 0/6 | 76 ± 3 | 58 ± 3 | 0 ± 0 |
| A.2 Syngeneic BMT + BCL-1 | 25 | 25 | 5 | 11 | 8/8 | 571 ± 70 | 63 ± 8 | 48 ± 13 |
| A.3 Syngeneic BMT + BCL-1 → WHI-P131 | 25 | 25 | 5 | 11 | 8/8 | 591 ± 51 | 44 ± 2 | 40 ± 4 |
| A.4 Allo BMT | 25 | 25 | 0 | 11 | 0/6 | 176 ± 17 | 9 ± 0 | 0 ± 0 |
| 23 | 0/4 | 111 ± 10 | ND | ND | ||||
| A.5 Allo BMT + BCL-1 | 25 | 25 | 5 | 11 | 0/7 | 163 ± 11 | 8 ± 1 | 0 ± 0 |
| 23 | 0/5 | 121 ± 8 | ND | ND | ||||
| A.6 Allo BMT + BCL-1 → WHI-P131 | 25 | 25 | 5 | 11 | 0/7 | 181 ± 11 | 6 ± 1 | 0 ± 0 |
| 23 | 0/6 | 102 ± 4 | ND | ND | ||||
| B.1 Allo BMT + BCL-1 | 25 | 25 | 10 | 15 | 0/3 | 115 ± 18 | 11 ± 1 | 0 ± 0 |
| 30 | 0/3 | 89 ± 12 | 8 ± 1 | 0 ± 0 | ||||
| B.2 Allo BMT + BCL-1 → WHI-P131 | 25 | 25 | 10 | 15 | 0/5 | 120 ± 5 | 11 ± 1 | 0 ± 0 |
| 30 | 0/5 | 98 ± 8 | 13 ± 1 | 0 ± 0 | ||||
| B.3 Allo BMT + BCL-1 | 25 | 25 | 15 | 15 | 0/4 | 124 ± 4 | 10 ± 1 | 0 ± 0 |
| 30 | 0/3 | 70 ± 9 | 13 ± 1 | 0 ± 0 | ||||
| B.4 Allo BMT + BCL-1 → WHI-P131 | 25 | 25 | 15 | 15 | 0/5 | 140 ± 18 | 9 ± 1 | 0 ± 0 |
| 30 | 0/5 | 106 ± 13 | 22 ± 6 | 0 ± 0 | ||||
| B.5 Allo BMT + BCL-1 | 25 | 25 | 20 | 15 | 0/5 | 113 ± 10 | 11 ± 1 | 0 ± 0 |
| 30 | 0/3 | 85 ± 7 | 15 ± 1 | 0 ± 0 | ||||
| B.6 Allo BMT + BCL-1 → WHI-P131 | 25 | 25 | 20 | 15 | 0/3 | 111 ± 27 | 9 ± 1 | 0 ± 0 |
| 30 | 0/3 | 97 ± 1 | 19 ± 5 | 0 ± 0 | ||||
| C.1 Allo BMT + BCL-1 | 25 | 2.5 | 5 | 15 | 0/5 | 148 ± 20 | 12 ± 1 | 0 ± 0 |
| 30 | 0/5 | 63 ± 11 | 25 ± 9 | 0 ± 0 | ||||
| C.2 Allo BMT + BCL-1 → WHI-P131 | 25 | 2.5 | 5 | 15 | 0/5 | 158 ± 9 | 12 ± 2 | 0 ± 0 |
| 30 | 0/5 | 80 ± 5 | 33 ± 3 | 0 ± 0 | ||||
| C.3 Allo BMT + BCL-1 | 25 | 5 | 5 | 15 | 0/5 | 133 ± 9 | 8 ± 1 | 0 ± 0 |
| 30 | 0/5 | 73 ± 5 | 16 ± 3 | 0 ± 0 | ||||
| C.4 Allo BMT + BCL-1 → WHI-P131 | 25 | 5 | 5 | 15 | 0/5 | 130 ± 9 | 7 ± 0 | 0 ± 0 |
| 30 | 0/5 | 81 ± 7 | 19 ± 2 | 0 ± 0 | ||||
| C.5 Allo BMT + BCL-1 | 25 | 10 | 5 | 15 | ND | ND | ND | 0 ± 0 |
| 30 | 0/5 | 85 ± 4 | 14 ± 1 | 0 ± 0 | ||||
| C.6 Allo BMT + BCL-1 → WHI-P131 | 25 | 10 | 5 | 15 | 0/4 | 160 ± 10 | 7 ± 1 | 0 ± 0 |
| 30 | 0/5 | 91 ± 10 | 15 ± 3 | 0 ± 0 | ||||
| C.7 Allo BMT + BCL-1 | 25 | 20 | 5 | 15 | 0/5 | 115 ± 12 | 6 ± 1 | 0 ± 0 |
| 30 | 0/3 | 79 ± 13 | ND | ND | ||||
| C.8 Allo BMT + BCL-1 → WHI-P131 | 25 | 20 | 5 | 15 | 0/4 | 100 ± 18 | 6 ± 1 | 0 ± 0 |
| 30 | 0/4 | 85 ± 3 | 17 ± 3 | 0 ± 0 | ||||
The BMT recipients were inoculated with the “standard” BM (25 × 106)/S (25 × 106) inoculum (A and B) or with an inoculum consisting of 25 × 106 BM cells and varying doses of splenocytes (C), and challenged with “standard” (5 × 106) (A and C) or varying doses of BCL-1 leukemia cells (B). The leukemia burden was determined on day 11 and 23 (A) or 15 and 30 (B and C) after BMT by measuring the spleen weight, histopathologic examination of spleen and liver, and by immunophenotypic analysis of B220+ spleen cells (N = 3-6 mice/group), as described in “Materials and methods.” BCL-1 cells were identified as B220+H-2Db− cells. WHI-P131 was used at a dose level of 50 mg/kg per day. P was < .001 for A.2 versus A.1, A.3 versus A.1, A.5 versus A.2, A.6 versus A.2, A.6 versus A.3, B.1 versus A.2, B.3 versus A.2, B.5 versus A.2, B.2 versus A.3, B.4 versus A.3, B.6 versus A.3, C.1 versus A.2, C.3 versus A.2, C.5 versus A.2, C.7 versus A.2, C.2 versus A.3, C.4 versus A.3, C.6 versus A.3, C.8 versus A.3 for both spleen size as well as B220+H-2Db− leukemic BCL-1 fraction of B220+ cells in the spleen. There were no statistically significant differences for either the spleen size or the B220+H-2Db− leukemic BCL-1 fraction of B220+ splenocytes among any of the subgroups of group B or group C examined at the same time point after BMT. In all allogeneic BMT groups, including A.4, A.5, A.6, and all subgroups of B and C, the spleen size was smaller for mice examined at later time points after BMT. A.4 group mice undergoing allogeneic BMT had larger spleen (P < .001) and fewer B220+ splenic B cells (P < .001) than A.1 group mice undergoing syngeneic BMT.
We next used immunophenotyping with a highly sensitive quantitative multiparameter flow cytometric method to examine the spleens of allotransplanted F1 mice for the presence of B220+H-2Db− BCL-1 leukemia cells on day 11 after BMT. Whereas 48% ± 13% of the nucleated spleen cell populations from BCL-1 challenged F1 mice undergoing syngeneic BMT were B220+H-2Db− BCL-1 cells (Table 4, group A.2), no discrete leukemic cell population could be identified in the spleens of allotransplanted F1 mice by flow cytometric immunophenotyping (Table4, group A.5; P < .001). The percentages of B220+ B-lineage cells in the spleen cell suspensions were 9% ± 0% for allotransplanted F1 mice not challenged with BCL-1 leukemia cells and 8% ± 1% for allotransplanted F1 mice that were challenged with BCL-1 leukemia cells (Table 4, groups A.4 and A.5). These B220+ cells were 100% H-2Db+ consistent with their nonleukemic origin (Table 4).
We next set out to confirm the absence of residual leukemic cells in allotransplanted F1 recipient mice challenged with BCL-1 cells using F1 to BALB/c adoptive transfer experiments. To this end, 1 × 106 splenocytes obtained from these F1 recipients either 11 days (n = 7) or 23 days (n = 5) after BMT/BCL-1 inoculation were injected into the caudal vein of secondary BALB/c recipients. Notably, 8 of 9 BALB/c mice inoculated with day 11 splenocytes and 8 of 8 BALB/c mice inoculated with day 23 splenocytes survived without any evidence of leukemia more than 100 days and were electively killed between days 101 and 109. In contrast, 100% of control BALB/c recipients (n = 9) inoculated with day 11 after BMT splenocytes from F1 mice undergoing syngeneic BMT (n = 8) developed fatal leukemia and died with an MST of 15 days (Table5). At the time of death, control BALB/c recipients were found to have massive splenomegaly, whereas BALB/c recipients of day 11 or day 23 splenocytes from allotransplanted F1 mice had normal size spleens (1610 ± 70 mg versus 125 ± 6 mg and 123 ± 5 mg, respectively; P < .001; Table 5). Because 10 BCL-1 cells kill BALB/c mice within 12 weeks, these results demonstrate that the splenocyte suspensions used in the adoptive transfer experiments contained fewer than 10/1 000 000 (< 0.001%) BCL-1 cells.
Detection of BCL-1 leukemic cells in spleen of BMT recipient mice by adoptive transfer to secondary BALB/c recipient mice
| Group . | Donors . | Recipients . | |||||
|---|---|---|---|---|---|---|---|
| Days after BCL-1/BMT . | N . | No. cells . | N . | No. of survivors/total mice . | MST (d) . | Spleen weight at time of death (mg) . | |
| A. Syngeneic BMT + BCL-1 | 11 | 8 | 1 × 106 | 9 | 0/9 | 15 | 1610 ± 70 |
| B. Allogeneic BMT + BCL-1 | 11 | 7 | 1 × 106 | 9 | 8/9 | > 100 | 125 ± 6 |
| 23 | 5 | 1 × 106 | 8 | 8/8 | > 100 | 123 ± 5 | |
| C. Allogeneic BMT + BCL-1 → WHI-P131 | 11 | 7 | 1 × 106 | 9 | 9/9 | > 100 | 122 ± 3 |
| 23 | 6 | 1 × 106 | 7 | 7/7 | > 100 | 137 ± 12 | |
| > 60 | 9 | 0.5-1 × 106 | 9 | 9/9 | > 100 | 117 ± 6 | |
| D. Syngeneic BMT + BCL-1 → WHI-P131 | 11 | 8 | 1 × 106 | 9 | 0/9 | 15 | 1500 ± 97 |
| Group . | Donors . | Recipients . | |||||
|---|---|---|---|---|---|---|---|
| Days after BCL-1/BMT . | N . | No. cells . | N . | No. of survivors/total mice . | MST (d) . | Spleen weight at time of death (mg) . | |
| A. Syngeneic BMT + BCL-1 | 11 | 8 | 1 × 106 | 9 | 0/9 | 15 | 1610 ± 70 |
| B. Allogeneic BMT + BCL-1 | 11 | 7 | 1 × 106 | 9 | 8/9 | > 100 | 125 ± 6 |
| 23 | 5 | 1 × 106 | 8 | 8/8 | > 100 | 123 ± 5 | |
| C. Allogeneic BMT + BCL-1 → WHI-P131 | 11 | 7 | 1 × 106 | 9 | 9/9 | > 100 | 122 ± 3 |
| 23 | 6 | 1 × 106 | 7 | 7/7 | > 100 | 137 ± 12 | |
| > 60 | 9 | 0.5-1 × 106 | 9 | 9/9 | > 100 | 117 ± 6 | |
| D. Syngeneic BMT + BCL-1 → WHI-P131 | 11 | 8 | 1 × 106 | 9 | 0/9 | 15 | 1500 ± 97 |
Splenocytes were obtained at the indicated time points from lethally irradiated F1 mice reconstituted with (A) syngeneic BM/S grafts and injected with BCL-1 cells, (B) allogeneic BM/S grafts and injected with BCL-1 cells, (C) same as group B, but treated with WHI-P131, (D) same as group A but treated with WHI-P131.
Having documented the GVL activity of the allografts against 5 × 106 BCL-1 leukemia cells, we next sought to determine if the GVL function of the allografts is sufficient to protect F1 mice challenged with a larger dose of BCL-1 leukemia cells. TBI-conditioned F1 mice transplanted with C57BL/6 BM/S allografts containing the standard dose of 25 × 106 splenocytes were inoculated with BCL-1 cells at dose levels of 10 × 106, 15 × 106, or 20 × 106 leukemia cells/mouse. Mice were electively killed either on day 15 or day 30 after inoculation of BCL-1 cells and their leukemia burden was assessed by gross as well as histopathologic examination of their spleen and liver, determination of spleen weight, and immunophenotyping of spleen mononuclear cells. None of the allotransplanted F1 mice, even those challenged with 20 × 106 leukemia cells, developed splenomegaly, showed histopathologic evidence of leukemic infiltrates in spleen or liver, or immunophenotypic evidence of B220+H-2Db− BCL-1 leukemia cells in their spleen (Table 4, groups B.1, B.3, and B.5). There were no statistically significant differences for either spleen size or the B220+H-2Db− leukemic BCL-1 fraction of B220+ splenocytes among any of the subgroups challenged with a specific BCL-1 dose.
We next examined if splenocyte doses lower than 25 × 106splenocytes/allograft would be sufficient to achieve a desirable GVL activity against BCL-1 leukemia. TBI-conditioned F1 mice were transplanted with C57BL/6 BM/S allografts containing 2.5 × 106, 5 × 106, 10 × 106, or 20 × 106 splenocytes and challenged with a standard dose intravenous inoculum of 5 × 106 BCL-1 cells. None of the allotransplanted F1 mice, even those transplanted with allografts containing 2.5 × 106 splenocytes developed splenomegaly, showed histopathologic evidence of leukemic infiltrates in spleen or liver, or immunophenotypic evidence of B220+H-2Db− BCL-1 leukemia cells in their spleen (Table 4, groups C.1, C.3, C.5, and C.7).
Taken together, these results provide strong experimental evidence for a potent in vivo GVL function of the allografts from C57BL/6 mice (H-2b+) against BCL-1 (H-2b−) leukemia cells. The GVL function of the C57BL/6 BM/S allografts against BCL-1 leukemia was resistant to WHI-P131. Similar to allotransplanted F1 mice treated with vehicle, allotransplanted F1 mice treated with WHI-P131 did not develop leukemia regardless of the BCL-1 dose (Table4, groups A.6, B.2, B.4, and B.6) or splenocyte dose of the BM/S allograft (Table 4, groups A.6, C.2, C.4, C.6, and C.8). Specifically, these mice that showed no splenomegaly at their postmortem examination, the histopathologic examination of their spleen or liver revealed no morphologic evidence of leukemic infiltrates, and no discrete leukemic cell population could be identified in their spleens by flow cytometric immunophenotyping (Table 4). The absence of leukemia in WHI-P131–treated allotransplant recipient mice was due to the GVL function of the allografts rather than an antileukemic effect of WHI-P131 because WHI-P131 treatment of BCL-1-challenged F1 mice undergoing syngeneic BMT did not prevent the development of leukemia and leukemia-associated splenomegaly (Table 4, group A.3). In adoptive transfer experiments, BALB/c recipients of day 11 or day 23 splenocytes from allotransplanted F1 mice challenged with 5 × 106BCL-1 leukemia cells and treated with WHI-P131 did not develop leukemia (MST > 100 days), whereas BALB/c recipients of day 11 or day 23 splenocytes from F1 mice undergoing syngeneic BMT died of rapidly progressive leukemia with an MST of 15 days (Table 5).
GVHD prophylaxis with WHI-P131 improves the survival outcome of allotransplanted mice challenged with BCL-1 leukemia
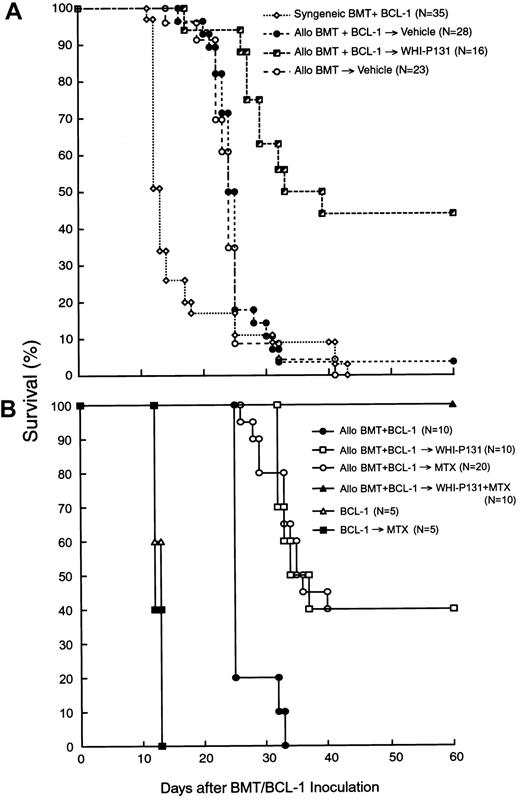
As shown in Table 1 and Figure 1A, allogeneic BMT altered the survival outcome of F1 mice after inoculation of BCL-1 leukemia cells. Unlike F1 mice undergoing syngeneic BMT (n = 22), which all developed rapidly progressive and fatal leukemia after inoculation of 5 × 106 BCL-1 cells with an MST of 14 days, none of the 28 F1 mice undergoing allogeneic BMT that were inoculated with the same number of BCL-1 cells died of leukemia. Furthermore, the survival outcome of allotransplanted F1 mice challenged with BCL-1 leukemia cells (MST, 25 days, n = 28) was virtually identical to the survival outcome of allotransplanted control F1 mice that were not inoculated with BCL-1 leukemia cells (MST, 24 days, n = 23; Table 1; Figure 1A). Thus, the challenge with BCL-1 leukemia cells did not worsen the survival of F1 mice undergoing allogeneic BMT. However, all of these mice eventually developed severe GVHD and died with an MST of 25 days (Table 1; Figure 1A). The average week 2 to 4 organ GVHD scores were 2.6 ± 0.1 for the liver, 1.9 ± 0.3 for the skin, 0.7 ± 0.1 for the small intestine, and 1.2 ± 0.2 for the large intestine (Table 2).
Effects of WHI-P131/JANEX-1 with or without MTX on survival.
The effects of the JAK3 inhibitor WHI-P131 (A) or WHI-P131 in combination with MTX (B) on the post-BMT survival outcome in a murine GVL model are shown. Irradiated (9.5 Gy) (BALB/cJxC57BL/6J) F1 (H-2d/b) recipients were given BM/S graft (25 × 106 cells of each) from syngeneic (syngeneic BMT) or allogeneic C57BL/6 (H-2b) donors (allo BMT). BMT recipients (GVL model) were injected with 5 × 106 leukemia/lymphoma BCL-1 cells on day 0. Recipients were treated with vehicle alone (A), WHI-P131 (50 mg/kg per day in 2 divided doses) alone (A,B), MTX (10 mg/m2 per day, single daily dose) alone (B), or WHI-P131 (50 mg/kg per day in 2 divided doses) plus MTX (10 mg/m2 per day, single daily dose; panel B) daily from day 0 until day 60 after BMT. Because there were no differences in survival rate of syngeneic BMT recipients treated with vehicle (n = 22) or with WHI-P131 (n = 13; Table1), they were summarized and presented as syngeneic BMT+BCl-1 group (n = 35; A). Controls (B) included mice inoculated with BCL-1 cells but not transplanted; some of these mice were treated with MTX alone.P < .0001 (WHI-P131–treated allogeneic BMT group versus vehicle-treated allogeneic BMT group, log-rank test, panel A);P < .001 (WHI-P131 or MTX versus no GVHD prophylaxis, log-rank test); P < .01 (WHI+MTX versus all other groups, log- rank test, panel B).
Effects of WHI-P131/JANEX-1 with or without MTX on survival.
The effects of the JAK3 inhibitor WHI-P131 (A) or WHI-P131 in combination with MTX (B) on the post-BMT survival outcome in a murine GVL model are shown. Irradiated (9.5 Gy) (BALB/cJxC57BL/6J) F1 (H-2d/b) recipients were given BM/S graft (25 × 106 cells of each) from syngeneic (syngeneic BMT) or allogeneic C57BL/6 (H-2b) donors (allo BMT). BMT recipients (GVL model) were injected with 5 × 106 leukemia/lymphoma BCL-1 cells on day 0. Recipients were treated with vehicle alone (A), WHI-P131 (50 mg/kg per day in 2 divided doses) alone (A,B), MTX (10 mg/m2 per day, single daily dose) alone (B), or WHI-P131 (50 mg/kg per day in 2 divided doses) plus MTX (10 mg/m2 per day, single daily dose; panel B) daily from day 0 until day 60 after BMT. Because there were no differences in survival rate of syngeneic BMT recipients treated with vehicle (n = 22) or with WHI-P131 (n = 13; Table1), they were summarized and presented as syngeneic BMT+BCl-1 group (n = 35; A). Controls (B) included mice inoculated with BCL-1 cells but not transplanted; some of these mice were treated with MTX alone.P < .0001 (WHI-P131–treated allogeneic BMT group versus vehicle-treated allogeneic BMT group, log-rank test, panel A);P < .001 (WHI-P131 or MTX versus no GVHD prophylaxis, log-rank test); P < .01 (WHI+MTX versus all other groups, log- rank test, panel B).
We then set out to determine if the survival outcome of allotransplanted F1 recipients challenged with BCL-1 leukemia cells could be improved by attenuating the severity of GVHD with the JAK3 inhibitor WHI-P131. Recipient mice were treated from the day of BMT/BCL-1 inoculation on with WHI-P131 (25 mg/kg per dose, twice daily, intraperitoneal injection; n = 16) or vehicle (n = 28). The organ-specific GVHD scores of WHI-P131–treated mice electively killed at 2 to 4 weeks were significantly lower than those of vehicle-treated control mice (Table 2). As shown in Figure 1A, GVHD prophylaxis using WHI-P131 markedly improved the post-BMT survival outcome. The probability of survival at 30 days after BMT was 11% ± 6% for vehicle-treated recipients (MST, 25 days) versus 63% ± 12% for recipients treated with WHI-P131 (MST, 36 days;P < .0001; Figure 1A and Table 1). Because WHI-P131 is devoid of antileukemic activity against BCL-1 leukemia cells (Table 1), this marked improvement in survival outcome was due to reduced incidence of GVHD-associated fatalities in the WHI-P131 group. All of the syngeneic BMT recipients treated with WHI-P131 died of leukemia with an MST of 12 days after the BCL-1 challenge (Table 1). Because of the sustained GVL function of the allografts, the challenge with BCL-1 leukemia cells did not worsen the post-BMT survival outcome of WHI-P131–treated allograft recipients (Table 1).
The long-term survivors in the WHI-P131–treated group showed no clinical signs of severe GVHD or leukemia. According to the GVHD scoring system, the average GVHD grades were 0.6 ± 0.1 for the liver, 0.0 ± 0.0 for the skin, 0.3 ± 0.1 for the small intestine, and 0.1 ± 0.1 for the large intestine (Table 2). Flow cytometric H-2Dd/b typing of splenocytes obtained from these 4 long-term survivors of the WHI-P131–treated group showed 95.3% ± 0.8% H-2Dd−/b+ donor cell engraftment (Table3), indicating that WHI-P131 does not prevent donor cell engraftment under these experimental conditions and the attenuation of GVHD in WHI-P131–treated recipient mice was not due to lack of donor cell engraftment.
GVHD prophylaxis with WHI-P131 plus MTX markedly improves the survival outcome of allotransplanted mice challenged with BCL-1 leukemia
We next set out to determine if the survival outcome of BCL-1 (5 × 106 cells/mouse) challenged F1 mice could be further improved by using a combination of WHI-P131 (50 mg/kg per day) plus MTX (10 mg/m2 per day), instead of WHI-P131 alone (50 mg/kg per day), for GVHD prophylaxis. As shown in Figure 1B and Table6, all 10 BCL-1–inoculated control F1 mice not receiving allografts died of leukemia in less than 2 weeks regardless of whether or not they were treated with MTX. Similarly, all 10 mice undergoing allogeneic BMT without any GVHD prophylaxis died with an MST of 25 days (Table 6). In contrast, when compared side-by-side, WHI-P131 alone (n = 10) as well as MTX alone (n = 20) conferred 40% long-term survival with an MST of 36 days (P < .0001 when compared to no GVHD prophylaxis). Notably, none of the 10 allotransplanted F1 mice treated with WHI-P131 plus MTX died within the 60-day post-BMT observation period (P < .0001 when compared to no prophylaxis andP = .01 when compared to WHI-P131 alone or MTX alone). Flow cytometric H-2Dd/b typing of splenocytes obtained from 7 long-term survivors treated with this effective 2-drug combination showed 95.6% ± 1.0% H-2Dd−/b+ donor cell engraftment (Table 3). Thus, GVHD prophylaxis with WHI-P131 plus MTX resulted in 100% survival of allotransplanted mice challenged with an otherwise invariably fatal dose of BCL-1 leukemia.
Effect of WHI-P131/JANEX-1 + MTX on post-BMT survival outcome in a mouse GVL/GVHD model
| Treatment protocol . | MST . | Cumulative proportion surviving (% ± SEM) . | P versus . | ||||
|---|---|---|---|---|---|---|---|
| N . | d . | 15 d . | 30 d . | 60 d . | C . | F . | |
| A. BCL-1 | 5 | 13 | 0 ± 0 | 0 ± 0 | 0 ± 0 | — | — |
| B. BCL-1MTX | 5 | 12 | 0 ± 0 | 0 ± 0 | 0 ± 0 | — | — |
| C. Allogeneic BMT + BCL-1 | 10 | 25 | 100 ± 0 | 20 ± 13 | 0 ± 0 | — | < .0001 |
| D. Allogeneic BMT + BCL-1 → WHI-P131 | 10 | 36 | 100 ± 0 | 100 ± 0 | 40 ± 16 | .0002 | .01 |
| E. Allogeneic BMT + BCL-1 → MTX | 20 | 36 | 100 ± 0 | 80 ± 9 | 40 ± 12 | < .0001 | .01 |
| F. Allogeneic BMT + BCL-1 → WHI-P131 + MTX | 10 | > 60 | 100 ± 0 | 100 ± 0 | 100 ± 0 | < .0001 | — |
| Treatment protocol . | MST . | Cumulative proportion surviving (% ± SEM) . | P versus . | ||||
|---|---|---|---|---|---|---|---|
| N . | d . | 15 d . | 30 d . | 60 d . | C . | F . | |
| A. BCL-1 | 5 | 13 | 0 ± 0 | 0 ± 0 | 0 ± 0 | — | — |
| B. BCL-1MTX | 5 | 12 | 0 ± 0 | 0 ± 0 | 0 ± 0 | — | — |
| C. Allogeneic BMT + BCL-1 | 10 | 25 | 100 ± 0 | 20 ± 13 | 0 ± 0 | — | < .0001 |
| D. Allogeneic BMT + BCL-1 → WHI-P131 | 10 | 36 | 100 ± 0 | 100 ± 0 | 40 ± 16 | .0002 | .01 |
| E. Allogeneic BMT + BCL-1 → MTX | 20 | 36 | 100 ± 0 | 80 ± 9 | 40 ± 12 | < .0001 | .01 |
| F. Allogeneic BMT + BCL-1 → WHI-P131 + MTX | 10 | > 60 | 100 ± 0 | 100 ± 0 | 100 ± 0 | < .0001 | — |
(BALB/cJxC57BL/6J)F1 (H-2d/b) recipients were lethally irradiated (9.5 Gy) and transplanted with BM/S grafts from syngeneic F1 or allogeneic C57BL/6 (H-2b) donors. The survival outcome of different treatment groups was compared. GVHD prophylaxis included WHI-P131 dose (50 mg/kg per day), MTX dose (10 mg/m2 per day), or vehicle alone. P (log-rank test) < .05 was considered significant.
Discussion
We recently reported the structure-based design of specific inhibitors of JAK3 as apoptosis-inducing antileukemic agents.18 The lead compound, WHI-P131/JANEX-1, inhibited JAK3 but not JAK1 or JAK2.18 Similarly, the ZAP/SYK family tyrosine kinase SYK, TEC family tyrosine kinase BTK, SRC family tyrosine kinase LYN, and receptor family tyrosine kinase IRK were not inhibited by WHI-P131. The use of this compound in biologic assays confirmed that JAK3 is a vital target in human leukemia cells and demonstrated that WHI-P131 triggers apoptosis in lymphoblastic as well as myeloblastic leukemia cells.18 WHI-P131 was very well tolerated by mice and monkeys, and plasma concentrations of WHI-P131 that are cytotoxic to human leukemia cells in vitro could be achieved at nontoxic dose levels.19 The antileukemic activity and lack of significant systemic toxicity of WHI-P131 suggest that this JAK3 inhibitor may be useful in the treatment of relapsed or therapy-refractory leukemia patients. Intriguingly, WHI-P131 did not cause any myelosuppression in mice or monkeys,19indicating that JAK3 does not have a critical nonredundant function in normal myelopoiesis.
The pivotal role of JAK3 in normal lymphocyte development and function along with the cytotoxic effects of WHI-P131 on leukemia cells prompted the hypothesis that this JAK3 inhibitor could be useful as a dual-function anti-GVHD agent with potent antileukemic activity. In a more recent study, we examined the effectiveness of targeting JAK3 with WHI-P131 for prevention and treatment of lethal GVHD across a major histocompatibility barrier in mice.20 WHI-P131 exhibited potent in vivo biologic activity in an aggressive acute GVHD model using BALB/c (H-2d) donor BM/S cells and H-2 disparate C57BL/6 (H-2b) recipient mice. WHI-P131 could improve the survival outcome even when the therapy was delayed until the third week after BMT.20
The purpose of the present study was to evaluate the effects of GVHD prophylaxis with the JAK3 inhibitor WHI-P131 on the GVL function of marrow allografts in mice undergoing BMT after being challenged with an otherwise invariably fatal dose of BCL-1 leukemia cells. We first confirmed that (1) severe GHVD can be induced in lethally irradiated (BALB/cJxC57BL/6J) F1 mice (H-2d/b) across the MHC barrier by injection of BM/S grafts from C57BL/6 mice (H-2b) and (2) WHI-P131 can improve survival in this BMT model by attenuating the severity of GVHD. We next set out to characterize the in vivo GVL function of the allografts from C57BL/6 mice (H-2b+) against BCL-1 (H-2b−) leukemia cells by comparing the BMT survival outcome of syngeneic versus allogeneic BMT recipients. Detailed studies using pathologic and histopathologic examinations, immunophenotyping, and adoptive transfer experiments aimed at identifying residual BCL-1 leukemia cells in allotransplanted F1 recipients provided strong experimental evidence for a potent in vivo GVL function of the allografts from C57BL/6 mice (H-2b+) against BCL-1 (H-2b−) leukemia cells.
We next set out to determine if the survival outcome of allotransplanted F1 recipients challenged with BCL-1 leukemia cells could be improved by attenuating the severity of GVHD with the JAK3 inhibitor WHI-P131. Notably, GVHD prophylaxis using WHI-P131 markedly improved the post-BMT survival outcome. Because WHI-P131 is devoid of antileukemic activity against BCL-1 leukemia cells, the improvement in survival outcome was due to reduced incidence of GVHD-associated fatalities combined with sustained GVL function of the allografts in the WHI-P131 group. The long-term survivors in the WHI-P131–treated group showed no clinical signs of severe GVHD or leukemia. Notably, adoptive transfer experiments demonstrated that the spleens of WHI-P131–treated allograft recipients contained less than 0.001% BCL-1 cells.
Even though the GVHD prophylaxis with WHI-P131 significantly improved the survival outcome of BCL-1–challenged F1 mice undergoing allogeneic BMT and prevented leukemic deaths, more than half of the mice died of GVHD. In a recent study, we found that the combination of WHI-P131 with the standard anti-GVHD drug MTX was more effective than WHI-P131 alone or MTX alone in preventing fatal GVHD. Therefore, we next set out to determine if the survival outcome of BCL-1–challenged F1 mice could be further improved by using a combination of WHI-P131 plus MTX for GVHD prophylaxis. Notably, GVHD prophylaxis with WHI-P131 plus MTX resulted in 100% survival of allotransplanted mice challenged with an otherwise invariably fatal dose of BCL-1 leukemia.
Taken together, our results provide strong experimental evidence that GVHD prophylaxis using WHI-P131 does not impair the GVL function of the allografts and consequently contributes to an improved post-BMT survival outcome of the recipient mice. In the present study, we used the JAK3− BCL-1 leukemia cells to avoid the antileukemic activity of WHI-P131 against JAK3+ leukemia cells as a confounding factor in the evaluation of its effects on the GVL function of marrow allografts. We hypothesize that the antileukemic activity of WHI-P131 against JAK3-expressing human leukemia cells18 may further enhance its ability to attenuate the severity of GVHD without increasing the risk of relapse post-BMT in clinical settings.
The authors wish to thank Angela L. Willardson and Randall Carpenter for excellent technical assistance.
M.C.-C. is a Michael Boyum Scholar in Bone Marrow Transplantation. F.M.U. is a Hughes Chair in Oncology.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Fatih M. Uckun, Parker Hughes Cancer Center, 2665 Long Lake Rd, Suite 300, St Paul, MN 55113; e-mail:fatih_uckun@ih.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal