Abstract
Chronic myelomonocytic leukemia (CMML) is a hematologic malignancy characterized by wide heterogeneity of clinical presentation and course. CMML shares myelodysplastic characteristics with features of myeloproliferative disorders. No treatment has proven effective in modifying the natural course of the disease. To improve the prognostic assessment of clinical outcome, the associations of patient and disease characteristics with survival times of 213 patients with CMML was investigated retrospectively. Median survival was 12 months. Univariate analysis identified low hemoglobin level; low platelet count; high white blood cell, monocyte, and lymphocyte counts; presence of circulating immature myeloid cells, high percentage of marrow blasts, low percentage of marrow erythroid cells, abnormal cytogenetics, and high levels of serum lactate dehydrogenase and β2-microglobulin as characteristics associated with shorter survival. Hemoglobin level below 120 g/L (12 g/dL), presence of circulating immature myeloid cells, absolute lymphocyte count above 2.5 × 109/L, and marrow blasts 10% or more were independently associated with shorter survival by multivariate analysis and were used to generate a prognostic score. The model identified 4 subgroups of patients with median survival of 24, 15, 8, and 5 months for low, intermediate-1, intermediate-2, and high risk, respectively. Researchers could not confer objective evidence suggesting that arbitrary divisions of CMML by white blood cell counts into “dysplastic” and “proliferative” categories reflect clinical entities differing in the risk of acute leukemia development, although a trend of shorter survival in patients with leukocytosis was observed. The prognostic model was compared with 6 previously published scoring systems for myelodysplastic syndrome/CMML. The reported results should provide an improved assessment of prognosis in CMML.
Introduction
Chronic myelomonocytic leukemia (CMML) is characterized by increased monocytes in the bone marrow and peripheral blood and a variable degree of marrow dysplasia. The classification of CMML remains a subject of debate.1-9 Because it is frequently accompanied by dysplastic hematopoiesis, CMML was classified as a subcategory within myelodysplastic syndromes (MDSs) by the French-American-British Cooperative Leukaemia Group (FAB) in 1982.10 However, CMML is more heterogeneous than other types of MDSs. Thus, while some patients present with only modest leukocytosis, others have high white blood cell (WBC) counts and organ involvement, eg, splenomegaly, serous effusions, and lymph node or skin infiltration. Accordingly, an arbitrarily chosen leukocyte count has been recently used to distinguish between a “dysplastic” type (MDS-CMML; WBC count ≤ 13 × 109/L) and a “proliferative” type (myeloproliferative disorder [MPD]-CMML; WBC count > 13 × 109/L).4,9 A recent proposal by the World Health Organization classification committee included CMML in a new category of MDS/MPD disorders.11
The natural course of CMML is variable, with reported life expectancy ranging from several months to several years. Numerous studies have been conducted to identify factors associated with these different prognoses. Indeed, analysis of prognostic factors may help us to understand the biology of the disease, develop risk-tailored treatment programs, and evaluate new treatments for defined groups. Most such studies have been carried out within overall populations of MDS patients; the largest number of patients with CMML included in a single study was 125.12
At least 19 studies12-30 that included more than 30 patients with CMML each sought to assess the prognostic value of laboratory and clinical variables and to delineate prognostic factors. However, the selection of patients for these studies was undoubtedly influenced by diagnostic criteria. Some reports included patients with up to 20% bone marrow blasts and adhered to the FAB criteria for CMML. Others included patients with organ infiltration and up to 30% bone marrow blasts. These differences may have been responsible for the variability of median survival times across studies. In our study, we emphasized a strict adherence to the FAB classification of CMML and used karyotyping to exclude patients with t(9;22). The objectives of this study were (1) to analyze survival in 213 patients diagnosed with CMML at The University of Texas M. D. Anderson Cancer Center (MDACC) and identify independent covariates associated with survival; (2) to examine the ability of published prognostic systems to stratify patients according to risk; (3) to design a new, simple, and clinically useful scoring system based on data from a large number of patients; and (4) to compare prognostic variables and survival in CMML patients with “dysplastic” and “proliferative” disease.
Patients and methods
Study group
From August 1966 through March 1999, 213 patients diagnosed with CMML were evaluated at MDACC. These patients were identified through an extensive search of our leukemia databases. Diagnostic criteria for CMML strictly adhered to those proposed by FAB in 1982 10: blood monocytes above 1 × 109/L; bone marrow blasts 20% or less associated with hematopoietic dysplastic features; peripheral blasts below 5%; and absence of Auer rods in myeloid cells. Blood differential counts were based on manual reading of Giemsa-stained blood smears at the hematopathology department of MDACC. A percentage of monocytes in the peripheral blood of 8% or more was taken as an additional criterion to exclude patients whose absolute monocytosis was not matched by a corresponding relative monocytosis—namely, those with very high leukocyte counts. The threshold of 8% was chosen for consistency with our previous reports.31 32
The median time between the first detection of hematologic abnormalities and the assignment of diagnosis at MDACC was 3 months (range, 0-109 months).
After referral to our institution, patients received either supportive care with or without hematopoietic growth factors (erythropoietin, granulocyte-macrophage colony-stimulating factor, interleukin-3, interleukin-4, steroids) (n = 71), α- or γ-interferon (n = 9), low-dose or single-agent chemotherapy (hydroxyurea with or without busulfan or mithramycin, low-dose cytarabine, topotecan, fludarabine, 6-mercaptopurine, thioguanine, oral idarubicin, oral etoposide, 9-nitrocamptothecin, azacitidine) (n = 68), or more intensive intravenous and/or combination chemotherapy (n = 65). Although the analysis of treatment-associated differences was not an objective of this study, we evaluated the differences in survival between these subgroups of patients. We also assessed the impact of the time between the first signs of an antecedent hematologic disorder (AHD) and the date of referral to our institution, because patients who were treated before the referral were usually the ones who were referred later. AHD was defined as a hematologic abnormality (platelet count < 150 × 109/L or > 440 × 109/L; WBC count < 3.9 × 109/L or > 10 × 109/L; absolute neutrophil count < 1.0 × 109/L; or hemoglobin level < 120g/L [12 g/dL]) documented at least 30 days prior to and verified at the time of referral to MDACC. Finally, to exclude possible time-related bias, we evaluated survival according to the decade of treatment.
Blood and bone marrow studies were performed on the date of admission to MDACC. With the exception of 9 patients seen before the introduction of chromosome banding at MDACC in 1973, all patients had cytogenetic analysis performed using the GTG banding technique on bone marrow and/or peripheral blood cells, which were routinely processed after 24 to 48 hours in culture. At least 20 metaphases were examined for each patient.
Prognostic factor analysis and statistical methods
Univariate analysis of prognostic factors.
Clinical, biochemical, and hematologic characteristics were analyzed for their association with survival. These characteristics were age, sex, presence of splenomegaly, history of previous malignancies, presence of AHD, hemoglobin level, platelet and WBC counts, peripheral blood differential counts (manual counting of 200 cells), serum lactate dehydrogenase (LDH), serum β2-microglobulin, bone marrow parameters (bone marrow differentials, myeloid–erythroid cell ratio, and karyotype), and presence of N- or K-ras point mutations. In the peripheral blood, myeloblasts, promyelocytes, myelocytes, and metamyelocytes were analyzed together as a single covariate (immature myeloid cells [IMCs]), whereas in the bone marrow blast percentage was regarded as a single covariate. However, patients with circulating blasts of 5% or more were excluded from the study.10
Estimates of survival were based on the Kaplan-Meier method and were calculated from the time of referral to the MDACC; curves were compared among groups using the log-rank test. For the purpose of reporting survival distributions for which plots are not presented, curves are summarized in tabular form by means of the 75th, 50th, and 25th percentile estimates. For covariates measured on a continuous scale, patients were grouped into 2 or 3 categories using selected cutoff points. WBC count, absolute monocyte and lymphocyte counts, platelet count, and marrow blasts percentage were further investigated for the form of their association with survival by martingale residual plots analysis. Various cutoff points, including those shown to be significant in previously published studies of CMML patients, were individually analyzed.
Correlation analysis.
Rank correlation coefficients were calculated to evaluate associations between pairs of continuous variables. These variables were compared for patients grouped separately by presence or absence ofras mutation, abnormal karyotype, or AHD using a Wilcoxon test.33
Multivariate analysis.
To evaluate the association of multiple patient and disease characteristics with survival, we applied both a (1) classification and regression tree (CART) analysis34,35 and a (2) Cox proportional hazards regression model.36
(1) The CART method, also termed “recursive partitioning,” searches for appropriate cutoff points for continuous covariates and considers the possibility of interactions among covariates.34 35This computer-intensive tool is nonparametric because it does not depend on any underlying distributional assumptions, ie, it does not assume any cutoff point to analyze the data.
Beginning with the total set of patients and measurements of select covariates, the program first determined for each possible predictor variable a cutoff point by which the population could be split into 2 subgroups most different in the survival-time outcome and then selected the single one of these variables that could identify 2 groups most different in their survival times. The process was repeated on resulting subgroups until no further partitioning was warranted, either because a subgroup was homogeneous for the survival-time variable or because the subgroup was too small to divide further. The final result was a survival tree (Figure 3).
(2) The proportional hazards model allows the relative prognostic importance of each factor to be evaluated while simultaneously considering the effects of other covariates. For the purpose of clinical utility, continuous covariates were regarded as dichotomous, with categories determined based on consideration of previously reported cutoff points in this disease as well as on inspection of residual plots to assess the functional forms of the associations of interest.37
Acute leukemia transformation.
A cause-specific method38 was used to calculate the incidence of transformation of CMML to acute myelogenous leukemia (AML). This method considered the separate competing hazards of developing AML or dying of CMML and computed the cumulative incidence of AML while allowing for the competing risk of death.
Other scoring systems
Patient data were also assessed using 5 previously published scoring systems for MDS12,14,22,28,39 and 1 for CMML21 that assign point scores for various parameters and classify patients into 2, 3, or 4 categories based on predicted survival times.
Among 141 MDS patients analyzed to design the Bournemouth14scoring system, 31 were CMML cases. Median age of the entire group was 73 years, and median survival of the CMML patients was 22 months. The modified Bournemouth21 system was derived from data on a series of 53 CMML patients who had a median age of 79 years and a median survival of 17 months. The Spanish22 scoring system was based on the data of 370 MDS patients, including 70 CMMLs. Median age was 68 years, and median survival of the CMML patients was 12 months. The 235 patients in the Düsseldorf28 study, which included 25 patients with CMML, had a median age of 72 years. Median survival of the CMML patients was 19 months. The Lille classification12 was derived from data on 408 MDS patients, 125 of whom had CMML. In this study, median age of the entire group was 65 years, and median survival of CMML patients was 21 months. Finally, the IPSS (International Prognostic Scoring System)39 was developed based on data on 816 patients with primary MDS. Concerning CMML, only those patients with a WBC count 12 × 109/L or less were included in the IPSS meta-analysis, for a total number of 126 and a median survival of 2.4 years.
Results
Study group
The study group comprised 150 men (70.4%) and 63 women (29.6%); the male-female ratio was 2.4. Median age of all 213 patients was 65 years (range, 20-88 years). The initial clinical and hematologic findings are summarized in Tables 1 and2. A total of 71% had an AHD. Splenomegaly was observed in 61 patients (29%).
Characteristics and initial laboratory values of 213 patients with CMML
| Variable . | Median . | Range . |
|---|---|---|
| Age, y | 65 | 20-88 |
| Male, no. (%) | 150 (70.4) | — |
| Female, no. (%) | 63 (29.6) | — |
| Hemoglobin, g/L (g/dL) | 102 (10.2) | 52-156 (5.2-15.6) |
| Platelet count, × 109/L | 87 | 4-706 |
| White blood cell count, × 109/L | 20.4 | 2.1-352 |
| Neutrophils, % | 46 | 3-81 |
| Neutrophils, × 109/L | 9.8 | 0.3-186.5 |
| Monocytes, % | 22 | 8-92 |
| Monocytes, × 109/L | 4.2 | 1.0-162 |
| Lymphocytes, % | 15 | 1-57 |
| Lymphocytes, × 109/L | 3.1 | 0.3-22.9 |
| Eosinophils, % | 0 | 0-30 |
| Basophils, % | 0 | 0-9 |
| Blasts plus promyelocytes, % | 0 | 0-22 |
| Myelocytes plus metamyelocytes, % | 3 | 0-32 |
| Peripheral blood IMCs, % | 3 | 0-38 |
| Bone marrow blasts, % | 4 | 0-19 |
| Bone marrow monocytes, % | 12.2 | 0.3-78 |
| Bone marrow lymphocytes, % | 5.8 | 0-47 |
| Bone marrow erythroid cells, % | 13.4 | 0.4-65.4 |
| Myeloid–erythroid cell ratio | 4.3 | 0.2-192 |
| LDH, U/L | 783 | 270-5310 |
| β2-microglobulin, mg/L* | 3.8 | 1-15.1 |
| Variable . | Median . | Range . |
|---|---|---|
| Age, y | 65 | 20-88 |
| Male, no. (%) | 150 (70.4) | — |
| Female, no. (%) | 63 (29.6) | — |
| Hemoglobin, g/L (g/dL) | 102 (10.2) | 52-156 (5.2-15.6) |
| Platelet count, × 109/L | 87 | 4-706 |
| White blood cell count, × 109/L | 20.4 | 2.1-352 |
| Neutrophils, % | 46 | 3-81 |
| Neutrophils, × 109/L | 9.8 | 0.3-186.5 |
| Monocytes, % | 22 | 8-92 |
| Monocytes, × 109/L | 4.2 | 1.0-162 |
| Lymphocytes, % | 15 | 1-57 |
| Lymphocytes, × 109/L | 3.1 | 0.3-22.9 |
| Eosinophils, % | 0 | 0-30 |
| Basophils, % | 0 | 0-9 |
| Blasts plus promyelocytes, % | 0 | 0-22 |
| Myelocytes plus metamyelocytes, % | 3 | 0-32 |
| Peripheral blood IMCs, % | 3 | 0-38 |
| Bone marrow blasts, % | 4 | 0-19 |
| Bone marrow monocytes, % | 12.2 | 0.3-78 |
| Bone marrow lymphocytes, % | 5.8 | 0-47 |
| Bone marrow erythroid cells, % | 13.4 | 0.4-65.4 |
| Myeloid–erythroid cell ratio | 4.3 | 0.2-192 |
| LDH, U/L | 783 | 270-5310 |
| β2-microglobulin, mg/L* | 3.8 | 1-15.1 |
Data available for 64 patients.
Initial findings of clinical abnormalities, abnormal karyotype, and ras point mutations
| Variable . | No. of patients (%*) . |
|---|---|
| AHD | 151 (70.9) |
| Splenomegaly | 61 (30.7) |
| Hepatomegaly | 38 (18.9) |
| Enlarged lymph nodes | 28 (13.9) |
| Previous malignancy | 34 (16) |
| Abnormal karyotype | 70 (34.1) |
| ras point mutation† | 25 (38.4) |
| Variable . | No. of patients (%*) . |
|---|---|
| AHD | 151 (70.9) |
| Splenomegaly | 61 (30.7) |
| Hepatomegaly | 38 (18.9) |
| Enlarged lymph nodes | 28 (13.9) |
| Previous malignancy | 34 (16) |
| Abnormal karyotype | 70 (34.1) |
| ras point mutation† | 25 (38.4) |
Percentages calculated on the basis of data availability.
Data available for 65 patients.
Cytogenetic data were available for 205 patients (Table3). Thirty-four percent had chromosomal abnormalities, which were limited to relatively few types. The most common abnormal karyotypes were monosomy 7 (7.8%) and trisomy 8 (6.3%). Only 6.3% of patients had complex karyotypes (≥ 3 abnormalities). Of the 65 patients examined by polymerase chain reaction–based DNA sequencing of N- and K-ras oncogenes, 25 (38%) had a point mutation.
Occurrence and frequency of chromosomal abnormalities
| Karyotype . | No. of patients . | % . |
|---|---|---|
| Total | 213 | — |
| Available data | 205 | 100 |
| Diploid | 135 | 65.9 |
| − 7 (single abnormality) | 16 | 7.8 |
| + 8 with or without 1 additional abnormality | 13 | 6.3 |
| Complex3-150 | 13 | 6.3 |
| Miscellaneous | 28 | 13.6 |
| − 5 and − 7 | 3 | 1.46 |
| + 21 | 2 | .98 |
| 20q- | 2 | .98 |
| 12p- | 2 | .98 |
| i(17) ± other single abnormalities | 2 | .98 |
| Other chromosomal abnormalities3-151 | 17 | 8.3 |
| Karyotype . | No. of patients . | % . |
|---|---|---|
| Total | 213 | — |
| Available data | 205 | 100 |
| Diploid | 135 | 65.9 |
| − 7 (single abnormality) | 16 | 7.8 |
| + 8 with or without 1 additional abnormality | 13 | 6.3 |
| Complex3-150 | 13 | 6.3 |
| Miscellaneous | 28 | 13.6 |
| − 5 and − 7 | 3 | 1.46 |
| + 21 | 2 | .98 |
| 20q- | 2 | .98 |
| 12p- | 2 | .98 |
| i(17) ± other single abnormalities | 2 | .98 |
| Other chromosomal abnormalities3-151 | 17 | 8.3 |
Complex karyotype, at least 3 chromosomal abnormalities.
Other chromosomal abnormalities were as follows: − 5, inv(Y), + 10, + 14, + 19, + X, 5q-, 11q-, 12q-, add(4) [q35], t(11;22), t(11;16), t(4;15), t(9;21), − Y, − F + G, − C.
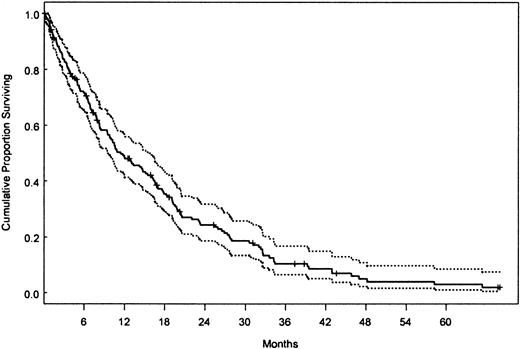
Patient survival
At the time of last follow-up, 167 patients had died. Median follow-up time for living patients was 10 months (range, 0-154 months). Median survival was 12 months (95% confidence interval [CI], 10-16). The survival curve (with 95% CI) for the whole study group is shown in Figure 1. No significant difference in survival time was observed between patients who received supportive treatment, interferon-based therapy, low-dose/single-agent chemotherapy, or intensive/combination chemotherapy, although there was a trend of shorter survival for the latter subgroup of patients (data not shown). Likewise, we did not observe differences in survival times between patients referred to our institution at the first occurrence of hematologic disorder and patients who were referred 1, 3, 6, or 12 months later and, also, among patients who were referred in the decades of the 1960s/1970s, 1980s, or 1990s (12%, 25%, and 63% of patients, respectively) (data not shown). Therefore, treatment did not appear to have a major impact on survival or interact with the influence of patient characteristics on survival. Although early survival experience of patients with MDS-CMML and MPD-CMML was similar (median 13 vs 12 months, respectively), a trend for increased risk was noted for the latter group after 16 months (Figure 2A,P = .02).
Kaplan-Meier survival curve of CMML patients.
Overall survival of all 213 patients with CMML (dotted lines denote 95% CI).
Kaplan-Meier survival curve of CMML patients.
Overall survival of all 213 patients with CMML (dotted lines denote 95% CI).
Kaplan-Meier survival curves of CMML patients grouped according to patient characteristics identified by univariate analysis.
Survival curves for patients grouped by (A) WBC counts (≤ or > 13 × 109/L); (B) hemoglobin (HGB) level (≥ or < 120 g/L [12 g/dL]); (C) presence or absence of peripheral blood IMCs, (D) absolute lymphocyte count (≤ or > 2.5 × 109/L); and (E) bone marrow blast percentage (< or ≥ 10%). In each graph, the P value reflects the difference between the 2 Kaplan-Meier curves (log-rank test). Due to missing data, the total number of patients does not equal 213 in panels C, D, and E. Pts indicates patients; surv, survival.
Kaplan-Meier survival curves of CMML patients grouped according to patient characteristics identified by univariate analysis.
Survival curves for patients grouped by (A) WBC counts (≤ or > 13 × 109/L); (B) hemoglobin (HGB) level (≥ or < 120 g/L [12 g/dL]); (C) presence or absence of peripheral blood IMCs, (D) absolute lymphocyte count (≤ or > 2.5 × 109/L); and (E) bone marrow blast percentage (< or ≥ 10%). In each graph, the P value reflects the difference between the 2 Kaplan-Meier curves (log-rank test). Due to missing data, the total number of patients does not equal 213 in panels C, D, and E. Pts indicates patients; surv, survival.
Forty patients (19%) developed AML after a median time of 7 months (range, 1-96 months) following referral (MDS-CMML 20%, MPD-CMML 18%,P = ns). The estimated incidence of transformation to AML in our CMML population was 15% at 1 year and 21% at 5 years.
Univariate analysis of prognostic factors
Patient characteristics investigated individually for possible associations with survival times are shown in Table4, which also shows median survival with 75th and 25th percentiles from Kaplan-Meier estimates. Although the presence of chromosomal abnormalities was associated with shorter survival time, we were unable to identify any prognostic significance for specific aberrations, likely because of the small numbers of patients with the respective karyotype. In addition, we were unable to observe any differences in survival time between patients with monosomy 7, trisomy 8, or complex karyotype (n = 42) and patients with other abnormalities (n = 28) (data not shown), making it difficult to identify a subpopulation of CMML patients with unfavorable karyotypes among the abnormal ones.
Univariate analysis of the effects of patient characteristics on survival
| Patient characteristics4-150 . | No. of patients . | No. of deaths . | Percentile of survival (mo) . | P(log-rank test) . | ||
|---|---|---|---|---|---|---|
| 75% . | 50% . | 25% . | ||||
| Age | ||||||
| 65 years or younger | 109 | 85 | 6 | 14 | 31 | NS |
| Older than 65 years | 104 | 82 | 4 | 10 | 20 | |
| Sex | ||||||
| Female | 63 | 52 | 4 | 16 | 28 | NS |
| Male | 150 | 115 | 5 | 11 | 25 | |
| Prior malignancy | ||||||
| No | 129 | 91 | 5 | 15 | 31 | NS |
| Yes | 34 | 30 | 7 | 10 | 16 | |
| AHD | ||||||
| No | 58 | 54 | 6 | 14 | 26 | NS |
| Yes | 151 | 109 | 5 | 11 | 27 | |
| Splenomegaly | ||||||
| No | 138 | 110 | 6 | 13 | 27 | NS |
| Yes | 61 | 46 | 4 | 11 | 27 | |
| Hemoglobin level | ||||||
| Lower than 120 g/L (12 g/dL) | 168 | 134 | 4 | 10 | 20 | < .01 |
| 120 g/L or higher (12 g/dL) | 45 | 33 | 13 | 21 | 41 | |
| Platelets count | ||||||
| No higher than 50 × 109/L | 62 | 45 | 3 | 7 | 15 | < .01 |
| 50-100 × 109/L | 54 | 45 | 4 | 10 | 19 | |
| Higher than 100 × 109/L | 93 | 74 | 8 | 19 | 33 | |
| White blood cells count | ||||||
| No higher than 10 × 109/L | 56 | 37 | 7 | 14 | 41 | < .01 |
| Higher than 10 × 109/L | 157 | 130 | 5 | 11 | 23 | |
| No higher than 13 × 109/L | 74 | 51 | 5 | 13 | 33 | .03 |
| Higher than 13 × 109/L | 139 | 116 | 5 | 12 | 20 | |
| Neutrophil count | ||||||
| No higher than 2.5 × 109/L | 40 | 30 | 3 | 8 | 38 | NS |
| Higher than 2.5 × 109/L | 165 | 130 | 5 | 12 | 23 | |
| No higher than 9 × 109/L | 99 | 74 | 5 | 13 | 31 | NS |
| Higher than 9 × 109/L | 106 | 86 | 5 | 11 | 20 | |
| Monocyte count | ||||||
| No higher than 4 × 109/L | 102 | 75 | 6 | 14 | 32 | .03 |
| Higher than 4 × 109/L | 111 | 92 | 5 | 11 | 21 | |
| Lymphocyte count | ||||||
| No higher than 2.5 × 109/L | 75 | 46 | 7 | 18 | 37 | < .01 |
| Higher than 2.5 × 109/L | 130 | 114 | 4 | 10 | 20 | |
| Peripheral blood IMCs | ||||||
| 0% | 43 | 31 | 7 | 24 | 39 | < .01 |
| More than 0% | 154 | 122 | 4 | 9 | 18 | |
| LDH | ||||||
| No higher than 700 U/L | 82 | 55 | 7 | 17 | 39 | < .01 |
| Higher than 700 U/L | 120 | 102 | 4 | 10 | 20 | |
| β2-microglobulin level4-151 | ||||||
| No higher than 4 mg/L | 36 | 20 | 6 | 15 | 23 | .02 |
| Higher than 4 mg/L | 28 | 20 | 4 | 6 | 11 | |
| BM blasts | ||||||
| Less than 5% | 115 | 89 | 7 | 17 | 28 | < .01 |
| 5%-10% | 56 | 43 | 5 | 10 | 27 | |
| 10% or more | 36 | 30 | 3 | 7 | 14 | |
| BM monocytes | ||||||
| No more than 20% | 137 | 104 | 5 | 12 | 26 | NS |
| More than 20% | 44 | 35 | 4 | 10 | 28 | |
| BM lymphocytes | ||||||
| No more than 9% | 149 | 113 | 5 | 14 | 27 | NS |
| More than 9% | 40 | 34 | 3 | 7 | 13 | |
| BM erythroid cells | ||||||
| No more than 10% | 68 | 58 | 4 | 8 | 20 | < .01 |
| More than 10% | 124 | 92 | 6 | 13 | 32 | |
| BM myeloid–erythroid cell ratio | ||||||
| No more than 6 | 126 | 93 | 5 | 11 | 31 | .05 |
| More than 6 | 66 | 57 | 4 | 11 | 20 | |
| Karyotype | ||||||
| Diploid | 135 | 100 | 6 | 16 | 28 | .01 |
| Abnormal | 70 | 59 | 3 | 8 | 18 | |
| ras point mutation‡ | ||||||
| Normal | 40 | 32 | 5 | 13 | 21 | NS |
| Mutation | 25 | 15 | 6 | 11 | 19 | |
| Patient characteristics4-150 . | No. of patients . | No. of deaths . | Percentile of survival (mo) . | P(log-rank test) . | ||
|---|---|---|---|---|---|---|
| 75% . | 50% . | 25% . | ||||
| Age | ||||||
| 65 years or younger | 109 | 85 | 6 | 14 | 31 | NS |
| Older than 65 years | 104 | 82 | 4 | 10 | 20 | |
| Sex | ||||||
| Female | 63 | 52 | 4 | 16 | 28 | NS |
| Male | 150 | 115 | 5 | 11 | 25 | |
| Prior malignancy | ||||||
| No | 129 | 91 | 5 | 15 | 31 | NS |
| Yes | 34 | 30 | 7 | 10 | 16 | |
| AHD | ||||||
| No | 58 | 54 | 6 | 14 | 26 | NS |
| Yes | 151 | 109 | 5 | 11 | 27 | |
| Splenomegaly | ||||||
| No | 138 | 110 | 6 | 13 | 27 | NS |
| Yes | 61 | 46 | 4 | 11 | 27 | |
| Hemoglobin level | ||||||
| Lower than 120 g/L (12 g/dL) | 168 | 134 | 4 | 10 | 20 | < .01 |
| 120 g/L or higher (12 g/dL) | 45 | 33 | 13 | 21 | 41 | |
| Platelets count | ||||||
| No higher than 50 × 109/L | 62 | 45 | 3 | 7 | 15 | < .01 |
| 50-100 × 109/L | 54 | 45 | 4 | 10 | 19 | |
| Higher than 100 × 109/L | 93 | 74 | 8 | 19 | 33 | |
| White blood cells count | ||||||
| No higher than 10 × 109/L | 56 | 37 | 7 | 14 | 41 | < .01 |
| Higher than 10 × 109/L | 157 | 130 | 5 | 11 | 23 | |
| No higher than 13 × 109/L | 74 | 51 | 5 | 13 | 33 | .03 |
| Higher than 13 × 109/L | 139 | 116 | 5 | 12 | 20 | |
| Neutrophil count | ||||||
| No higher than 2.5 × 109/L | 40 | 30 | 3 | 8 | 38 | NS |
| Higher than 2.5 × 109/L | 165 | 130 | 5 | 12 | 23 | |
| No higher than 9 × 109/L | 99 | 74 | 5 | 13 | 31 | NS |
| Higher than 9 × 109/L | 106 | 86 | 5 | 11 | 20 | |
| Monocyte count | ||||||
| No higher than 4 × 109/L | 102 | 75 | 6 | 14 | 32 | .03 |
| Higher than 4 × 109/L | 111 | 92 | 5 | 11 | 21 | |
| Lymphocyte count | ||||||
| No higher than 2.5 × 109/L | 75 | 46 | 7 | 18 | 37 | < .01 |
| Higher than 2.5 × 109/L | 130 | 114 | 4 | 10 | 20 | |
| Peripheral blood IMCs | ||||||
| 0% | 43 | 31 | 7 | 24 | 39 | < .01 |
| More than 0% | 154 | 122 | 4 | 9 | 18 | |
| LDH | ||||||
| No higher than 700 U/L | 82 | 55 | 7 | 17 | 39 | < .01 |
| Higher than 700 U/L | 120 | 102 | 4 | 10 | 20 | |
| β2-microglobulin level4-151 | ||||||
| No higher than 4 mg/L | 36 | 20 | 6 | 15 | 23 | .02 |
| Higher than 4 mg/L | 28 | 20 | 4 | 6 | 11 | |
| BM blasts | ||||||
| Less than 5% | 115 | 89 | 7 | 17 | 28 | < .01 |
| 5%-10% | 56 | 43 | 5 | 10 | 27 | |
| 10% or more | 36 | 30 | 3 | 7 | 14 | |
| BM monocytes | ||||||
| No more than 20% | 137 | 104 | 5 | 12 | 26 | NS |
| More than 20% | 44 | 35 | 4 | 10 | 28 | |
| BM lymphocytes | ||||||
| No more than 9% | 149 | 113 | 5 | 14 | 27 | NS |
| More than 9% | 40 | 34 | 3 | 7 | 13 | |
| BM erythroid cells | ||||||
| No more than 10% | 68 | 58 | 4 | 8 | 20 | < .01 |
| More than 10% | 124 | 92 | 6 | 13 | 32 | |
| BM myeloid–erythroid cell ratio | ||||||
| No more than 6 | 126 | 93 | 5 | 11 | 31 | .05 |
| More than 6 | 66 | 57 | 4 | 11 | 20 | |
| Karyotype | ||||||
| Diploid | 135 | 100 | 6 | 16 | 28 | .01 |
| Abnormal | 70 | 59 | 3 | 8 | 18 | |
| ras point mutation‡ | ||||||
| Normal | 40 | 32 | 5 | 13 | 21 | NS |
| Mutation | 25 | 15 | 6 | 11 | 19 | |
NS indicates not significant; BM, bone marrow.
Patient characteristics at the time of admission to MDACC.
Data available for 64 patients.
Data available for 65 patients.
Figure 2 illustrates the survival curves for the WBC count, hemoglobin level, presence of circulating IMCs, absolute lymphocyte count, and bone marrow blast percentage. We found no evidence of association between survival time and age, sex, bone marrow monocyte and lymphocyte percentages, presence of splenomegaly, previous malignancies, or presence of AHD. The presence of N- or K-ras point mutations was also not associated with differences in survival times. Although the DNA sequencing of N- and K-ras oncogenes was performed in only 65 patients, the median survival of those patients (12 months) was identical to that of the whole study population, thus validating the inclusion of the ras mutation covariate in the survival analysis.
Low hemoglobin level (< 120 g/L [12 g/dL]; Figure 2A) and thrombocytopenia (≤ 100 × 109/L) were each associated with shorter survival. High total WBC count (> 10 × 109/L) was also associated with shorter survival. We initially tested the total WBC cutoff point of 13 × 109/L or less because this threshold was chosen by FAB4 and more recently by Germing et al9 to differentiate between MDS-CMML and MPD-CMML. This threshold appeared to divide patients into 2 groups with slightly different survival times, but the difference in the survival was significant only after 16 months (Figure 2B). The WBC count of 10 × 109/L showed the greatest impact on survival in our series. An absolute monocyte count above 4 × 109/L correlated with shorter survival, but the association was of borderline significance (Table 4).
The presence of IMCs in the peripheral blood was associated with shorter survival time (Table 4 and Figure 2C), as was the absolute lymphocyte count of above 2.5 × 109/L (Table 4and Figure 2D). While other cutoff points for lymphocyte count also stratified patients by survival time, the 2.5 × 109/L value provided the best possible discrimination, as determined by univariate analysis and martingale residual plots analysis. Evidence of increased risk for shorter survival time was also shown for LDH levels above 700 U/L and for β2-microglobulin levels above 4 mg/L, although the latter parameter was available in only 64 patients. The percentage of bone marrow blasts was associated with survival time; although a level of more than 5% was significant for predicting a shorter survival, the level that had the greatest impact on decreasing survival time was 10% or more (Table 4 and Figure 2E). Other than blasts, bone marrow characteristics associated with shorter survival were a lower percentage of erythroid cells (≤ 10%) and a higher myeloid–erythroid cell ratio (> 6).
Correlation analysis
To consider associations between individual patient characteristics shown to have a significant effect on survival, we computed correlation coefficients for pairs of patient characteristics. The total WBC counts correlated positively with absolute neutrophil, monocyte, and lymphocyte counts; serum LDH and β2-microglobulin levels; and history of AHD. Monocyte counts correlated also with absolute neutrophil and lymphocyte counts and with β2-microglobulin levels. Higher absolute lymphocyte counts associated slightly with presence of circulating IMCs, higher β2-microglobulin levels, and history of AHD. A strong correlation was observed between absolute lymphocyte counts and LDH. Hemoglobin values and platelet counts did not correlate with other hematologic parameters (eg, WBC count, circulating IMCs, and bone marrow blast percentages) but correlated inversely with the presence of chromosomal abnormalities. Chromosomal abnormalities also correlated with the presence of circulating IMCs. The bone marrow blast percentage correlated significantly only with β2-microglobulin level. Noteworthy are the correlations between the presence of aras point mutation and the presence of circulating IMCs, LDH level, β2-microglobulin level, and absolute lymphocyte count.
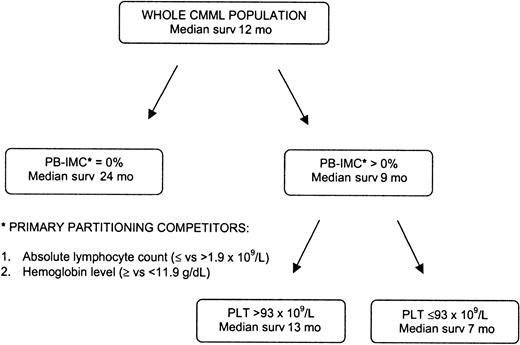
Multivariate analysis
To identify independent prognostic factors for survival time, variables for which there was indication of a prognostic role by the univariate analysis were included in the CART procedure. The results of this multivariate analysis, which does not assume any cutoff point for continuous covariates, are shown in Figure3. The presence or absence of circulating IMCs was identified as the primary discriminator of survival time, with absolute lymphocyte count (≤ and > 1.9 × 109/L) and hemoglobin level (≥ and < 119 g/L [11.9 g/dL]) as the following main competitors for such primary partitioning; patients with no circulating IMCs were identified as having longer survival (median 24 months), with no further partitioning warranted. The process continued in the subset of patients with circulating IMCs above 0% and identified platelet counts of more than 93 × 109/L and 93 × 109/L or less as providing the best further discrimination of survival, with medians of 13 and 7 months, respectively (Figure 3).
CART survival model.
This model identified the presence of peripheral blood IMCs as the strongest independent variable determining shorter survival, followed by the absolute lymphocyte count above 1.9 × 109/L and the hemoglobin level below 119 g/dL (11.9 g/dL) as main competitor variables. The process continued in the subset of patients with circulating IMCs above 0% and identified platelet counts of more than 93 × 109/L and 93 × 109/L or less as providing the best further discrimination of survival.
CART survival model.
This model identified the presence of peripheral blood IMCs as the strongest independent variable determining shorter survival, followed by the absolute lymphocyte count above 1.9 × 109/L and the hemoglobin level below 119 g/dL (11.9 g/dL) as main competitor variables. The process continued in the subset of patients with circulating IMCs above 0% and identified platelet counts of more than 93 × 109/L and 93 × 109/L or less as providing the best further discrimination of survival.
To provide an alternative analysis of the association between multiple patient characteristics and survival times, which would also allow evaluation of the relative prognostic importance of each factor while correcting for the effects of other covariates, we applied a proportional hazards model. This model considered the following terms, based on previous information from the literature, and the results of analyzing individual covariates in the data set: hemoglobin (< 120 vs ≥ 120 g/L [< 12 vs ≥ 12 g/dL]), platelet count (≤ 100 × 109/L vs >100 × 109/L), presence of circulating IMCs (0% vs > 0%), total WBC count (≤ 10 × 109/L vs > 10 × 109/L), absolute monocyte count (≤ 4 × 109/L vs > 4 × 109/L), absolute lymphocyte count (≤ 2.5 × 109/L vs > 2.5 × 109/L), bone marrow blast percentage (< 10% vs ≥ 10%), bone marrow erythroid cell percentage (≤ 10% vs > 10%), serum LDH level (≤ 700 vs > 700 U/L), and cytogenetics (normal vs abnormal karyotype). Substitutions of 10% or more bone marrow blast value by at least 5%, 100 × 109/L platelet count by 50 × 109/L, and more than 10 × 109/L WBC count by more than 13 × 109/L were also tested in the model.
Table 5 summarizes the results for the proportional hazards model where a backward-selection procedure was applied. The model identified hemoglobin level, absolute lymphocyte count, the presence of peripheral blood IMCs, and bone marrow blast percentage as covariates independently associated with survival time. Given the unexpected significance of lymphocyte counts in this analysis, we performed a subsequent multiple regression analysis with omission of this term. The resulting model explained less variation in survival, and another covariate was not selected in place of the lymphocyte term (data not shown). This finding confirmed our observation that lymphocytes have an autonomous association with survival, at least in our data set.
Associations between survival time and patient characteristics identified using backward-selection procedure
| Patient characteristics . | Hazard ratio . | 95% CI . | P . |
|---|---|---|---|
| Hemoglobin level less than 120 g/L (12 g/dL) | 1.8 | 1.2-2.8 | < .01 |
| Lymphocyte count higher than 2.5 × 109/L | 1.8 | 1.2-2.7 | < .01 |
| Peripheral blood IMCs more than 0% | 1.8 | 1.2-2.8 | < .01 |
| Bone marrow blasts no more than 10% | 1.9 | 1.2-2.8 | < .01 |
| Patient characteristics . | Hazard ratio . | 95% CI . | P . |
|---|---|---|---|
| Hemoglobin level less than 120 g/L (12 g/dL) | 1.8 | 1.2-2.8 | < .01 |
| Lymphocyte count higher than 2.5 × 109/L | 1.8 | 1.2-2.7 | < .01 |
| Peripheral blood IMCs more than 0% | 1.8 | 1.2-2.8 | < .01 |
| Bone marrow blasts no more than 10% | 1.9 | 1.2-2.8 | < .01 |
In an attempt to more reliably distinguish CMML from Philadelphia chromosome–negative CML and atypical CML, the FAB group proposed in 1994 additional criteria that could be applied to cases in which the initial patient characteristics did not suggest a clear diagnosis.6,31,40 Guidelines initially proposed by Galton2 in 1992 were included in this “modified FAB” classification.4 Using these guidelines, we sought to confirm the validity of our results by applying the proportional hazards model to a subpopulation of patients selected accordingly. For this analysis, the original FAB diagnostic criteria for CMML were expanded as follows: monocytes above 10%, circulating IMCs below 10%, and basophils below 2%. In our study population, 124 patients (58%) met all the expanded criteria. Compared with survival times calculated for all 213 patients in the study, median survival for this subset of patients was longer (16 months; 95% CI, 10.6-19.0), and it was closer to those cited in previously reported CMML series.12,13,15,17,20,21,24,25 30 The results obtained by the analysis of this restricted CMML subpopulation are comparable to those obtained in the analysis of the whole CMML series, with one exception: Bone marrow blast percentage was not significant in a multivariate model that contained hemoglobin level below 120 g/L (12 g/dL), absolute lymphocyte count above 2.5 × 109/L, and presence of peripheral blood IMCs (data not shown).
Scoring systems
Although many staging systems have been devised based on MDS populations that included patients with CMML, only one, based on data from 53 patients,21 was designed exclusively for CMML. We applied 6 scoring systems12,14,21,22,28,39 widely used to predict survival in MDS and/or CMML to determine whether they were effective in stratifying our patient population (Table6). All these models identified a distinct population of “good prognosis” patients, with survival times ranging from 18 to 21 months. The proportion of patients in this low-risk group varied between 7% (Düsseldorf) and 45% (Bournemouth). Only the Düsseldorf scoring system was able to meaningfully stratify patients into 3 distinct subcategories—but with only 7% of cases in the low-risk group. For other systems, differences in median survival times of intermediate- and high-risk groups were too small to be meaningful. The least distinct stratification was obtained using the IPSS, which we applied only in patients with WBC counts below 12 × 109/L (67 [29%] of 213 patients) for consistency with the analysis performed by Greenberg et al.39 To stratify patients according to life expectation, we designed a simple scoring system based on the variables that were identified as having independent association with survival time (Table 5)—namely, hemoglobin level, lymphocyte count, presence of peripheral blood IMCs, and bone marrow blast percentage. Because such risk factors were roughly equivalent in importance (as shown by the proportional hazards regression analysis), we assigned equal weight to each risk factor. One point was assigned for each of the following variables: hemoglobin below 120 g/L (12 g/dL), absolute lymphocyte count above 2.5 × 109/L, peripheral blood IMCs above 0%, and bone marrow blasts 10% or more. These scores were combined as explained below for a maximum total of 4 points to create risk categories. Only 7 patients had none of the poor prognostic features; therefore, this small group was combined with those having 1 unfavorable feature.
Survival of patients with CMML according to risk groups based on various scoring systems
| Scoring system . | Risk group . | No. of patients (%) . | No. of deaths . | Percentiles of survival (mo) . | P (log-rank test) . | ||
|---|---|---|---|---|---|---|---|
| 75% . | 50% . | 25% . | |||||
| Bournemouth14 | Low | 92 (45) | 74 | 8 | 18 | 33 | < .01 |
| Intermediate | 102 (50) | 77 | 4 | 8 | 18 | ||
| High | 10 (5) | 9 | 2 | 5 | 11 | ||
| Modified Bournemouth21 | Low | 72 (37) | 58 | 8 | 19 | 33 | .01 |
| High | 124 (63) | 96 | 4 | 8 | 19 | ||
| Spanish22 | Low | 61 (30) | 49 | 10 | 20 | 34 | < .01 |
| Intermediate | 114 (56) | 88 | 4 | 10 | 22 | ||
| High | 29 (14) | 23 | 3 | 5 | 10 | ||
| Düsseldorf28 | Low | 15 (7) | 11 | 9 | 21 | 39 | < .01 |
| Intermediate | 119 (61) | 92 | 6 | 15 | 31 | ||
| High | 62 (32) | 50 | 3 | 7 | 12 | ||
| Lille12 | Low | 67 (34) | 53 | 9 | 19 | 34 | < .01 |
| Intermediate | 109 (56) | 83 | 4 | 9 | 20 | ||
| High | 20 (10) | 16 | 1 | 7 | 11 | ||
| IPSS6-15039 | Low | 18 (30) | 9 | 8 | 20 | 48 | .03 |
| Intermediate-1 | 27 (44) | 17 | 5 | 14 | 57 | ||
| Intermediate-2 | 11 (18) | 7 | 1 | 5 | 7 | ||
| High | 5 (8) | 5 | 1 | 6 | 8 | ||
| Scoring system . | Risk group . | No. of patients (%) . | No. of deaths . | Percentiles of survival (mo) . | P (log-rank test) . | ||
|---|---|---|---|---|---|---|---|
| 75% . | 50% . | 25% . | |||||
| Bournemouth14 | Low | 92 (45) | 74 | 8 | 18 | 33 | < .01 |
| Intermediate | 102 (50) | 77 | 4 | 8 | 18 | ||
| High | 10 (5) | 9 | 2 | 5 | 11 | ||
| Modified Bournemouth21 | Low | 72 (37) | 58 | 8 | 19 | 33 | .01 |
| High | 124 (63) | 96 | 4 | 8 | 19 | ||
| Spanish22 | Low | 61 (30) | 49 | 10 | 20 | 34 | < .01 |
| Intermediate | 114 (56) | 88 | 4 | 10 | 22 | ||
| High | 29 (14) | 23 | 3 | 5 | 10 | ||
| Düsseldorf28 | Low | 15 (7) | 11 | 9 | 21 | 39 | < .01 |
| Intermediate | 119 (61) | 92 | 6 | 15 | 31 | ||
| High | 62 (32) | 50 | 3 | 7 | 12 | ||
| Lille12 | Low | 67 (34) | 53 | 9 | 19 | 34 | < .01 |
| Intermediate | 109 (56) | 83 | 4 | 9 | 20 | ||
| High | 20 (10) | 16 | 1 | 7 | 11 | ||
| IPSS6-15039 | Low | 18 (30) | 9 | 8 | 20 | 48 | .03 |
| Intermediate-1 | 27 (44) | 17 | 5 | 14 | 57 | ||
| Intermediate-2 | 11 (18) | 7 | 1 | 5 | 7 | ||
| High | 5 (8) | 5 | 1 | 6 | 8 | ||
Applied only to patients with WBC counts ≤ 12 × 109/L.
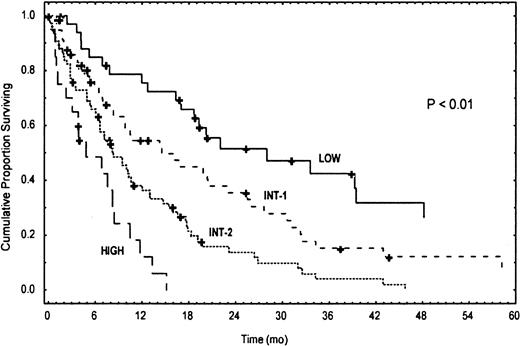
Of the 213 patients included in our CMML population, we were able to assign a score to 190 (23 patients had some missing data, which did not allow a definite risk allocation) and to stratify them into 4 distinct subgroups based on levels of risk: low (score = 0-1), intermediate-1 (score = 2), intermediate-2 (score = 3), and high (score = 4) (M. D. Anderson Prognostic Score [MDAPS]). The corresponding Kaplan-Meier survival curves are shown in Figure4. Low-risk patients had a median survival of 24 months, compared with 15 and 8 months for intermediate-1 and intermediate-2 risk, respectively, and only 5 months for high-risk patients (Table 7). As additional verification, we tested our scoring system on the subset of CMML patients selected according the “modified FAB” classification.4 Of the 124 patients who met the expanded criteria, we could assign a score in 118 patients. Table8 outlines the results of this assessment, which are similar to those obtained in the analysis of the whole CMML series (Table 7 and Figure 4). Both in the whole study population and in this subset of patients, subdivision into “dysplastic” and “proliferative” subgroups according to WBC counts provided no additional benefit to prognostic stratification. Finally, to rule out any possible major interaction between treatment and the influence of patient characteristics on survival, we analyzed the distribution of treatment modalities among patients stratified according to the MDAPS. Indeed, patients of the 4 risk categories were evenly distributed among the different treatment modality groups.
Kaplan-Meier survival curves of CMML patients according to their risk classification by MDAPS.
A total of 213 patients had a diagnosis of CMML; 23 patients had some missing data, which did not allow assignment of a definite score according to risk factors. The P value reflects the difference between the 4 Kaplan-Meier curves.
Kaplan-Meier survival curves of CMML patients according to their risk classification by MDAPS.
A total of 213 patients had a diagnosis of CMML; 23 patients had some missing data, which did not allow assignment of a definite score according to risk factors. The P value reflects the difference between the 4 Kaplan-Meier curves.
Survival of 190 patients with CMML according to risk groups based on the MDAPS
| Risk group . | No. of patients (%) . | No. of deaths . | Percentiles of survival (mo) . | P (log-rank test) . | ||
|---|---|---|---|---|---|---|
| 75% . | 50% . | 25% . | ||||
| Low | 35 (18) | 21 | 12 | 24 | 51 | < .01 |
| Intermediate-1 | 60 (32) | 43 | 6 | 15 | 31 | |
| Intermediate-2 | 75 (39) | 65 | 3 | 8 | 18 | |
| High | 20 (11) | 18 | 1 | 5 | 8 | |
| Risk group . | No. of patients (%) . | No. of deaths . | Percentiles of survival (mo) . | P (log-rank test) . | ||
|---|---|---|---|---|---|---|
| 75% . | 50% . | 25% . | ||||
| Low | 35 (18) | 21 | 12 | 24 | 51 | < .01 |
| Intermediate-1 | 60 (32) | 43 | 6 | 15 | 31 | |
| Intermediate-2 | 75 (39) | 65 | 3 | 8 | 18 | |
| High | 20 (11) | 18 | 1 | 5 | 8 | |
Survival of a 118-patient subpopulation8-150 with CMML according to risk groups based on the MDAPS
| Risk group . | No. of patients (%) . | No. of deaths . | Percentiles of survival (mo) . | P (log-rank test) . | ||
|---|---|---|---|---|---|---|
| 75% . | 50% . | 25% . | ||||
| Low | 30 (25) | 17 | 16 | 26 | 58 | < .01 |
| Intermediate-1 | 42 (36) | 31 | 4 | 14 | 29 | |
| Intermediate-2 | 35 (30) | 29 | 3 | 9 | 18 | |
| High | 11 (9) | 9 | 3 | 5 | 8 | |
| Risk group . | No. of patients (%) . | No. of deaths . | Percentiles of survival (mo) . | P (log-rank test) . | ||
|---|---|---|---|---|---|---|
| 75% . | 50% . | 25% . | ||||
| Low | 30 (25) | 17 | 16 | 26 | 58 | < .01 |
| Intermediate-1 | 42 (36) | 31 | 4 | 14 | 29 | |
| Intermediate-2 | 35 (30) | 29 | 3 | 9 | 18 | |
| High | 11 (9) | 9 | 3 | 5 | 8 | |
Analysis based on patients who met the following criteria: IMCs below 10%, monocytes above 10%, and basophils above 2%.
Age-related effects
In univariate analysis, age was not significantly associated with survival time (Table 4). To ascertain that the impact of the age was not hidden in a subpopulation of patients, CMML patients were stratified by age within each risk group and also within “dysplastic” and “proliferative” categories. Statistical analysis failed to document any significant differences in the survival of patients aged 65 years or less versus more than 65 years. Only in the risk categories low and intermediate-1 was there a trend for longer survival (27 vs 21 months and 15 vs 9 months, respectively) in the relatively younger subgroup of patients; such differences, however, did not reach statistical significance. Similarly, a trend of longer survival for patients aged 65 years or less was noted in the “dysplastic” but not in the “proliferative” group of patients.
Discussion
Using 2 different statistical approaches, we identified 4 independent covariates whereby CMML patients could be stratified according to survival time. Hemoglobin level, absolute lymphocyte count, presence of circulating IMCs, and bone marrow blast percentage were equally significant. We used these covariates to derive a simple classification system that provided discrimination by survival time, enabling us to identify 4 subgroups of patients with different degrees of risk. Patients included in our study were selected based on well-defined and widely accepted diagnostic criteria for CMML.10 They represent the largest CMML series reported so far by either a single institution or cooperative group and only the second attempt to devise a risk-based scoring system for patients with CMML. The importance of these independent prognostic factors and the validity of our scoring system in stratifying patients according to survival expectations were confirmed in a subpopulation of 118 patients who met the modified and more stringent FAB criteria for CMML.4 The only exception was represented by the bone marrow blasts, which in this subset of patients lost their statistical significance, likely due to the reduced sample size.
In an attempt to further validate our findings, we tested the MDAPS also prospectively, in a cohort of 51 patients newly diagnosed with CMML who were referred to MDACC after the closing time of this study. Among these, 35 were alive at the time of last follow-up, with a median time from referral of only 2 months; median projected overall survival was 17 months. Although the analysis was limited by the inherent briefness of the follow-up times, we were able to confirm the validity of low hemoglobin level, presence of peripheral blood IMCs, and high absolute lymphocyte count in predicting for shorter survival, whereas such association was not proven for the marrow blasts. Nonetheless, the MDAPS identified 4 subgroups of patients similarly allocated in the corresponding risk categories: low = 12 (24%); intermediate-1 = 15 (29%); intermediate-2 = 20 (39%); high = 4 (8%). A consistent stratification by different survival times was achieved when the high-risk patients were combined with the larger subgroup of patients with intermediate-2 risk, most likely due to the small size of the former subgroup. Prediction of the clinical outcome of patients with a disease such as heterogeneous as CMML is difficult, and reported survival times vary widely. Published prognostic risk analysis models were based on relatively small CMML populations, and the studies often grouped CMML patients with other subcategories of MDS patients. Some of the independent variables that were identified as being significant in predicting outcome of CMML patients were similar to those identified in patients with MDS, eg, hemoglobin level, platelet count, and cytogenetics. However, other variables associated with survival of MDS patients, such as age and percentage of bone marrow blasts, showed prognostic value in some CMML studies13,15 but not in others.30 41
In our study, karyotype, platelet count, and WBC count were all highly significant in univariate analysis but lost their significance to hemoglobin level, presence of circulating IMCs, lymphocyte count, and bone marrow blast percentage when entered into multivariate analysis. Like in other studies,15,20,24,42 the frequency of abnormal karyotype in our CMML population was low (34%). Hence, the value of karyotype in the stratification of a predominantly diploid population of patients was expected to be lower than in an MDS population. In all published studies in CMML, karyotype failed to show a significant independent correlation with survival13,16,20,23-25 and was considered only in the IPSS,39 which was designed primarily for MDS. Interestingly, neither the extent of monocyte involvement of the bone marrow nor peripheral blood monocytosis, considered to be the hallmark of CMML, proved to be a significant independent variable in our population. However, monocytosis was reported as an independent prognostic variable in only 2 previous studies.20 41
In our series of CMML patients, we also tested 6 previously published scoring systems12,14,21,22,28 39 for predicting survival. Despite the diversity of factors included, all 6 systems were useful to some extent in separating our CMML population into 2 or 3 groups according to the expected survival time. Each system identified a low-risk group of patients with a median survival between 18 and 21 months. However, the proportion of patients in this category varied depending on the scoring system used (Table 6). Only the Düsseldorf system was able to clearly divide patients into 3 prognostic groups by survival times. By stratifying patients into 4 distinct subgroups (consisting of 18%, 32%, 39%, and 11% of patients), the MDAPS appears advantageous and may be more suitable for use in applying risk-adapted treatment strategies. Our finding that lymphocyte count was a variable independently associated with survival in CMML warrants confirmation. The question of the accuracy and reliability of the classification of cells on the blood smears could be raised. However, all differential counts were performed manually and by the same specialized laboratory; thus, the possibility of having classified monocytes or IMCs as lymphocytes is unlikely. To our knowledge, this study is the first to show a correlation between the peripheral blood lymphocyte count and the prognosis of patients with CMML and the first to consider this variable in a prognostic scoring system. The observation would lend a support to the notion of direct or indirect lymphocyte involvement in CMML; whether the prognostic significance reflects participation in the malignant clone, involvement in the malignant process, or rather a component of a reactive process remains unclear. The importance of ras mutations in the pathophysiology of CMML, as a biological marker of the disease or as a prognostic indicator, is unknown. Among hematologic malignancies, CMML has the highest frequency of point mutations of the ras gene family. In our analysis, ras mutations were identified in 38% of the 65 patients tested, a frequency considerably higher than in Philadelphia chromosome–negative CML (20%; M.B., unpublished data, December 2000). In our study, the presence of a ras mutation significantly correlated with the presence of circulating IMCs, the absolute lymphocyte count, and serum LDH and β2-microglobulin levels. Patients with a rasmutation tended to have shorter survival than patients with normalras, although the difference was not statistically significant, possibly due to the limited sample size. This information may be important when evaluating responses, eg, in patients treated with farnesyl transferase inhibitors specifically designed to targetras oncogene signaling pathways.
The serum β2-microglobulin level is another factor that has not been previously reported to have prognostic significance in CMML. Although in our study population serum β2-microglobulin was measured in only 64 patients (whose median survival was 10 months), the univariate analysis identified a subgroup of 28 patients with levels above 4 mg/L as having a much shorter survival (median 6 months) than that of the 36 patients with levels of 4 mg/L or less (median 15 months). Furthermore, serum β2-microglobulin was the only covariate that correlated with the percentage of bone marrow blasts. These results, together with the known prognostic importance of β2-microglobulin in myeloma,43 lymphoma,44 and chronic lymphocytic leukemia,45 encourage extended investigation of the influence of this covariate in CMML.
In our series, the rate of transformation of CMML to AML was comparable to findings in previous reports,12,20,24,26 confirming that the frequency of blastic transformation in patients with CMML is between 14% and 20%. This frequency is much lower than that in MDS categories such as refractory anemia with excess blasts (RAEB) and RAEB in transformation, wherein more than 50% of patients may develop AML.39 In this respect, our finding of an identical rate of transformation in “dysplastic” and “proliferative” subgroups of CMML is also of interest.
Our study shows that some of the survival-associated prognostic factors for CMML differ from those previously reported for MDS (eg, age, WBC count, LDH level, karyotypic profile, splenomegaly, organ involvement), further supporting the notion that CMML is a clinicopathological entity generally presenting with distinct characteristics.
The simplicity of the proposed scoring system, which is able to stratify patients according to life expectation, allows rapid prognostic assessment and should be useful in management decision making, selection of therapeutic approach, assignment of CMML patients to therapeutic trials, and in determining the value of therapeutic interventions. Ultimately, objective biological and molecular characterization of CMML will be necessary for identification of disease-specific prognostic factors, better description of the disease, and a more reliable and objective distinction between CMML, MDS, and bcr/abl− MPD. Recent reports highlighting a potentially important role of receptor tyrosine kinases in the pathophysiology of MPDs46-50 hold particular promises not only for prognostic classification of CMML but also for new strategies of therapy.
We are grateful to professor Peter Greenberg for having reviewed this manuscript, providing constructive criticism, and offering suggestions that led to its unquestionable improvement. We express our appreciation to Shang Ying Liang for the preliminary analysis of the data. The invaluable assistance of Sherry A. Pierce and Mary Beth Rios with data processing is greatly appreciated. Our thanks go also to Kate O'Suilleabhain for reviewing, correcting, and editing this manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Miloslav Beran, Dept of Leukemia, Box 428, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: mberan@mdanderson.org.

![Fig. 2. Kaplan-Meier survival curves of CMML patients grouped according to patient characteristics identified by univariate analysis. / Survival curves for patients grouped by (A) WBC counts (≤ or > 13 × 109/L); (B) hemoglobin (HGB) level (≥ or < 120 g/L [12 g/dL]); (C) presence or absence of peripheral blood IMCs, (D) absolute lymphocyte count (≤ or > 2.5 × 109/L); and (E) bone marrow blast percentage (< or ≥ 10%). In each graph, the P value reflects the difference between the 2 Kaplan-Meier curves (log-rank test). Due to missing data, the total number of patients does not equal 213 in panels C, D, and E. Pts indicates patients; surv, survival.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/3/10.1182_blood.v99.3.840/5/m_h80322086002.jpeg?Expires=1769819337&Signature=4vVLrxRCmM4NEVEHf5E~yrFWVsNXjgqG9bIgS~r1~IVGKJpzPK8BoMdcTp-AIviIvLB7GFLiB18JsxyJaiGUt~XA54tAxDyTwtdpU6CTJLK05Q9s~zkJEk2zzqelKkeF65ve~vffKjp0QSJ9BZIEobrAKZgUxvhnz~s2KRjZGTpwBGmWGxPtH7Z7T0A0fGPcGZVgRd7thaDnULO4eD5b6WVB8iXLYa2eL2GnG~hoqIoDrmvgaERcv-XKlX6CiGD9bIXlhdXjnWPPeyXXlKUiRGDkmrgi6~oLc1fz2Wke0Xds~X-ehLgPclwwkiggcmLnWE3vhwAA35BuxISJE-eKUQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal