Abstract
The ability of factor VIIa to initiate thrombin generation and clot formation in blood from healthy donors, blood from patients with hemophilia A, and in anti–factor IX antibody–induced (“acquired”) hemophilia B blood was investigated. In normal blood, both factor VIIa–tissue factor (TF) complex and factor VIIa alone initiated thrombin generation. The efficiency of factor VIIa was about 0.0001 that of the factor VIIa–TF complex. In congenital hemophilia A blood and “acquired” hemophilia B blood in vitro, addition of 10 to 50 nM factor VIIa (pharmacologic concentrations) corrected the clotting time at all TF concentrations tested (0-100 pM) but had little effect on thrombin generation. Fibrinopeptide release and insoluble clot formation were only marginally influenced by addition of factor VIIa. TF alone had a more pronounced effect on thrombin generation; an increase in TF from 0 to 100 pM increased the maximum thrombin level in “acquired” hemophilia B blood from 120 to 480 nM. Platelet activation was considerably enhanced by addition of factor VIIa to both hemophilia A blood and “acquired” hemophilia B blood. Thus, pharmacologic concentrations of factor VIIa cannot restore normal thrombin generation in hemophilia A and hemophilia B blood in vitro. The efficacy of factor VIIa (10-50 nM) in hemophilia blood is dependent on TF.
Introduction
The blood-coagulation cascade is initiated when cryptic tissue factor (TF) is expressed and exposed to circulating blood and binds plasma factor VIIa. The resulting factor VIIa–TF complex activates the serine protease zymogens factor IX and factor X. The factor Xa that is initially produced generates picomolar amounts of thrombin, which activates platelets and cleaves procofactors factors V and VIII. Factor VIIIa forms a complex on a membrane surface with serine protease factor IXa and activates factor X at a 50- to 100-fold higher rate than the factor VIIa–TF complex. The factor Xa produced, in complex with its cofactor, factor Va, and an appropriate membrane surface forms the prothrombinase complex, which is the primary activator of prothrombin. The thrombin produced amplifies its own generation by activating factor XI and completing the activation of platelets and procofactors. Thrombin cleaves fibrinogen and activates factor XIII to form the insoluble isopeptide cross-linked fibrin clot. The coagulation cascade is down-regulated by the stoichiometric inhibitors antithrombin III (AT-III) and tissue factor pathway inhibitor (TFPI) and by the dynamic protein C system.1
Genetic and acquired deficiencies in coagulation proteins lead to hemorrhagic syndromes.2-6 The most common bleeding disorders result from deficiencies of factor VIII (hemophilia A) or factor IX (hemophilia B) coagulant activity. In the past, the principal treatments for hemophilia relied on partially purified concentrates of coagulation factors.7,8 These concentrates, however, have been associated with thromboembolic complications and viral infections.9-11 During the past decade, plasma-derived, monoclonal antibody–purified factor VIII12 and factor IX13 concentrates that contain negligible amounts of other coagulation factors, as well as recombinant factors VIII14,15 and IX,16 have become available. In addition, substantial progress in the treatment of hemophilias has been achieved in animal models by using gene therapy,17-19 and clinical trials employing this approach in patients have begun.20 21
In a considerable proportion of patients with hemophilia receiving replacement therapy, inhibitory antibodies directed against the missing factor develop,15,22-25 and this complicates further administration of the deficient protein. For patients with antibodies against factor VIII or factor IX, an alternative treatment using supraphysiologic concentrations of factor VIIa was suggested.26,27 During the past decade, recombinant factor VIIa has been used successfully for hemophilia treatment in patients with and without inhibitors,28-32 especially those undergoing surgical procedures.30-32 Moreover, recombinant factor VIIa has been suggested for treatment of almost all bleeding disorders and for enhancement of normal hemostasis in patients without coagulation defects.33 The mechanism by which hemostasis is established by high doses of factor VIIa is not known, although it has been hypothesized that factor VIIa can trigger thrombin generation in a TF-independent manner.34 35
Several models of TF-initiated blood coagulation have been developed in our laboratory.36-38 In all these models, thrombin generation can be divided into 2 phases.39 The initiation phase follows addition of TF and is characterized by generation of thrombin at low nanomolar concentrations, which leads to activation of platelets and almost quantitative proteolysis of factors V and VIII. Femtomolar to picomolar amounts of factors VIIa, IXa, Xa, and XIa are produced during this phase, the duration of which is regulated primarily by the factor VIIa–TF complex and TFPI.36,37,40,41 Subsequently, a propagation phase occurs, which is characterized by rapid activation of prothrombin; increased rates of activation of factors VII, IX, X, and XI; and formation of solid clots. The rate of thrombin generation during the propagation phase depends primarily on the factor Xa produced by the factor IXa–factor VIIIa complex.39 As a consequence, thrombin generation during the initiation phase is virtually unaffected by the absence of factor VIII or factor IX.36,37,39 Thrombin generation during the propagation phase is significantly suppressed in hemophilia A and hemophilia B because of decreased generation of factor Xa.36,37,41 Addition of factor VIII to a physiologic concentration (0.7 nM) to hemophilia A blood restores normal thrombin generation.41 In this study, we evaluated the ability of high concentrations of factor VIIa alone and in the presence of TF to generate thrombin in blood from healthy donors, blood from patients with hemophilia A, and “acquired” hemophilia B blood and in a synthetic blood coagulation model.
Materials and methods
Materials
Human coagulation factors VII, X, IX and prothrombin were isolated from fresh-frozen plasma by using methods described by Bajaj et al42 and were purged of traces of contaminants and active enzymes as described previously.40 Human factor V and AT-III were isolated from fresh-frozen plasma.43,44Recombinant factor VIII and recombinant TF (residues 1-242) were provided by Dr Shu Len Liu and Dr Roger Lundblad (Baxter Healthcare, Duarte, CA). Recombinant human factor VIIa was from Novo Pharmaceuticals (Copenhagen, Denmark). Recombinant full-length TFPI produced in Escherichia coli was provided by Dr K. Johnson (Chiron, Emeryville, CA). Corn trypsin inhibitor (CTI) was isolated from popcorn as described previously.41 Washed platelets were prepared by using the procedure of Mustard et al.45The TF/lipid reagent was prepared as described previously.41 Phosphatidylserine (PS), phosphatidylcholine (PC), and EDTA were from Sigma (St Louis, MO). Phospholipid vesicles (PCPS) composed of 25% PS and 75% PC were prepared as described previously.46 D-hexahydrotyrosyl-Ala-Arg p-nitroanilide (Spectrozyme TH) was from American Diagnostica (Greenwich, CT). D-Phe-Pro-Arg-chloromethyl ketone (FPRck) was synthesized in house. The enzyme-linked immunosorbent assay (ELISA) thrombin–AT-III (TAT) kit (Enzygnost) was from Behring (Marburg, Germany). Osteonectin ELISA plates were coated in house. Monoclonal anti-osteonectin and anti-factor IX (α-IX-91) antibodies were developed in the Biochemistry Antibody Core Laboratory at the University of Vermont. Anti-TF antibody 5G9 was from Dr T. Edgington (Scripps, La Jolla, CA). Fibrinogen, fibrinopeptide A (FPA), fibrinopeptide B (FPB), and solid-clot analyses were done as described previously.47
Human donors
Three healthy donors and one patient with hemophilia A were recruited and advised about the study according to a protocol approved by the University of Vermont Human Studies Committee,38 41and their consent to participate was obtained. Healthy donors with a history of thrombosis or hemorrhage or regular aspirin or drug use were excluded. Donors selected as controls had normal values for prothrombin time (PT; 11.6-13.0 seconds), international normalized ratio (INR; 1.0-1.1), fibrinogen (2.4-3.4 mg/mL), blood-coagulation proteins, and platelet counts (1.8-2.7 × 108/mL).
The donor with hemophilia A, a 21-year-old man, tested positive for hepatitis C. However, he had normal values for fibrinogen (2.5 mg/mL), platelet count (2.1 × 108/mL), PT (12.8 seconds), and INR (1.0), although he had a severe deficiency of factor VIII (VIII:C < 1%). His levels of factor IX were elevated (209%); those of other coagulation proteins were in the normal range. He had a history of bleeding and joint pain and routinely self-administered recombinant factor VIII products when symptomatic. Blood was drawn 1 week after his most recent factor VIII injection, and his factor VIII levels on the days of the experiments were less than 2%. There was no evidence of inhibitors (eg, anti-factor VIII antibodies).
“Acquired” hemophilia B blood
The equivalent of acquired hemophilia B was induced in fresh CTI-inhibited normal whole blood in vitro by adding 50 μg/mL α-IX-91. At this concentration, the antibody prolonged the activated partial thromboplastin time (APTT) of normal plasma from 38 to 115 seconds. The APTT for commercial factor IX–deficient plasma (< 1% of factor IX; George King Biomedical, Overland Park, KS) was 112 seconds. The titer of the α-IX-91 at 50 μg/mL was 27 Bethesda units.48
Synthetic blood coagulation model
Procofactor solution.
Relipidated TF (50 pM; omitted when desired) was incubated with 4 μM PCPS (omitted in platelet experiments) in HEPES-buffered saline (HBS; 20 mM HEPES and 150 mM sodium chloride) and 2 mM calcium chloride (CaCl2) for 10 minutes at 37°C. Factor V (40 nM), factor VIII (1.4 nM; omitted when desired), and 4 × 108/mL platelets (in platelet experiments only) were added to the relipidated TF before initiation of the reaction.
Zymogen-inhibitor solution.
Prothrombin (2.8 μM), factor VII (20 nM), factor VIIa (0.2 nM), factor X (340 nM), factor IX (180 nM), TFPI (5 nM), and AT-III (6.8 μM) were preheated in HBS and 2 mM CaCl2 at 37°C for 3 minutes. In some experiments, additional amounts of factor VIIa (10-240 nM) were added.
The reaction was started by mixing equal volumes of both solutions, which resulted in physiologic concentrations of the proteins and platelets and a final TF concentration of 25 pM. After initiation of the reaction, 10-mL aliquots were withdrawn from the reaction mixture at selected time points, placed in 20 mM EDTA in HBS (pH 7.4) containing 0.2 mM Spectrozyme TH, and assayed immediately for thrombin activity. Hydrolysis of the substrate was monitored by the change in absorbance at 405 nm by using a Vmax spectrophotometer (Molecular Devices, Sunnyvale, CA). Thrombin generation was calculated from a standard curve prepared by serial dilutions of α-thrombin.
TF-initiated clotting of fresh human blood
Single-tube clotting time test.
The protocol used was that described by Holmes et al.49Fresh human blood (1 mL) was added to a tube containing 100 μg/mL CTI (CTI prevents the contact pathway of blood coagulation by inhibiting factor XIIa) and various concentrations (0-40 μg/mL) of antibody 5G9 and recombinant factor VIIa (0-60 nM) in the absence or presence of 1 pM or 10 pM TF. The tube was placed in a Hemochron activated-coagulation-time instrument (International Technidyne, Edison, NJ). The time to clot was detected by displacement of a magnet within the rotating tube by formation of fibrin strands.
Multiple-tube experiments.
The protocol used was a modification of that of Rand et al.38 Experiments were done in 32 tubes placed on a rocking table enclosed in a temperature-controlled (37°C) glove box. Fresh CTI-inhibited (100 μg/mL CTI) blood was used after venipuncture and immediate delivery into reagent-loaded tubes.
Thirty of 32 tubes (2 series/experiment; 16 tubes/series) were loaded with CTI and 12.5 pM relipidated TF in HBS and 2 mM CaCl2. Two phlebotomy control tubes (1 tube/series) contained no TF. Recombinant factor VIIa (10 or 50 nM; all tubes, experiment series only) and an equivalent volume of factor VIIa dilution buffer (HBS and 2mM CaCl2; all tubes, control series only) were loaded. The zero-time tube for each series was pretreated with 1 mL 50 mM EDTA and 10 μL 10 mM FPRck (diluted in 10 mM hydrochloric acid). After blood was delivered, the tubes were periodically (1-20 minutes) quenched with EDTA and FPRck.
In the “acquired” hemophilia B experiments, all tubes were loaded in duplicate with CTI and various concentrations of TF (0-100 pM) and factor VIIa (0, 10, and 50 nM). All tubes in experiment series were loaded with 50 μg/mL α-IX-91; those in control series were loaded with α-IX-91 dilution buffer (HBS and 2 mM CaCl2). Tubes were treated with EDTA and FPRck 10 minutes after solid clot was observed.
In all multiple-tube experiments, no more than 35 μL of reagents was loaded in each tube. The clotting time was observed visually by 2 observers and was recorded when clumps were observed on the side of the tube. After the experiment, tubes were centrifuged and the supernatants were aliquoted for further analyses. ELISAs for TAT (thrombin generation) and osteonectin (platelet activation) were done. FPA and FPB (clot formation) were assessed by high-performance liquid chromatography and fibrinogen and fibrin by Western blot analysis.47 Solid clots were lyophilized, weighed, solubilized, and analyzed by gel electrophoresis.47
Results
Factor VIIa and TF in normal blood
Thrombin generation in the synthetic blood coagulation model.
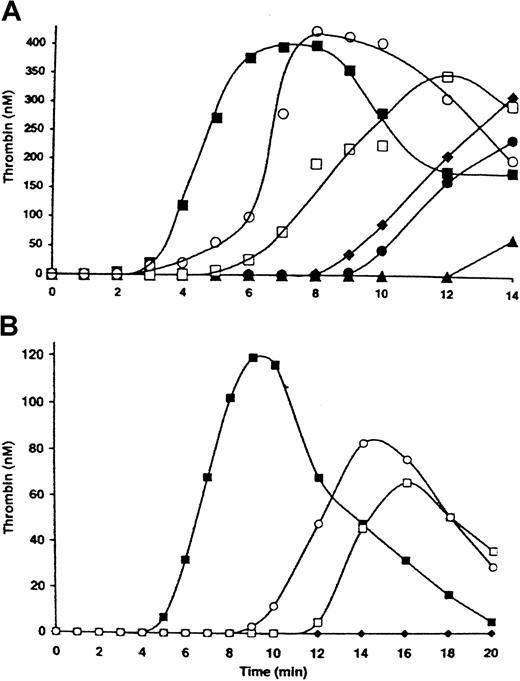
Figure 1A (▪) shows thrombin generation in the presence of 2 μM PCPS (1-2 μM PCPS is equivalent to 2 × 108/mL platelets in supporting thrombin generation50) initiated by 25 pM TF with factor VIIa at a physiologic concentration (0.1 nM). The initiation phase lasted approximately 3 minutes, and maximum levels of thrombin (∼ 390 nM) were achieved 7 minutes after initiation of the reaction. The maximum rate of thrombin generation was 2.5 nM/second. In the absence of TF and with 10 nM factor VIIa (Figure 1A; ▴), the initiation phase was prolonged to 12 minutes. Increases in factor VIIa concentrations (up to 120 nM) in the absence of TF shortened the initiation phase in a concentration-dependent manner. At the highest concentration of factor VIIa tested (120 nM; Figure 1A; ○), the initiation phase was extended (∼ 5 minutes) relative to that observed in the presence of 25 pM TF and 0.1 nM factor VIIa. Thus, an equivalent maximum rate of thrombin generation (2.7 nM/second) in the absence of TF was achieved only with a 1200-fold increase (to 120 nM) in factor VIIa.
Factor VIIa titration in a synthetic blood coagulation model in a complete system.
Thrombin generation was initiated either by 25 pM TF in the presence of factors V, VIII, VII, VIIa, IX, and X, prothrombin, AT-III, and TFPI at physiologic concentrations (▪) or by factor VIIa at 10 (▴), 20 (●), 30 (♦), 60 (■), and 120 nM (○) in the absence of TF. The reaction was conducted in the presence of either 2 μM PCPS (A) or 2 × 108/mL platelets (B).
Factor VIIa titration in a synthetic blood coagulation model in a complete system.
Thrombin generation was initiated either by 25 pM TF in the presence of factors V, VIII, VII, VIIa, IX, and X, prothrombin, AT-III, and TFPI at physiologic concentrations (▪) or by factor VIIa at 10 (▴), 20 (●), 30 (♦), 60 (■), and 120 nM (○) in the absence of TF. The reaction was conducted in the presence of either 2 μM PCPS (A) or 2 × 108/mL platelets (B).
When PCPS was replaced by washed platelets (2 × 108/mL; Figure 1B), the initiation phase in the reaction initiated by 25 pM relipidated TF lasted almost 5 minutes (Figure 1B; ▪). The maximum thrombin level (120 nM) was achieved 9 minutes after initiation of the reaction. In the absence of TF, factor VIIa at a concentration of 30 nM (Figure 1B; ♦) did not produce detectable amounts of thrombin for 20 minutes. At the highest factor VIIa concentration tested (120 nM), the initiation phase of thrombin generation lasted 8 minutes and the maximum thrombin level was 80 nM. Thus, in the presence of either platelets or phospholipids, factor VIIa in the absence of TF was able to trigger thrombin generation. However, the efficiency of the factor VIIa–TF complex was about 104-fold higher than that of factor VIIa alone. This observation is in agreement with the finding by Komiyama et al51 indicating that TF increases the factor IX– and factor X–activating efficiency of factor VIIa by 3 to 4 orders of magnitude.
Clotting assays of CTI-inhibited whole blood.
In the presence of 100 μg/mL CTI, fresh, otherwise untreated human blood clotted in more than 1000 seconds (Figure2; ▪). Without addition of TF, addition of factor VIIa at concentrations of 10 and 20 nM (Figure 2; ▪) decreased the clotting time to 822 seconds and 517 seconds, respectively. A further increase in factor VIIa concentration to 60 nM reduced the clotting time to 336 seconds. Addition of the anti-TF antibody 5G9 at concentrations of 0 to 40 μg/mL in the presence of 50 nM factor VIIa had no effect on clotting time, indicating that clot formation was initiated by factor VIIa alone and not by contaminating TF in the blood drawn. Addition of 10 pM TF without exogenous factor VIIa produced an almost identical clotting time (334 seconds; Figure 2; ▴). These results suggest that TF with endogenous (∼ 0.1 nM) factor VIIa is about 6 × 103-fold more potent as an initiator than factor VIIa. This idea is consistent with observations in the synthetic model. Addition of 10 nM factor VIIa with 10 pM TF decreased the clotting time to 196 seconds. At this TF concentration, further increases in factor VIIa concentration had little effect on clotting time. Addition of 1 pM TF (Figure 2; ●) with 10 or 20 nM factor VIIa produced clotting times of 571 seconds and 300 seconds, respectively. Further increases in factor VIIa concentration (up to 50 nM) had no additional effect on clotting time.
Factor VIIa titration in normal blood.
Clotting of CTI-inhibited (0.1 mg/mL) whole blood was initiated in a Hemochron instrument by various concentrations of factor VIIa in the absence of TF (▪) or the presence of 1 pM (●) and 10 pM (▴) TF.
Factor VIIa titration in normal blood.
Clotting of CTI-inhibited (0.1 mg/mL) whole blood was initiated in a Hemochron instrument by various concentrations of factor VIIa in the absence of TF (▪) or the presence of 1 pM (●) and 10 pM (▴) TF.
Factor VIIa and TF in hemophilia
Thrombin generation in the synthetic hemophilia A model.
Thrombin generation initiated by 25 pM TF in the presence of 2 × 108/mL platelets is shown in Figure3. In a complete system (Figure 3; ▪), detectable thrombin generation was observed after the initiation phase (∼ 5 minutes) and reached a maximum level of 158 nM. The maximum rate of thrombin formation was 2.0 nM/second. In a similar experiment in the absence of factor VIII (Figure 3; ○), only 4 nM thrombin was observed at the end of the experiment (20 minutes). Addition of 5 nM factor VIIa in the absence of factor VIII (Figure 3; ▴) decreased the duration of the initiation phase to approximately 12 minutes and increased maximum thrombin levels to 10 nM. An increase in factor VIIa concentration to 10 nM (Figure 3; ♦) had no further effect on the initiation phase, although maximum thrombin levels increased to 20 nM. Increasing the factor VIIa concentration to 40 nM (Figure 3; ■) had no additional effect on thrombin generation. Similar profiles for thrombin generation were observed when 2 μM PCPS was substituted for platelets (data not shown).
Thrombin generation in synthetic blood coagulation model in the absence of factor VIII.
Thrombin generation on 2 × 108/mL platelets was initiated by 25 pM TF in the presence of factors V, VIII, VII, VIIa, IX, and X, prothrombin, AT-III, and TFPI at physiologic concentrations (▪) or in the absence of factor VIII and the presence of 0.1 (○), 5 (▴), 10 (♦), and 40 nM (■) factor VIIa.
Thrombin generation in synthetic blood coagulation model in the absence of factor VIII.
Thrombin generation on 2 × 108/mL platelets was initiated by 25 pM TF in the presence of factors V, VIII, VII, VIIa, IX, and X, prothrombin, AT-III, and TFPI at physiologic concentrations (▪) or in the absence of factor VIII and the presence of 0.1 (○), 5 (▴), 10 (♦), and 40 nM (■) factor VIIa.
“Acquired” hemophilia B blood.
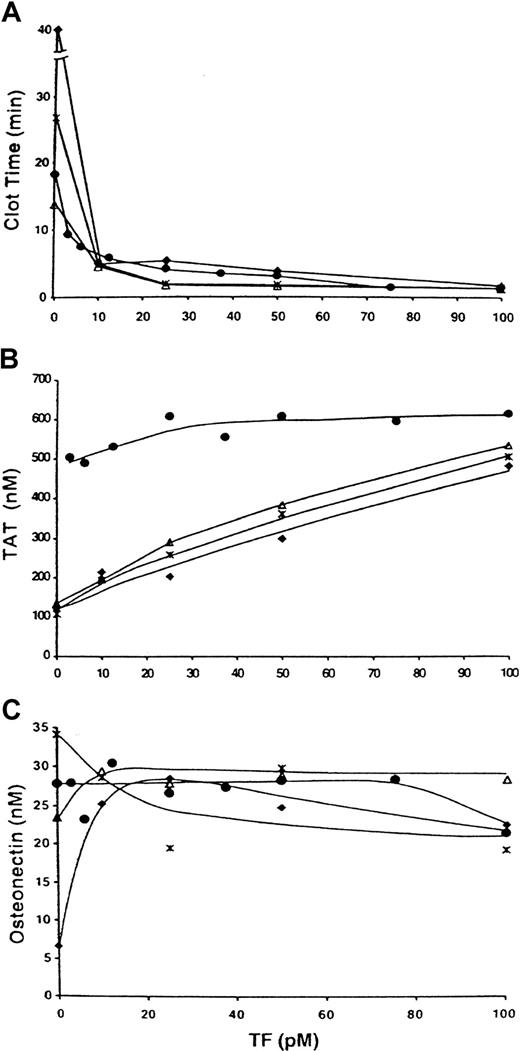
In the absence of TF, CTI-inhibited normal blood clotted in 18 minutes (Figure 4A; ●). Addition of 3.1 pM TF decreased the clotting time to 9.4 minutes. At 25 pM TF, the clotting time was 3.9 minutes. Increases in TF to 75 to 100 pM further decreased the clotting time of normal blood (to 1.4 minutes). No clot was observed after 40 minutes in the “acquired” hemophilia B blood in the absence of TF (Figure 4; ♦). Addition of 10 pM TF decreased the clotting time to 4.8 minutes. At 100 pM TF, a clotting time of 1.7 minutes was observed, ie, a time comparable to that in normal blood. Factor VIIa in the absence of TF decreased the clotting time in acquired hemophilia B blood to 26.7 minutes and 13.9 minutes (10 nM factor VIIa [Figure 4; ∗] and 50 nM [Figure 4; ▵] factor VIIa, respectively). The combination of 10 pM TF and factor VIIa led to further decreases in clotting times (4.9 minutes at 10 nM factor VIIa and 4.4 minutes at 50 nM, ie, to levels observed in normal blood at a similar TF concentration but without exogenous factor VIIa). At 10 to 100 pM TF, clotting times of the “acquired” hemophilia B blood with factor VIIa additions were similar to those in normal blood. No substantial differences in clotting times were observed at any TF concentration when the factor VIIa concentration was increased from 10 to 50 nM.
TF titration in normal blood and “acquired” hemophilia B blood.
Clotting of CTI-inhibited (0.1 mg/mL) normal blood (●) and acquired hemophilia B blood at various TF concentrations. Factor VIIa at concentrations of 0 (♦), 10 (∗), and 50 nM (▵) was added to the hemophilia B blood. Panel A shows clotting times; panel B, maximum TAT levels; and panel C, maximum osteonectin levels.
TF titration in normal blood and “acquired” hemophilia B blood.
Clotting of CTI-inhibited (0.1 mg/mL) normal blood (●) and acquired hemophilia B blood at various TF concentrations. Factor VIIa at concentrations of 0 (♦), 10 (∗), and 50 nM (▵) was added to the hemophilia B blood. Panel A shows clotting times; panel B, maximum TAT levels; and panel C, maximum osteonectin levels.
In normal blood, maximum thrombin levels were independent of TF concentration at concentrations of this protein above 20 pM (Figure 4B; ●). In acquired hemophilia B blood, in either the absence or presence of additional factor VIIa at concentrations of 10 and 50 nM, increases in TF concentration cause raised the maximum thrombin levels at all concentrations of TF tested. In the absence of TF and exogenous factor VIIa (Figure 4B; ♦), 120 nM thrombin was generated after 40 minutes of reaction. In contrast, addition of 10pM TF produced thrombin levels of 210 nM, and at 100 pM TF, almost 500 nM thrombin was generated. Addition of 10 or 50 nM factor VIIa to “acquired” hemophilia B blood only slightly increased thrombin levels at all TF concentrations tested (0-100 pM).
Platelet activation in normal blood was complete after 11 to 28 minutes at all TF concentrations tested (Figure 4C; ●). In “acquired” hemophilia B blood in the absence of TF and exogenous factor VIIa (Figure 4C; ♦), about 25% of osteonectin was released after 40 minutes of reaction. Addition of 10 pM TF to the “acquired” hemophilia B blood drove platelet activation to completion. In the absence of TF and the presence of 10 or 50 nM exogenous factor VIIa (Figure 4C; ∗ and ▵, respectively), platelet activation in “acquired” hemophilia B blood was complete at the end of the experiment (37 minutes and 24 minutes, respectively).
Congenital hemophilia A blood.
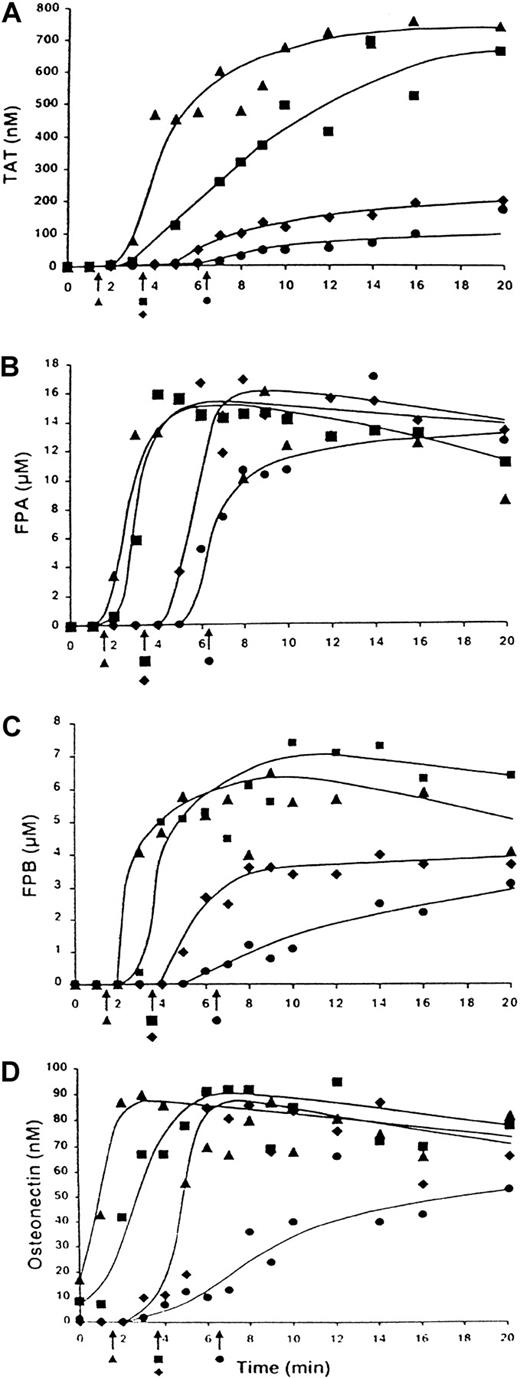
The influence of 10 nM factor VIIa on clotting of normal (contemporaneous control) and hemophilia A blood is shown in Figure5. In all experiments, clotting of normal and hemophilia A blood was initiated with 12.5 pM TF. In the absence of exogenous factor VIIa, normal blood clotted in 3.25 minutes (Figure 5; ▪). Addition of 10 nM factor VIIa decreased the clotting time to 1.9 minutes (Figure 5; ▴). Blood from the patient with hemophilia A (factor VIII < 1%) clotted in 6.4 minutes in the absence of exogenous factor VIIa (Figure 5; ●), and addition of 10 nM factor VIIa to this blood normalized the clotting time (3.3 minutes; Figure 5; ♦). No further decrease in clotting time was observed when factor VIIa concentration was increased to 50 nM (data not shown).
TF-initiated clotting of normal blood and congenital hemophilia A blood in the presence of factor VIIa.
Clotting of CTI-inhibited (0.1 mg/mL) normal blood initiated with 12.5 pM TF (▪) and addition of 10 nM factor VIIa (▴) and of hemophilia A blood with (♦) and without (●) addition of 10 nM factor VIIa. Panel A shows TAT generation over time; panel B, FPA release; panel C, FPB release; and panel D, osteonectin release. Arrows indicate clotting times.
TF-initiated clotting of normal blood and congenital hemophilia A blood in the presence of factor VIIa.
Clotting of CTI-inhibited (0.1 mg/mL) normal blood initiated with 12.5 pM TF (▪) and addition of 10 nM factor VIIa (▴) and of hemophilia A blood with (♦) and without (●) addition of 10 nM factor VIIa. Panel A shows TAT generation over time; panel B, FPA release; panel C, FPB release; and panel D, osteonectin release. Arrows indicate clotting times.
Thrombin generation in normal blood occurred at a maximum rate of 1.1 nM/second (Figure 5A; ▪). The maximum level of the TAT complex observed at the end of the experiment (20 minutes) was 660 nM. The concentration of thrombin at clotting was between 10 and 20 nM. Addition of 10 nM factor VIIa to normal blood (Figure 5A; ▴) increased both the maximum rate of generation and the level of thrombin (3.1 nM/second and 750 nM, respectively). Approximately 5 nM thrombin was observed at clotting. An increase in factor VIIa concentration to 50 nM had no further effect on thrombin generation rate, with an increase in maximum thrombin level (to almost 1.0 μM). The thrombin concentration at clotting was about 5 nM.
Thrombin generation in hemophilia A blood (Figure 5A; ●) occurred after an extended initiation phase (∼ 6.5 minutes) and at a maximum of 0.27 nM/second rate during the propagation phase. The maximum thrombin level was 20% that of the normal value (150 nM). The concentration of thrombin at clotting was about 10 nM. Addition of 10 nM factor VIIa to hemophilia A blood (Figure 5A; ♦) in vitro slightly decreased the duration of the initiation phase, increased the maximum rate of thrombin generation (to 0.72 nM/second), and slightly increased the maximum thrombin level (to 200 nM). The thrombin level at clotting was less than 5 nM. Factor VIIa at a concentration of 50 nM did not increase the maximum rate of thrombin generation further (0.58 nM/second) but did increase the maximum thrombin level 360 nM. The thrombin concentration at clotting was less than 3 nM.
FPA release in normal blood (Figure 5B; ▪) was observed before clot formation, with about 70% (11 μM) of the FPA released at clotting. Addition of 10 nM factor VIIa to normal blood (Figure 5B; ▴) had almost no effect on FPA release. However, clotting occurred with less than 1 μM FPA released. In the hemophilia A blood (without exogenous factor VIIa; Figure 5B; ●), about 50% (7 μM) of the FPA was released at clotting. Addition of 10 nM factor VIIa to this blood (Figure 5B; ♦) slightly accelerated FPA release; however, no FPA was detected at clotting. FPA release occurred at a similar maximum rate (5-8 μM/minute) and was complete in all experiments.
Release of FPB in normal blood (Figure 5C; ▪) started just before clot formation, with 1.8 μM (∼ 25% of all FPB released) released at clotting (3.25 minutes). Addition of 10 nM factor VIIa to normal blood (Figure 5C; ▴) slightly shortened the inception of FPB release; however, no FPB was detected at clotting. In normal blood, FPB release occurred at a maximum rate of 4.1 μM/minute and the maximum level of this peptide was 7.4 μM. Addition of 10 nM factor VIIa to this blood had almost no effect on these parameters of FPB release. In hemophilia A blood without exogenous factor VIIa (Figure 5C; ●), about 25% (0.8 μM) of the FPB was released at clotting. The rate of FPB release was less than 10% that observed in normal blood (0.35 vs 4.1 μM/minute). The maximum level of FPB (3.0 μM) was about 40% that in normal blood (7.4 μM). Addition of 10 nM factor VIIa to hemophilia A blood (Figure5C; ♦) increased both the rate of FPB release (to 1.3 μM/minute) and the final concentration of this peptide (to 3.7 μM) but did not restore these to normal levels. Thus, addition of factor VIIa to normal or hemophilia A blood affected clotting time more than it influenced release of FPA or FPB.
Analyses of the platelet-activation data based on osteonectin release (Figure 5D) showed that at the clotting of normal blood (Figure 5D; ▪), platelet activation was almost complete. Addition of 10 nM factor VIIa to normal blood (Figure 5D; ▴) caused a notable acceleration in platelet activation, which was comparable to the decrease in clotting time, ie, platelet activation was almost complete at this point. In hemophilia A blood without factor VIIa (Figure 5D; ●), clotting was observed when less than 20% (16 nM) of platelet osteonectin was released. The maximum rate of osteonectin release in hemophilia A blood was 20% that in normal blood (0.1 nM/second vs 0.5 nM/second). Addition of 10 nM factor VIIa to hemophilia A blood (Figure 5D; ♦) corrected the maximum rate of platelet activation.
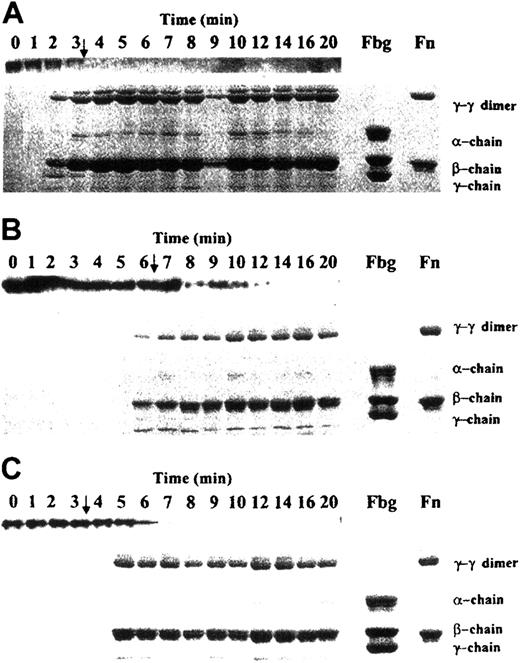
Analyses of soluble fibrinogen and solubilized fibrin clots (Figure6) showed that at the clotting of normal blood (Figure 6A; arrow), fibrinogen was almost depleted from the solution (Figure 6A; upper lane) and a solid cross-linked clot was formed (lower panel). Addition of 10 nM factor VIIa to normal blood had almost no effect on the time course of fibrinogen depletion and solid-clot formation (data not shown). In hemophilia A blood (Figure6B), fibrinogen depletion from the solution was delayed and residual amounts of this protein remained until the 12-minute time point (ie, for approximately 6 minutes after solid clots were observed), whereas in normal blood, no soluble fibrinogen was detected within 1 minute after clotting (Figure 6A). Addition of 10 nM factor VIIa to hemophilia A blood (Figure 6C), although it corrected clotting time to normal (arrows in Figure 6A and Figure 6C), did not restore the time course for fibrinogen depletion or solid-clot formation (no solid clots were formed for > 1 minute after clotting) observed in normal blood. Fibrinogen was present in the solution phase for about 4 minutes after clotting. Thus, the pattern of soluble fibrinogen depletion and solid-clot formation observed in hemophilia A blood with factor VIIa addition resembled that in hemophilia A blood without factor VIIa addition and proceeded more slowly than in normal blood.
Depletion of fibrinogen and solid clot formation during TF-initiated clotting of whole blood.
Samples for fibrinogen and solid-clot analyses were obtained from experiments shown in Figure 5. Upper lanes in each panel represent soluble fibrinogen; lower parts represent solubilized solid clots. Panel A shows normal blood without factor VIIa addition; panel B, congenital hemophilia A blood without factor VIIa addition; and panel, C congenital hemophilia A blood with 10 nM factor VIIa addition. Arrows indicate clotting times. Fbg indicates fibrinogen; Fn, fibrin.
Depletion of fibrinogen and solid clot formation during TF-initiated clotting of whole blood.
Samples for fibrinogen and solid-clot analyses were obtained from experiments shown in Figure 5. Upper lanes in each panel represent soluble fibrinogen; lower parts represent solubilized solid clots. Panel A shows normal blood without factor VIIa addition; panel B, congenital hemophilia A blood without factor VIIa addition; and panel, C congenital hemophilia A blood with 10 nM factor VIIa addition. Arrows indicate clotting times. Fbg indicates fibrinogen; Fn, fibrin.
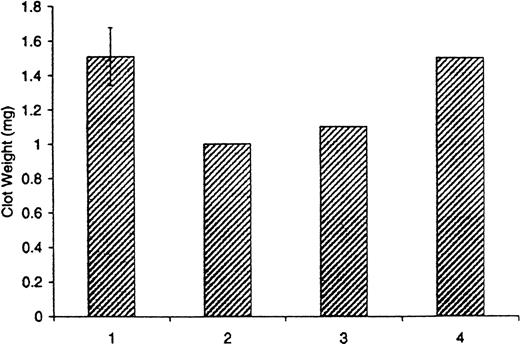
The average clot weight derived from 11 experiments conducted in normal blood in which clotting was initiated by TF was 1.5 ± 0.2 mg (Figure7; bar 1). Clots formed in hemophilia A blood (2 experiments with the same patient's blood done 20 months apart) at the same TF concentration were smaller and weighed an average of 1.0 mg (Figure 7; bar 2). Addition of factor VIIa at concentrations of 10 and 50 nM to hemophilia A blood in which clotting was initiated by TF had little effect on clot weights (Figure 7; bar 3). They were similar to those observed in hemophilia A blood without addition of factor VIIa (compare bars 2 and 3 in Figure 7). On the other hand, addition of 0.7 nM recombinant factor VIII (mean physiologic concentration) to the same hemophilia A blood (contemporaneous control) increased the clot weight to normal values (Figure 7; bar 4).
Clot weights in normal blood and congenital hemophilia A blood initiated by TF.
Clots were obtained from blood to which 12.5 pM TF was added, according to the procedure described by Brummel et al.47 Bar 1 represents a composite of 11 experiments in normal blood; bar 2, a composite of 2 experiments in congenital hemophilia A blood; bar 3, a composite of 2 experiments contemporaneous to those in bar 2 when 10 and 50 nM factor VIIa was added to congenital hemophilia A blood; and bar 4, a contemporaneous control when 0.7 nM factor VIII was added to congenital hemophilia A blood.
Clot weights in normal blood and congenital hemophilia A blood initiated by TF.
Clots were obtained from blood to which 12.5 pM TF was added, according to the procedure described by Brummel et al.47 Bar 1 represents a composite of 11 experiments in normal blood; bar 2, a composite of 2 experiments in congenital hemophilia A blood; bar 3, a composite of 2 experiments contemporaneous to those in bar 2 when 10 and 50 nM factor VIIa was added to congenital hemophilia A blood; and bar 4, a contemporaneous control when 0.7 nM factor VIII was added to congenital hemophilia A blood.
Discussion
A safe and efficacious hemostatic agent must act only at the site of a vascular lesion. The success of in vivo treatment of bleeding episodes with recombinant factor VIIa, particularly episodes related to hemophilia A and hemophilia B, indicates that this enzyme meets the above requirement. The mechanism by which factor VIIa establishes normal hemostasis in factor VIII and factor IX deficient blood is unknown. The findings of this study indicate that in both normal blood and the synthetic model representing normal blood, factor VIIa alone, in the absence of TF, can initiate thrombin generation. In hemophilia A blood and “acquired” hemophilia B blood, however, pharmacologic concentrations of factor VIIa could not restore normal thrombin generation or formation of solid cross-linked clots.
In the normal synthetic system, the concentrations of factor VIIa required to provide thrombin generation profiles comparable to those observed in TF-initiated reactions were approximately 104-fold higher. In whole blood (with approximately 0.1 nM factor VIIa), similar clotting times were observed when coagulation was initiated by 10 pM TF or by 60 nM factor VIIa. In the synthetic model in the presence of 2 μM phospholipids or 2 × 108/mL platelets, factor VIIa concentrations had to be increased above 120 nM to show the efficiency provided by 25 pM factor VIIa–TF complex. These data suggest that the factor VIIa–TF complex is approximately 5 to 6 × 103-fold more efficient than factor VIIa as an initiator of the blood-coagulation cascade. This ratio is based on an assumption that all TF added to the reaction is in a complex with factor VIIa. However, the decrease in clotting time of normal blood caused by addition of factor VIIa in the presence of TF suggests that a fraction of TF is free and that 5-6 × 103-fold is an underestimate. Steady-state data derived by Komiyama et al51 showed that TF increases the efficiency of factor VIIa in the proteolyses of its natural substrates factor IX and factor X by approximately 104-fold. This increase in efficiency toward individual substrates is similar to that observed in the current study for the entire coagulation cascade.
Addition of excess factor VIIa to whole blood in the presence of limiting TF affected clotting time until the saturation of TF was achieved. Further increases in factor VIIa had no additional effect on clotting time. The concentration of factor VIIa required to produce the shortest clotting time was inversely dependent on the TF concentration. For example, at 1 pM TF, the shortest clotting time was at 20 nM factor VIIa, whereas at 10 pM TF, the shortest clotting time was at 10 nM factor VIIa. These data suggest that in the presence of TF, the role of factor VIIa is kinetic saturation of TF. Additional excesses of factor VIIa had limited (if any) role in the blood-coagulation process. The superior role of factor VIIa in complex compared with that of factor VIIa acting alone in thrombin generation and clot formation was also observed in congenital hemophilia A blood and “acquired” hemophilia B blood.
During the past 2 decades, recombinant factor VIIa has been tested in the treatment of a variety of bleeding disorders.26-33,52-59 It has primarily been used to treat hemophilia patients with acquired inhibitors.28-32 The in vivo concentrations of factor VIIa for this treatment range from 3 to 20 nM29,60-63 (based on 50 000 units/mg specific activity of recombinant factor VIIa63). The mechanism by which factor VIIa at these concentrations maintains normal hemostasis is unknown. In reports from one laboratory, it was postulated that factor VIIa alone, in the absence of TF, can generate factor IXa and factor Xa on the surface of activated platelets at concentrations sufficient to provide normal hemostasis in hemophilia.34 35 The data of this study, however, indicate that factor VIIa at pharmacologic concentrations is a potent hemostatic agent only in the presence of TF.
Addition of factor VIIa to the synthetic blood-coagulation model in the absence of factor VIII slightly increased thrombin generation but never raised it to levels observed with factor VIII. The initiation phase remained prolonged, and thrombin generation during the propagation phase was significantly suppressed. Increasing the concentration of factor VIIa from 10 to 40 nM did not enhance thrombin generation. These observations were valid for experiments conducted with phospholipids or platelets. The results suggest that the limited positive effect caused by supraphysiologic factor VIIa concentrations was due to overcoming factor VII competition and the kinetic saturation of TF in the reaction.64 The efficiency of factor VIIa observed in the studies in Roberts' laboratory34 35 reflects differences in either experimental design or protein preparations. Even negligible amounts of enzymes in the synthetic reaction mixtures containing platelets may cause a “spontaneous” generation of thrombin in the absence of both factor VIIa and TF.
Similarly, in the hemophilia A blood and “acquired” hemophilia B blood, addition of factor VIIa had little effect on thrombin generation, ie, the duration of the initiation phase and the rate of thrombin generation during the propagation phase. The maximum levels of thrombin were only marginally affected by this addition. An increase in factor VIIa concentration from 10 to 50 nM had almost no additional effect on the parameters of thrombin generation.
Pharmacologic concentrations of factor VIIa had a negligible effect on clot formation in hemophilia A blood. Addition of factor VIIa slightly decreased the time for FPA and FPB release, slightly increased the rate of formation and final levels of FPB, and slightly accelerated solid-clot formation and cross-linking. However, none of these variables was corrected to levels observed in blood from healthy donors. Additionally, the smaller clot weights observed in hemophilia blood were not increased by addition of factor VIIa. The only process that was considerably altered by addition of factor VIIa to normal or hemophilia blood was platelet activation. Addition of 10 nM factor VIIa led to more rapid platelet activation in hemophilia A blood and acquired hemophilia B blood, producing rates observed in normal blood.
The mechanism by which factor VIIa addition accelerates platelet activation is unknown. One of the possibilities is that high concentrations of factor VIIa can activate platelets directly. More likely, addition of factor VIIa slightly accelerates thrombin generation due to the kinetic saturation of TF and, as a result, increases thrombin during the initiation phase. The additional amount of thrombin cannot alter the entire process of coagulation and clot formation substantially but can accelerate platelet activation. This assumption is supported by our recent study indicating that subnanomolar concentrations of thrombin are required for relatively rapid platelet activation in whole blood.65
We thank Joshua D. Dee and Shyla L. Tessmer for their technical assistance; Dr Shu Len Liu and Dr Roger Lundblad for providing TF and factor VIII; Dr Kirk Johnson for providing TFPI; and Dr Thomas Edgington for providing anti-TF antibody.
Supported by grants R01 HL34575, P01 HL46703, and PHS T32 HL07594 from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kenneth G. Mann, Dept of Biochemistry, University of Vermont, Given Bldg, Room C401, 89 Beaumont Ave, Burlington, VT 05405-0068; e-mail: kmann@zoo.uvm.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal