We have read with interest the paper of Huhn et al on rituximab therapy of patients with B-cell chronic lymphocytic leukemia (CLL).1 Seven of 28 heavily pretreated patients showed a partial remission (NCI criteria), lasting for a median of 20 weeks. Huhn et al conclude that rituximab as a single agent might play a role in patients with poor marrow reserve for whom other options have failed. We would like to comment on the role of this therapy in refractory patients with concomitant acute hemolitic anemia (AHA). About 4% of CLL patients develop AHA,2 mainly in those with active disease, and few data exist regarding the efficacy and safety of rituximab in this clinical setting.3
We recently employed rituximab as rescue therapy for a patient with resistant B-cell chronic lymphocytic leukemia (B-CLL) and secondary AHA. A 52-year-old patient, diagnosed in January 1996 as having stage Rai II classical B-CLL, was admitted to our department after 24 months in disease progression (huge splenomegaly, anemia, and lymphocyte count above 50 000/μL) complicated by AHA (warm antibody type). Thereafter, he was given the following therapy: chlorambucil and prednisone, fludarabine+mitoxantrone+dexamethasone (FND), and cyclophosphamide+adriamycin+vincristine+prednisone (CHOP). After each line of therapy the patient attained only a short (less than 6 months') partial remission (PR), followed by tumor regrowth (splenomegaly, lymphocytosis, and anemia). In August 2000 he was admitted to our department again for disease progression. Physical examination revealed overt jaundice and hepato- and splenomegaly (15 cm below costal margin). Laboratory data were as follows: hematocrit, 21.3%; hemoglobin level, 6.6 g/dL; reticulocyte count, 320 × 109/L; mean corpuscular volume, 106 fL; platelet count, 166 × 109/L; white blood cell count, 32 × 109/L with 38% lymphocytes, 50% prolymphocytes, and 12% neutrophils; bilirubin level, 35 μM; lactate dehydrogenase level, 894 IU/L (normal is below 460 IU/L); and a direct antiglobulin test (DAT) positive for both complement (3+/4+) and IgG (4+/4+). The immunophenotype of peripheral blood lymphoid cells showed a typical B-CLL pattern (CD19+/CD5+/CD23+/CD10−) with unusual CD20 bright fluorescence intensity. He was naı̈ve for hepatitis viruses and had an antibody pattern indicating a remote infection of Epstein Barr virus and cytomegalovirus. The patient did not respond to a 10-day course of 6-metilprednisolone IV (250 mg/d). Then, after obtaining the informed consent, we began a therapy with rituximab (Mabthera) at 375mg/m2/wk for 4 weeks, and tapered prednisone over 2 weeks. The follow-up is shown in Figure1.
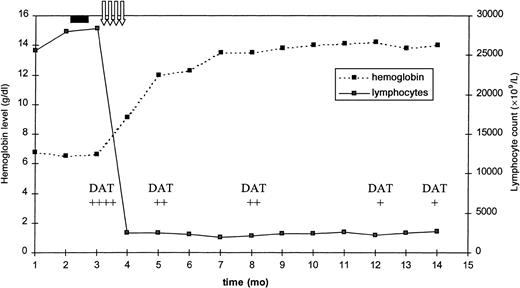
Changes in hemoglobin level, lymphocyte count, and DAT score following rituximab therapy.
The solid black rectangle indicates prednisone therapy; the vertical arrows, rituximab therapy; and DAT, direct antiglobulin test.
Changes in hemoglobin level, lymphocyte count, and DAT score following rituximab therapy.
The solid black rectangle indicates prednisone therapy; the vertical arrows, rituximab therapy; and DAT, direct antiglobulin test.
The first infusion of rituximab produced a marked reduction of the lymphocytosis, and after 5 days the hemoglobin level started to increase. No side effects related to rituximab infusion were recorded. At the end of week 8, the patient was re-evaluated. There were no signs of active AHA (reticulocyte count and hemoglobin, lactate dehydrogenase, and haptoglobin levels within range), and DAT was slightly positive (score +/4+). According to NCI criteria, the patient was judged to be in PR, because of the persistence of the splenomegaly, while having normal hemogram elements and a bone marrow interstitial lymphocyte infiltration of about 15%. After 12 months of follow-up the patient is still in PR, and this compares favorably to the median disease progression time of 20 weeks reported by Huhn et al.1 The rapid response of AHA to rituximab markedly contrasts to the slow response to conventional therapy (median, 4.5 months), as reported by Mauro et al.2 The almost simultaneous response of CLL and AHA could be interpreted as due to the clearance of both the neoplastic and the autoreactive clones.3 Further studies are warranted to clarify the anti-CD20 role in the treatment of CLL in general and in patients with secondary AHA in particular.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal