Abstract
Minimal residual disease (MRD) following sequential administration of CHOP and rituximab was studied in previously untreated patients with follicular lymphoma. At diagnosis, the presence of Bcl-2/IgH-positive cells in the peripheral blood (PB) and/or bone marrow (BM) was demonstrated in all patients (n = 128) by polymerase chain reaction (PCR) analysis. Patients who achieved a clinical response following CHOP but remained PCR-positive were eligible for rituximab (375 mg/m2 intravenously, weekly for 4 weeks). After CHOP, 57% achieved a complete response (CR), 37% a partial response (PR), and 6% were nonresponders (NR). At this stage, patients proving PCR-negative (n = 41) or failing to achieve a clinical response (n = 8) were excluded from rituximab treatment. Seventy-seven patients received rituximab and entered a scheduled MRD follow-up program. At the first molecular follow-up (+12 weeks), 59% had converted to PCR negativity in the BM and PB, with a further increase documented at the second control (+28 weeks) with 74% PCR negative. At the last molecular follow-up (+44 weeks), 63% of the patients remained PCR negative. At 3 years, the estimated overall survival of all patients is 95% (95% confidence interval [CI], 86-98). For patients achieving PCR-negative status following CHOP and therefore excluded from rituximab treatment, freedom from recurrence (FFR) was 52% (95% CI, 28-71). For patients treated with rituximab, a durable PCR-negative status was associated with a better clinical outcome since FFR was 57% (95% CI, 23-81) compared with 20% (95% CI, 4-46) in patients who never achieved or lost the molecular negativity (P < .001).
Introduction
Follicular lymphoma (FL) accounts for about 30% of newly diagnosed non-Hodgkin lymphomas (NHLs) and occurs most commonly in middle-aged patients with a median age at diagnosis of 50 years.1 The neoplastic clone of the great majority (up to 80%) of FL patients bears the t(14;18) translocation in which the Bcl-2 proto-oncogene on chromosome 18 is translocated to the immunoglobulin heavy chain (IgH) region on chromosome 14, creating a hybrid Bcl-2/IgH gene.2 The molecular cloning of this translocation has allowed development of a polymerase chain reaction (PCR)–based assay for the diagnosis and monitoring of minimal residual disease (MRD).3 Despite the prolonged median survival time of these patients,1,4,5 both conventional and experimental therapeutic approaches have failed to modify the overall survival of the disease,6 although high-dose therapy might provide some advantage at least in terms of freedom from disease progression.7-10 The results of several clinical trials indicate that the absence in the bone marrow (BM) and peripheral blood (PB) of neoplastic cells bearing the Bcl-2/IgH rearrangement during the follow-up is strongly associated with a reduced risk of recurrence and clearly suggests a need to improve the management of MRD.3,11-13 The protocols that allow a more thorough eradication of the neoplastic clone have been those based on high-dose chemoradiotherapy and transplantation of in vitro purged autologous hematopoietic stem cells.14 Unfortunately, these procedures have been associated with an unexpectedly high incidence of secondary myelodysplastic syndromes and acute leukemias15-17 which nowadays can possibly be avoided. In this respect, vaccine and genetic therapies, as well as immunotherapy with monoclonal antibodies, might provide new powerful strategies aimed at an effective eradication of the disease without major toxicity.18-21 Among the different monoclonal antibodies tested in clinical trials, the chimeric mouse/human anti-CD20 antibody rituximab (Hoffmannn-La Roche, Basel, Switzerland) has so far been the one most extensively studied.22-28 This molecule is an IgG1-kappa antibody containing murine light and heavy chain variable regions, and human gamma 1 heavy chain and kappa light chain constant regions. The antibody reacts specifically with the CD20 antigen, which appears during the pre–B-cell development stage of B-cell differentiation, is expressed on the surface of malignant and normal B cells, and is not present on stem cells and plasma cells. In addition, the CD20 antigen is not shed and does not appear to undergo modulation in response to antibody binding, thus representing an ideal target for a monoclonal antibody-based immunotherapy. Moreover, preliminary results indicate that rituximab can reverse to a negative status the BM and PB of patients with PCR-detectable Bcl-2/IgH-positive cells. Most importantly, recently published results suggest that in some newly diagnosed FL patients, both anthracycline-containing chemotherapeutic protocols29,30 as well as rituximab given alone31 can induce molecular remissions. The present multicenter study was designed to evaluate whether the sequential administration of rituximab to patients who proved persistently PCR positive after CHOP chemotherapy can induce and maintain a prolonged negativity of the Bcl-2/IgH chimeric gene in BM and PB cells.
Patients and methods
The study was an open label, multicenter, phase II study aimed at assessing whether in patients with FL carrying the t(14;18) translocation the neoplastic clone can be reduced and potentially eliminated through the sequential administration of CHOP chemotherapy and rituximab as first-line treatment. The local ethics review committees approved the protocol at each center and all patients gave written informed consent before entering the study. The study was conducted according to good clinical and laboratory practice rules, and the principles of the Declaration of Helsinki.
Eligibility, study design, and molecular monitoring of MRD
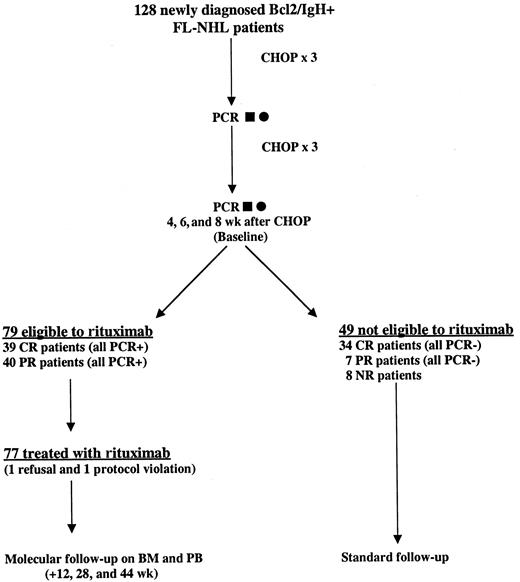
Nine Italian centers were selected for the study. To be eligible, patients were required to have a histologically proven diagnosis of CD20+ FL according to the REAL classification and a positive PCR analysis (BM and/or PB) for Bcl-2/IgH. All samples were centralized and analyzed in 2 laboratories (Bergamo and Torino). Patients were required to be older than 18 years of age and younger than 65 years of age, with a clinical stage II-IV (according to Ann Arbor staging system), and an Eastern Cooperative Oncology Group (ECOG) performance status of 0-1. Patients with such characteristics were assigned to receive 6 cycles of CHOP (cyclophosphamide 750 mg/m2, doxorubicin 50 mg/m2, vincristine 1.4 mg/m2, and prednisone 100 mg/m2) chemotherapy. Thereafter, to be eligible for rituximab treatment, patients had to be in partial or complete clinical remission and still PCR positive in the BM and/or PB as demonstrated on 2 different molecular analyses performed 4 and 6 weeks after the sixth cycle of CHOP (baseline). If one of the 2 tests proved negative, a third molecular assessment was performed within 2 weeks and this third evaluation conclusively established the positivity or negativity of the molecular marker. Patients treated with rituximab (375 mg/m2 weekly intravenous infusions for 4 weeks) were examined at the referral center every week to check efficacy and safety parameters. The molecular follow-up had to be performed always on BM and PB samples, and was carried out at 12, 28, and 44 weeks after the baseline (Figure1). Patients who were not eligible to receive rituximab were treated off study at each clinical center according to local protocols.
Treatment scheme and flow chart of the study.
One hundred twenty-eight newly diagnosed patients with FL with a PCR-proved rearrangement in the BM and PB at diagnosis were treated with CHOP chemotherapy. A PCR analysis was repeated on BM (▪) and PB (●) after the third cycle, as well as 4 and 6 weeks after the sixth cycle of chemotherapy (baseline). In case of discrepancies between the molecular results at baseline, a third assay was performed (8 weeks) after CHOP and this result conclusively established the positivity or negativity of the molecular marker. Only clinically responsive but persistently PCR-positive patients were eligible for rituximab treatment.
Treatment scheme and flow chart of the study.
One hundred twenty-eight newly diagnosed patients with FL with a PCR-proved rearrangement in the BM and PB at diagnosis were treated with CHOP chemotherapy. A PCR analysis was repeated on BM (▪) and PB (●) after the third cycle, as well as 4 and 6 weeks after the sixth cycle of chemotherapy (baseline). In case of discrepancies between the molecular results at baseline, a third assay was performed (8 weeks) after CHOP and this result conclusively established the positivity or negativity of the molecular marker. Only clinically responsive but persistently PCR-positive patients were eligible for rituximab treatment.
Patients were not included if they had other NHL subtypes, had progressive or stable disease after CHOP, had received prior chemotherapy for the lymphoma, had clinically significant abnormal cardiac, liver, or renal functions unrelated to the lymphoma, history of other cancers, major surgery within the last 4 weeks, opportunistic infections, HIV-positive serology, unacceptable hematologic status, or central nervous system (CNS) involvement.
End points and response criteria
Primary end points of the study were the efficacy of rituximab to induce the negativity of the Bcl-2/IgH chimeric gene in the BM and PB cells of patients in partial or complete clinical remission after CHOP chemotherapy and with molecularly detectable residual disease, as well as the safety of rituximab when sequentially administered after CHOP chemotherapy. A secondary end point was to evaluate the clinical activity of the sequential CHOP-rituximab therapy. Clinical restaging including computed tomography (CT) scan and bone marrow biopsy was performed at the end of CHOP chemotherapy and at the last molecular follow-up (week +44 from baseline). Response categories were the following: complete remission (CR) defined as the complete disappearance of all clinically detectable disease (including BM infiltration) and/or reduction to less than 1 × 1 cm in size of all lymph nodes visible on CT scans of neck, chest, abdomen; and pelvis. Partial response (PR) was defined as a more than 50% reduction in the sum of the products of bidimensional measurements for all measurable lesions. Progressive disease (PD) was considered an increase in size of more than 25% of previously documented disease or the appearance of disease at any site. Toxicity was evaluated and registered according to the World Health Organization (WHO) recommendations.
Study population and statistical analyses
All registered patients were analyzed for the overall survival (OS), considered as the time between entry into trial and death; and freedom from recurrence (FFR), considered as the time from response evaluation of CHOP chemotherapy to relapse or progression. The evaluation of clinical and molecular response to Rituximab was carried out in all patients who completed at least one Rituximab infusion according to an intention-to-treat analysis. To identify prognostic variables for remission duration, the following factors were examined using the log-rank test and the Cox proportional hazards regression model32: age, sex, serum lactate dehydrogenase (LDH), bulky disease, extranodal sites other than BM, BM infiltration, remission status after CHOP chemotherapy (CR vs PR), detection of Bcl-2/IgH rearranged cells in follow-up BM samples (12, 28, and 44 weeks from baseline). OS and FFR curves were computed by the life table method according to Kaplan-Meier.33
Molecular evaluation of Bcl-2/IgH rearrangement
The molecular analysis of patients with FL bearing the t(14;18) chromosome abnormality was carried out and evaluated by PCR analysis of the Bcl-2/IgH rearrangement according to published methods with only minor modifications.34 The following standardized protocol was used by the 2 laboratories (Bergamo and Torino) in which the molecular analysis was centralized. Briefly, the initial amplification was carried out in a 50 μL final volume using 1 μg DNA with the following oligonucleotides: 5′-CAGCCTTGAAACATTGATGG-3′ for the major breakpoint region (MBR) or 5′-CGTGCTGGTACCACTCCTG-3′ for the minor cluster region (mcr) and 5′-ACCTGAGGAGACGGTGACC-3′ for the JH consensus region. Samples were amplified with an initial denaturation at 95°C for 10 minutes, followed by either 27 cycles of denaturation for the MBR or 30 cycles for the mcr. Each cycle was performed with 1 minute of denaturation at 94°C and 1 minute of annealing at 55°C for the MBR amplification or 1 minute at 58°C and 1 minute of extension at 72°C for the mcr. The final extension period was prolonged to 10 minutes. A nested PCR reaction (30 cycles) was performed using 1 μL of a 1:10 dilution of the first-round amplification product using oligonucleotide primers internal to the original primers: 5′-TATGGTGGTTTGACCTTTAG-3′ for the MBR or 5′-GGACCTTCCTTGGTGTGTTG-3′ for the mcr and 5′-ACCAGGGTCCCTTGGCCCCA-3′ for the JH consensus region, with 1 minute of denaturation at 94°C, 1 minute of annealing at 58°C, and 1 minute of extension at 72°C. The final extension period was again prolonged to 10 minutes. A 25-μL aliquot of PCR product was analyzed on a 2% agarose gel containing ethidium bromide in Tris-borate electrophoresis buffer and visualized under UV light. For MBR and mcr, a reproducible sensitivity level of 10−5 and 10−4 respectively, was obtained. This sensitivity level was repeatedly reproducible in the 2 laboratories using paired blind samples.
Results
Clinical and molecular response to CHOP chemotherapy
One-hundred twenty-eight previously untreated patients with a molecularly proven diagnosis of Bcl-2/IgH-positive FL were assigned to receive 6 cycles of CHOP chemotherapy (Table1). The presence of Bcl-2/IgH-positive neoplastic cells within the PB and/or BM mononuclear cells was demonstrated by a standard nested PCR assay in all patients. One-hundred fifteen (90%) were rearranged within the MBR and 13 (10%) within the mcr. The molecular evaluation after 3 and 6 CHOP chemotherapy cycles documented the clearance of the Bcl-2/IgH chimeric gene in about one third of cases (Table2). The evaluation of clinical response after CHOP showed that 73 patients (57%) achieved CR, 47 (37%) PR, and 8 (6%) were considered nonresponsive (NR). The 41 patients who proved PCR negative in the BM and PB on 2 determinations (baseline), as well as the 8 resistant patients, were excluded from rituximab treatment. The correlation between this clinical response after CHOP chemotherapy and the molecular status in the BM is shown in Table3. Seventy-nine patients having either a complete (n = 39) or partial clinical remission (n = 40) and who tested persistently PCR positive in the BM or PB were eligible for rituximab treatment.
Clinical characteristics of the patients
| . | CHOP (128 patients) . | Rituximab (79 patients) . |
|---|---|---|
| Median age, y (range) | 51 (28-66) | 50 (28-66) |
| Aged over 60 y | 15 | 9 |
| Sex, male | 78 | 48 |
| Type of BCL2/IgH rearrangement | ||
| MBR | 115 | 71 |
| mcr | 13 | 8 |
| Disease stage at diagnosis | ||
| I | 1 | 0 |
| II | 12 | 4 |
| III | 26 | 16 |
| IV | 89 | 59 |
| Bone marrow involvement | 87 | 58 |
| Extranodal sites (other than BM) | ||
| 1 | 23 | 11 |
| 2 or more | 6 | 6 |
| PS more than 2 | 0 | 0 |
| B symptoms | 13 | 8 |
| Bulky disease* | 29 | 23 |
| Serum LDH | ||
| Normal | 106 | 67 |
| Elevated | 17 | 11 |
| Unknown | 5 | 1 |
| IPI | ||
| 0 to 1 | 82 | 49 |
| At least 2 | 37 | 29 |
| Unknown | 9 | 1 |
| Response to CHOP chemotherapy | ||
| CR | 73 | 39 |
| PR | 47 | 40 |
| NR | 8 | 0 |
| . | CHOP (128 patients) . | Rituximab (79 patients) . |
|---|---|---|
| Median age, y (range) | 51 (28-66) | 50 (28-66) |
| Aged over 60 y | 15 | 9 |
| Sex, male | 78 | 48 |
| Type of BCL2/IgH rearrangement | ||
| MBR | 115 | 71 |
| mcr | 13 | 8 |
| Disease stage at diagnosis | ||
| I | 1 | 0 |
| II | 12 | 4 |
| III | 26 | 16 |
| IV | 89 | 59 |
| Bone marrow involvement | 87 | 58 |
| Extranodal sites (other than BM) | ||
| 1 | 23 | 11 |
| 2 or more | 6 | 6 |
| PS more than 2 | 0 | 0 |
| B symptoms | 13 | 8 |
| Bulky disease* | 29 | 23 |
| Serum LDH | ||
| Normal | 106 | 67 |
| Elevated | 17 | 11 |
| Unknown | 5 | 1 |
| IPI | ||
| 0 to 1 | 82 | 49 |
| At least 2 | 37 | 29 |
| Unknown | 9 | 1 |
| Response to CHOP chemotherapy | ||
| CR | 73 | 39 |
| PR | 47 | 40 |
| NR | 8 | 0 |
Mass (> 10 cm) in one location or mediastinal mass (> 1/3 of the thoracic diameter).
MBR indicates major breakpoint region; mcr, minor cluster region; BM, bone marrow; PS, performance status; LDH, lactate dehydrogenase; IPI, International Prognostic Index; CR, complete remission; PR, partial remission; and NR, nonresponsive.
Molecular analysis of Bcl-2/IgH chimeric gene before, during, and after CHOP chemotherapy
| . | PCR-negative . | |
|---|---|---|
| Bone marrow (%) . | Peripheral blood (%) . | |
| At diagnosis | 0/128 | 0/128 |
| After 3 cycles of CHOP | 32/111 (29)* | 35/113 (30) |
| After 6 cycles of CHOP | 43/118 (36)† | 43/121 (35) |
| . | PCR-negative . | |
|---|---|---|
| Bone marrow (%) . | Peripheral blood (%) . | |
| At diagnosis | 0/128 | 0/128 |
| After 3 cycles of CHOP | 32/111 (29)* | 35/113 (30) |
| After 6 cycles of CHOP | 43/118 (36)† | 43/121 (35) |
In 13 patients the molecular evaluation on bone marrow aspirates was not performed because of patients' and physicians' refusal. In 4 patients the harvested marrow was not adequate for molecular analysis.
Seven patients were not evaluated because they were clinically not responsive to CHOP. In one patient the harvested marrow was not adequate for molecular analysis. In 2 patients the evaluation was not performed because of the patients' and physicians' refusal.
Clinical and molecular response after CHOP chemotherapy
| Clinical response (%) . | Molecular status3-150 . | |
|---|---|---|
| PCR+ . | PCR− . | |
| Complete 73 (57) | 39 | 34 |
| Partial 47 (37) | 40 | 7 |
| Absent 8 (6) | 8 | 0 |
| Clinical response (%) . | Molecular status3-150 . | |
|---|---|---|
| PCR+ . | PCR− . | |
| Complete 73 (57) | 39 | 34 |
| Partial 47 (37) | 40 | 7 |
| Absent 8 (6) | 8 | 0 |
The molecular status was assessed on mononuclear cells from bone marrow (n = 118) and peripheral blood (n = 121).
PCR indicates polymerase chain reaction.
Clinical response to rituximab
The clinical characteristics of the 79 Bcl-2/IgH-positive patients eligible to receive rituximab are summarized in Table 1. The median age was 50 years; 61% of patients were male. The majority of patients (95%) had stage III or IV disease before CHOP chemotherapy, with 23 patients (29%) having bulky disease and 58 (73%) histologically documented BM infiltration. Of these patients, 77 were treated with rituximab whereas 2, already in CR after CHOP, did not receive the assigned treatment because of refusal and protocol violation. Both eligible and noneligible patients remain under regular follow-up by the study group. Of the 77 patients treated with the chimeric antibody, 74 received the 4 scheduled doses of the drug whereas 2 patients discontinued treatment after the first infusion. At the time of this analysis, 76 patients treated with rituximab had completed the molecular follow-up and could be clinically re-evaluated to correlate the clinical response and the molecular results. Of these patients, 53 (69%) were in CR, 10 (13%) in PR, and 13 (17%) had relapsed or showed evidence of disease progression. For 1 patient, this analysis was too early. Among the 37 patients already in CR after CHOP, only 2 relapsed. For 1 patient, this analysis was too early. Of the 40 patients who were in PR after CHOP, rituximab treatment was followed by the achievement of a clinical CR in 18 patients. Ten patients remained in PR, 11 showed evidence of disease progression, and for 1 patient this analysis was too early.
Molecular response to rituximab
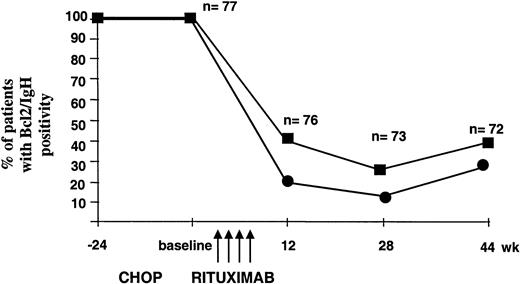
All 77 patients treated with rituximab entered the MRD follow-up program, which was performed on BM and PB samples collected at week +12, +28, and +44 after baseline. At the first molecular follow-up, 59% of the evaluable patients (n = 76) showed a complete molecular response (PCR negativity in the BM and PB). Interestingly, several cases still PCR positive at the first follow-up control converted to negativity at the second molecular analysis (74%, n = 73), thus indicating a prolonged biologic activity of the recombinant antibody capable of inducing an apparent progressive in vivo clearance of the neoplastic clone. At week +44, the percentage of PCR-negative patients declined to 63% of the 72 evaluable patients. Notably, at all time points the disease appeared to be more resistant in the BM compared with the PB (Figure 2). At the end of the molecular follow-up, 75% of patients who achieved or maintained CR status proved PCR negative in the BM and 89% in the PB. As expected, most patients who remained in PR or suffered disease progression or relapse were found to be PCR positive in both the BM and the PB. However, among patients with evidence of relapse or progression, 2 were PCR negative in the BM and 4 in the PB; in these latter patients the clinical relapse was isolated and never systemic (Table4).
Progressive clearance of Bcl-2/IgH-positive cells after rituximab administration.
PCR analysis was performed at the indicated time points on patients' BM (▪) and PB (●) mononuclear cells. As negative control, a sample containing all PCR reagents without DNA was amplified in parallel with DNA extracted from PB mononuclear cells obtained from a pool of healthy donors. In each experiment, DNA extracted at diagnosis and DNA from the Bcl-2/IgH-positive DoHH2 cell line were used as positive controls.
Progressive clearance of Bcl-2/IgH-positive cells after rituximab administration.
PCR analysis was performed at the indicated time points on patients' BM (▪) and PB (●) mononuclear cells. As negative control, a sample containing all PCR reagents without DNA was amplified in parallel with DNA extracted from PB mononuclear cells obtained from a pool of healthy donors. In each experiment, DNA extracted at diagnosis and DNA from the Bcl-2/IgH-positive DoHH2 cell line were used as positive controls.
Correlation between clinical and molecular response after sequential CHOP and rituximab
| Clinical response after rituximab (% of 77 patients) . | Molecular response . | |||
|---|---|---|---|---|
| Bone marrow . | Peripheral blood . | |||
| PCR− (%) . | PCR+ (%) . | PCR− (%) . | PCR+ (%) . | |
| Complete 53 (69) | 40 (75) | 13 (25) | 47 (89) | 5 (11) |
| Partial 10 (13) | 3 (30) | 7 (70) | 3 (30) | 7 (70) |
| Rel/Prog 13 (17) | 2 (15) | 11 (85) | 4 (30) | 9 (70) |
| Too early 1 | — | — | — | — |
| Clinical response after rituximab (% of 77 patients) . | Molecular response . | |||
|---|---|---|---|---|
| Bone marrow . | Peripheral blood . | |||
| PCR− (%) . | PCR+ (%) . | PCR− (%) . | PCR+ (%) . | |
| Complete 53 (69) | 40 (75) | 13 (25) | 47 (89) | 5 (11) |
| Partial 10 (13) | 3 (30) | 7 (70) | 3 (30) | 7 (70) |
| Rel/Prog 13 (17) | 2 (15) | 11 (85) | 4 (30) | 9 (70) |
| Too early 1 | — | — | — | — |
The analysis was performed at the last molecular follow-up (wk +44 in 69 patients). For patients with evidence of an early disease relapse or progression, the last molecular follow-up was at wk +12 in 3 patients and at wk +28 in 1 patient.
Treatment outcome
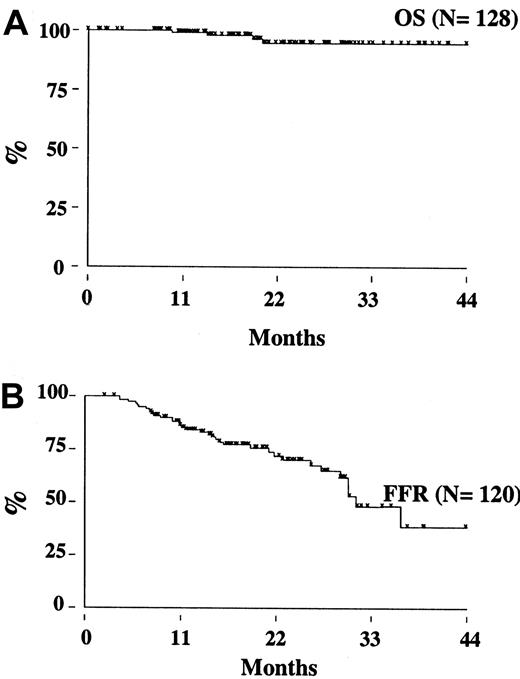
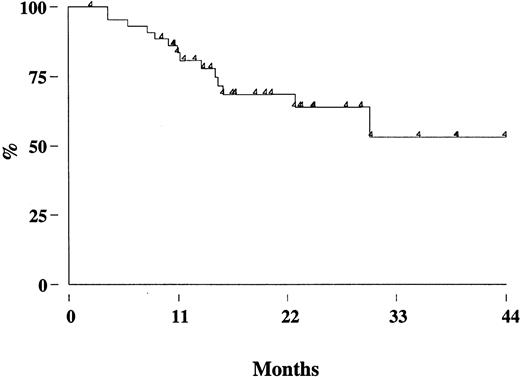
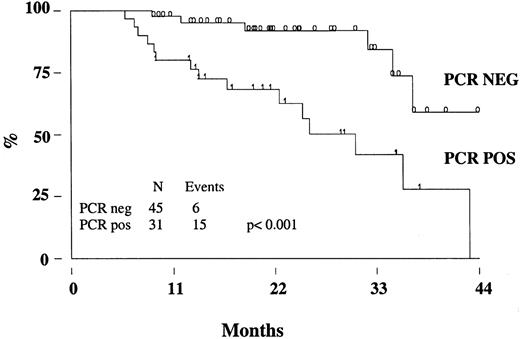
Two patients (one in the rituximab cohort) died from disease progression, 2 from another malignancy (metastatic renal cell carcinoma and Hodgkin disease), so that the estimated OS at 3 years is 95% (95% CI, 86-98) (Figure 3A). With a median follow-up of 17 months (range, 8-39 months), the estimated 3-year FFR is 44% (95% CI, 28-59) (Figure 3B). The FFR of patients who achieved PCR-negative status at the end of CHOP chemotherapy and who did not receive rituximab was 52% (95% CI, 28-71) (Figure4). For patients treated with rituximab, the achievement of a durable PCR-negative status was associated with a significantly better clinical outcome; at 3 years, in fact, the FFR was 57% (95% CI, 23-81), compared with 20% (95% CI, 4-46,P < .001) recorded in patients who failed to achieve a molecular response (Figure 5). Interestingly, provided that PCR-negative status in the BM was maintained until the last molecular follow-up (+44 weeks), a low frequency of recurrence or relapse was observed equally in patients who achieved the molecular response at the first molecular follow-up or later. Moreover, patients who never converted to PCR negativity or who reverted to positivity after rituximab had a similarly poor clinical outcome (Table 5).
Overall survival and freedom from recurrence for the study population.
(A) Overall survival. (B) Freedom from recurrence.
Overall survival and freedom from recurrence for the study population.
(A) Overall survival. (B) Freedom from recurrence.
FFR of patients achieving PCR-negative status (BM and PB) after CHOP chemotherapy.
These patients were excluded from rituximab treatment.
FFR of patients achieving PCR-negative status (BM and PB) after CHOP chemotherapy.
These patients were excluded from rituximab treatment.
FFR of patients treated with rituximab according to the PCR status.
The molecular analysis was performed on BM cells at the end of the molecular follow-up. PCR neg indicates PCR negativity, PCR pos; PCR positivity.
FFR of patients treated with rituximab according to the PCR status.
The molecular analysis was performed on BM cells at the end of the molecular follow-up. PCR neg indicates PCR negativity, PCR pos; PCR positivity.
Relationship between kinetics of the molecular response and disease recurrence
| PCR negativization in the bone marrow following rituximab . | Event rate (%) . | χ2 . | Log rank . |
|---|---|---|---|
| Rapid and sustained (at wk +12, +28, and +44) | 4/33 (12) | — | — |
| Late but sustained (at wk +28 and +44) | 2/12 (17) | 0.69 | 0.84 |
| Transient (at wk +12 and +28 but not +44) | 8/17 (47) | 0.006 | 0.003 |
| Absent | 7/14 (50) | 0.005 | 0.01 |
| PCR negativization in the bone marrow following rituximab . | Event rate (%) . | χ2 . | Log rank . |
|---|---|---|---|
| Rapid and sustained (at wk +12, +28, and +44) | 4/33 (12) | — | — |
| Late but sustained (at wk +28 and +44) | 2/12 (17) | 0.69 | 0.84 |
| Transient (at wk +12 and +28 but not +44) | 8/17 (47) | 0.006 | 0.003 |
| Absent | 7/14 (50) | 0.005 | 0.01 |
Prognostic factors for patients treated with rituximab
To identify prognostic variables for patients treated with rituximab, a number of factors were examined in univariate and multivariate analyses. By univariate analysis, male sex, presence of extranodal sites, absence of a complete clinical response after CHOP, and lack of a durable (44 weeks from baseline) clearance of Bcl-2/IgH-positive cells were all predictors of disease recurrence (Table 6). The prognostic value of the molecular status at 44 weeks was confirmed also by a Cox time-dependent multivariate analysis which showed that the absence of Bcl-2/IgH rearrangement in the BM at the last molecular control was strongly associated with a reduced risk of recurrence (hazards ratio, 0.3; 95% CI, 0.12-1; P = .04). All other clinical factors, with the exception of sex, did not have a predictive impact on outcome (Table7).
Univariate analysis of 77 patients treated with rituximab
| Variable . | Univariate analysis . | |
|---|---|---|
| Event rate (%) . | P6-150 . | |
| Sex | ||
| M | 16/47 (34) | .03 |
| F | 5/30 (17) | |
| Age | ||
| 60 y or younger | 19/68 (28) | .99 |
| Older than 60 y | 2/9 (22) | |
| LDH | ||
| Yes | 6/11 (55) | .09 |
| No | 15/65 (23) | |
| Bulky | ||
| Yes | 7/23 (30) | .59 |
| No | 14/54 (26) | |
| Extra nodal sites other BM | ||
| Less than 2 | 18/71 (25) | .01 |
| 2 or more | 3/6 (50) | |
| BM infiltration | ||
| Yes | 16/56 (29) | .5 |
| No | 5/21 (24) | |
| Clinical response after CHOP | ||
| CR | 6/37 (16) | .01 |
| PR | 15/40 (38) | |
| IPI | ||
| 0 to 1 | 11/48 (23) | .29 |
| At least 2 | 10/28 (36) | |
| (PCR on BM at wk +12) | ||
| Yes | 12/31 (39) | .09 |
| No | 9/45 (20) | |
| (PCR+ on BM at wk +44) | ||
| Yes | 15/31 (48) | .001 |
| No | 6/45 (13) | |
| Variable . | Univariate analysis . | |
|---|---|---|
| Event rate (%) . | P6-150 . | |
| Sex | ||
| M | 16/47 (34) | .03 |
| F | 5/30 (17) | |
| Age | ||
| 60 y or younger | 19/68 (28) | .99 |
| Older than 60 y | 2/9 (22) | |
| LDH | ||
| Yes | 6/11 (55) | .09 |
| No | 15/65 (23) | |
| Bulky | ||
| Yes | 7/23 (30) | .59 |
| No | 14/54 (26) | |
| Extra nodal sites other BM | ||
| Less than 2 | 18/71 (25) | .01 |
| 2 or more | 3/6 (50) | |
| BM infiltration | ||
| Yes | 16/56 (29) | .5 |
| No | 5/21 (24) | |
| Clinical response after CHOP | ||
| CR | 6/37 (16) | .01 |
| PR | 15/40 (38) | |
| IPI | ||
| 0 to 1 | 11/48 (23) | .29 |
| At least 2 | 10/28 (36) | |
| (PCR on BM at wk +12) | ||
| Yes | 12/31 (39) | .09 |
| No | 9/45 (20) | |
| (PCR+ on BM at wk +44) | ||
| Yes | 15/31 (48) | .001 |
| No | 6/45 (13) | |
Log rank.
For abbreviations, see Table 1.
Multivariate analysis of factors predictive of FFR for 77 patients treated with rituximab
| . | Freedom from recurrence . | ||
|---|---|---|---|
| Hazards ratio . | 95% confidence interval . | P . | |
| PCR− vs PCR+ (BM at 12 wk) | 0.54 | 0.18-1.61 | .271 |
| PCR− vs PCR+ (BM at 44 wk) | 0.34 | 0.12-1.00 | .049 |
| Sex (F vs M) | 0.17 | 0.05-0.62 | .007 |
| IPI (0-1 vs ≥ 2) | 0.63 | 0.21-1.87 | .403 |
| Absence of | |||
| BM infiltration | 0.41 | 0.12-1.38 | .151 |
| Bulky disease | 0.74 | 0.27-2.02 | .555 |
| Extranodal sites | 0.52 | 0.10-2.60 | .426 |
| . | Freedom from recurrence . | ||
|---|---|---|---|
| Hazards ratio . | 95% confidence interval . | P . | |
| PCR− vs PCR+ (BM at 12 wk) | 0.54 | 0.18-1.61 | .271 |
| PCR− vs PCR+ (BM at 44 wk) | 0.34 | 0.12-1.00 | .049 |
| Sex (F vs M) | 0.17 | 0.05-0.62 | .007 |
| IPI (0-1 vs ≥ 2) | 0.63 | 0.21-1.87 | .403 |
| Absence of | |||
| BM infiltration | 0.41 | 0.12-1.38 | .151 |
| Bulky disease | 0.74 | 0.27-2.02 | .555 |
| Extranodal sites | 0.52 | 0.10-2.60 | .426 |
For abbreviations, see Table 1.
Toxicity
All 77 patients treated with rituximab were considered in the evaluation of toxicity. In 2 patients, treatment was stopped after the first infusion because of grade III liver toxicity secondary to hepatitis B reactivation in one patient, and a severe allergic reaction with grade III hypotension in the other. The other most frequent adverse events attributed to rituximab were usually infusion related; they never exceeded grade 2 in severity and included fever, chills, and pruritus. These side effects were most frequently reported after the first infusion and substantially decreased in their frequency after the subsequent infusions. No patient required hospitalization as a consequence of rituximab treatment.
Discussion
This study was designed to investigate whether the sequential administration of 6 cycles of CHOP chemotherapy and 4 weekly intravenous infusions of rituximab could induce a significant and durable eradication of Bcl-2/IgH-positive cells from the BM and PB of previously untreated patients with FL. Our results indicate that in about one third of patients, CHOP alone can induce a molecular negativity of the BM and PB. In most of these patients, the clearance of the neoplastic clone seems to be rapid and frequently achieved after the first 3 cycles of chemotherapy. It is unclear whether this is a result of a particular sensitivity to chemotherapy or whether it reflects a lower tumor burden in the BM or PB. As expected, however, in the majority the neoplastic clone was not cleared by conventional chemotherapy and this group of patients was eligible for sequential rituximab administration. Remarkably, a significant proportion of these patients became PCR negative and indeed some patients experienced a long-standing molecular remission. The clearance of the Bcl-2/IgH chimeric signal after rituximab was progressive, since several cases became negative at the second molecular follow-up. This may be explained by pharmacokinetic data demonstrating that measurable amounts of rituximab can be found for a long period of time after the end of treatment.35
Recent studies have reported that rituximab, alone31,36 or in combination with standard dose systemic chemotherapy,37,38 is effective in the treatment of indolent NHL and capable of inducing a molecular Bcl-2 conversion in the PB and/or BM. Our study allowed us to demonstrate the unique capacity of rituximab to eliminate malignant cells from the marrow and/or blood of patients who had a persistent Bcl-2/IgH rearrangement following CHOP. Although a contribution of a delayed effect of CHOP cannot be excluded, the majority of patients who achieved a molecular response with chemotherapy did so within the first 3 cycles. Therefore, we are relatively confident that the subsequent elimination of MRD is due to the efficacy of rituximab rather than to a long-lasting effect of chemotherapy. Moreover, we provided evidence that rituximab can induce not only a durable molecular remission, but also a measurable improvement of the clinical response. The association between a better FFR and the achievement of a molecular response is confirmed by the results obtained in patients whose BM converted to PCR negativity after CHOP alone. Thus, our results support the concept that an improved FFR is associated with the achievement of PCR negativity. Whether this is reached with chemotherapy alone or with chemotherapy followed by rituximab may not be relevant. Our results also suggest that there may be no benefit from combining rituximab with chemotherapy but rather from its use in those patients who failed to achieve a molecular remission with chemotherapy alone. Therefore, rituximab can represent an excellent nontoxic, non–cross reacting agent for the management of MRD in patients with FL. Although the high proportion of complete (marrow and blood) or partial (only blood) molecular responses observed is promising, only a prolonged follow-up will conclusively demonstrate whether the PCR-negative state achieved by this approach bears the same significant improvement of clinical outcome observed with either conventional or high-dose treatments.13,14,29,30 In this respect, the lack of major toxicity associated with rituximab may be of particular importance when considering the risk of secondary myelodysplasia and acute leukemia, which have been observed by several groups after high-dose chemoradiotherapy.15,39 Despite the high proportion of patients who reverted to PCR negativity, in our experience the molecular conversion was transient in some patients and in others a clinical relapse or a progression of the disease was documented despite the molecular negativity of the BM and the PB. Most importantly and at variance from what was reported after high-dose chemotherapy and autologous transplantation with in vitro or in vivo purged peripheral blood stem cells (PBSCs),13,14 the FFR curve even among molecularly responding patients did not show any convincing plateau. This result underlines the fact that beyond the purging effect documented in the blood and marrow, a significant proportion of neoplastic cells may escape the pharmacologic activity of the drug because of their deep lymph node location. On the same line is the observation that the neoplastic clone showed a more pronounced resistance within the BM compared with PB. A plausible, though not exhaustive, explanation is that cells circulating in the blood are more easily accessible to the complement40 as well as to immune-mediated lysis (by natural killer [NK] cells, macrophages). In support of this notion is also the observation that a negativization of the PCR assay was more frequently recorded in the blood compared with the marrow of those patients in whom the concomitant persistence of measurable disease could be demonstrated, clinically or instrumentally. Thus, although blood PCR has been reported to be as informative as marrow for the detection of MRD,29 our results are rather in keeping with studies suggesting that molecular analysis of MRD performed on marrow samples provide more reliable prognostic implications.11
In conclusion, this study confirms the clinical activity of rituximab and documents that the sequential administration of CHOP chemotherapy followed by rituximab can induce complete BM and PB responses in more than 70% of patients. The clinical impact of achieving a molecular response has still to be fully appreciated41 since long term remissions have been reported in patients with a persistently detectable Bcl-2/IgH rearrangement,42 and indeed this rearrangement may occasionally be found in the normal PB lymphocytes.43 Further studies are required to identify the most effective chemotherapy programs to combine with rituximab, the optimal time for the administration of rituximab, and to address the issue of the correlation between molecular remission and clinical outcome.
Supported in part by grants from Associazione Italiana per la Ricerca contro il Cancro (AIRC), Consiglio Nazionale per le Ricerche (CNR) (target project on biotechnology no. 97.01278.PF449), Ministero dell'Università e della Ricerca Scientifica (MURST), Associazione Paolo Belli, and Associazione Italiana contro le Leucemie (AIL).
G.D. and E.G. are employed by Roche, SpA whose product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Alessandro Rambaldi, Divisione di Ematologia, Ospedali Riuniti, Largo Barozzi 1, 24100 Bergamo, Italy; e-mail: arambaldi@ospedaliriuniti.bergamo.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal