This report describes a new low-frequency alloantigen, Oea, responsible for a case of neonatal alloimmune thrombocytopenia (NAIT). In a population study none of 600 unrelated blood donors was an Oea carrier. By immunochemical studies the Oea antigen could be assigned to platelet glycoprotein (GP) IIIa. Sequencing of GPIIIa complementary DNA from an Oea (+) individual showed deletion of a lysine residue at position 611 (ΔLys611). Analysis of 20 Oea(−) and 3 Oea (+) individuals showed that the ΔLys611 form of GPIIIa was related to the phenotype. Anti-Oea reacted with the ΔLys611, but not with the wild-type isoforms on stable transfectants expressing GPIIIa, indicating that ΔLys611 directly induces the expression of Oea epitopes. Under nonreducing conditions the Pro33ΔLys611 variant migrated with a slightly decreased molecular weight compared to the Pro33Lys611 isoform suggesting that ΔLys611 has an influence on the disulfide bonds of GPIIIa. The Pro33ΔLys611 GPIIIa could undergo conformational changes and bind to fibrinogen in a similar manner as the Pro33Lys611 isoform. No difference was found in the tyrosine phosphorylation of pp125FAK, suggesting that ΔLys611 has no effect on integrin function. In contrast to all other low-frequency antigens, the ΔLys611 isoform was associated with the HPA-1b, but not with the high frequency HPA-1a allele. Comparison with GPIIIa DNA from nonhuman primates indicated that the HPA-1a allele represents the ancestral form of GPIIIa. It can be assumed that the Oea form did arise as a result of a mutational event from an already mutated GPIIIa allele.
Introduction
The platelet membrane glycoprotein (GP) IIb-IIIa complex, also referred to as αIIbβ3, belongs to the integrin family of heterodimeric adhesion receptors implicated in cell-cell and cell-matrix interactions.1 As receptor for ligands containing the Arg-Gly-Asp sequence such as fibrinogen and von Willebrand factor, GPIIb-IIIa plays an essential role in hemostasis by mediating platelet aggregation and platelet spreading on vascular matrices.2 GPIIb-IIIa primarily functions as a responsive adhesion receptor serving as a bidirectional conduit for inside-out and outside-in signaling processes across the plasma membrane. Whereas intracellular signals modulate the ligand-binding function of GPIIb-IIIa (inside-out), signals generated by ligation and clustering of GPIIb-IIIa regulate the extent of platelet aggregation and spreading (outside-in).3
The mature GPIIIa subunit is a disulfide bond-rich single polypeptide consisting of 762 amino acids encompassing a large extracellular domain, a single transmembrane domain, and a cytoplasmic tail. The extracellular domain carries an I-domain–like ligand-binding region (residues 110-294) and a cysteine-rich repeat region (423-622) that contains 31 of 56 cysteine residues. All cysteine residues are located in highly conserved regions of the molecule and play an important role in preserving the 3-dimensional structure, because pertubation or absence of one or more of these residues could affect stability or ligand-binding function.4,5 Recently, Yan and Smith demonstrated that a selected group of the cysteines located within the extracellular cysteine-rich domain remains unpaired. The redox status of these cysteine residues can directly influence the activation state of GPIIb-IIIa.6
Several mutations of GPIIIa receptor lead to the loss of either expression or function of the GPIIb-IIIa and are therefore responsible for Glanzmann thrombasthenia, an inherited bleeding disorder characterized by the failure of platelet aggregation.7
Glycoprotein IIIa represents one of the most polymorphic molecules on the platelet surface. So far, 7 human platelet alloantigen systems (HPA-1, -4, -6, -7, -8, -10, and -11) could be characterized on this receptor. The HPA systems are induced by single point mutations of the high frequency GPIIIa Leu33 isoform (HPA-1a; PlA1, Zwa), leading to the generation of 6 less frequently occurring GPIIIa alleles.8
Glycoprotein IIIa complementary DNA (cDNA) of human and other species showed a remarkably high degree of homology. Alignment analysis showed that the deduced amino acid sequence of canine and mouse corresponds to the high-frequency human GPIIIa isoform.9
Platelet alloantigens can elicit the production of alloantibodies and lead to neonatal alloimmune thrombocytopenia (NAIT), posttransfusion purpura, and platelet transfusion refractoriness.10 NAIT is induced by maternal immunization against a fetal HPA and subsequent transplacental transfer of the maternal antibody into the fetal circulation. In the white population about 75% of the NAIT cases are caused by immunization against HPA-1a (PlA1, Zwa) carried on the GPIIIa Leu33 isoform.11
In recent years, several groups reported that polymorphism of GPIIIa is not only immunologically relevant but may also contribute as a risk factor in the development of coronary heart disease.12,13Individuals carrying the HPA-1b allele seem to have increased risk for adverse cardiovascular events. At present, however, there is only limited evidence that integrin signaling may be affected by GPIIb-IIIa polymorphisms.14 15
In this study, we describe the biochemical, molecular, and functional characterization of a new immunogenic variant of GPIIIa, which is involved in NAIT.
Patients, materials, and methods
Case report
The 27-year-old mother (Ms Oe) had a healthy child after her first pregnancy. The second pregnancy ended with an abortion. After her third uneventful pregnancy she gave birth to a male infant (3540 g, Apgar score 1/5/10). The neonate exhibited cutaneous signs of a thrombocytopenic hemorrhagic diathesis: multiple hematoma and petechiae on head, back, and arms. Six hours postpartum the platelet count was 36 000/μL and further declined to a nadir of 29 000/μL a few hours later. Initially, septicemia was suspected and antibiotic therapy was initiated, but no other signs of a severe infection were detectable. During treatment with intravenous IgG over 2 days the platelet count increased to 63 000/μL. For 10 further days the patient received corticosteroids. Two weeks later the platelet count had increased to normal values and the newborn could be dismissed in good health.
Blood samples
Blood samples from the mother, the father, and the child were referred to our laboratory shortly after delivery because of suspected NAIT. Platelets were isolated from EDTA-anticoagulated blood by differential centrifugation and stored at 4°C in isotonic saline containing 0.1% NaN3. For the characterization of platelet alloantibodies, platelets were selected from a large number of donors with known HPAs and ABO blood group antigens.
All samples were obtained with informed consent and with the approval of the National Blood Service ethics review board.
Phenotyping
Phenotyping of HPAs was performed by the monoclonal antibody–specific immobilization of platelet alloantigens (MAIPA assay) as previously described.16
Monoclonal antibodies and peptides
Monoclonal antibodies (mAbs) Gi5 and Gi9 specific for GPIIb-IIIa and GPIa-IIa, respectively, were produced in our laboratory.17 The mAb FMC25 directed against GPIX subunit of GPIb-IX-V complex was provided by Dr Heddy Zola (Adelaide, Australia).18 The mAb AP3 specific for GPIIIa and mAb D3 against a ligand-induced binding site (LIBS) on GPIIIa were kindly provided by Dr Peter Newman (Milwaukee, WI) and Dr Lisa Jennings (Memphis, TN), respectively.19 20 High-performance liquid chromatography–purified RGDW and RGEW peptides were purchased from Bachem (Heidelberg, Germany). The mAbs 77 and PY20 directed against pp125FAK and phosphotyrosine, respectively, as well as mAb PAC-1 against activated GPIIb-IIIa complex were purchased from Becton Dickinson (Heidelberg, Germany).
Immunoblot analysis
Platelets were isolated from acid-citrate-dextrose anticoagulated blood by differential centrifugation. Platelets (4 × 106) were lysed in 2% sodium dodecyl sulfate (SDS), 30 mM N-ethylmaleinimide, 100 mM phenylmethylsulfonyl fluoride (PMSF) for 5 minutes at 100°C. The lysate was separated in a 7.5% SDS–polyacrylamide gel electrophoresis (SDS-PAGE) and blotted on a nitrocellulose membrane. Then, 200 μL eluate prepared from either maternal (anti-Oea) or control sera (anti–HPA-1a, AB serum) was incubated with the membrane for 90 minutes at room temperature. An alkaline phosphatase–labeled rabbit anti–human IgG (Dako, Hamburg, Germany) was added and bound antibodies were visualized with 5-bromo-4-chloro-3-indolyl phosphate (BCIP) substrate solution.
Immunoprecipitation
Platelets and Chinese hamster ovary (CHO) stable transfectants were surface labeled with 5 mmol/L NHS-LC biotin (Paesel, Frankfurt, Germany) as previously described.21 Then, 5 × 108 labeled platelets in 500 μL phosphate-buffered saline (PBS) were digested with 500 μL chymotrypsin (1 mg/mL, Sigma, Taufkirchen, Germany) at 37°C overnight and the reaction was terminated by adding 100 μL PMSF (5 mg/mL). After washings labeled platelets were lysed in 1 mL solubilization puffer (25 mM Tris, 10 mM EDTA, 100 mM NaCl, 1% Triton X-100, 2 mM PMSF, 1 mM leupeptin, and 2 mM N-ethylmaleimide) for immunoprecipitation.
Isolation of platelet messenger RNA and leukocyte DNA
Total platelet RNA was isolated from 20 mL EDTA anticoagulated blood as previously described.21 Genomic DNA was obtained from leukocyte 3 mL EDTA anticoagulated blood using QIAquick-Kit (Qiagen, Hilden, Germany).
Analysis of GPIIIa-specific cDNA
Platelet RNA (100 μL) was reverse transcribed using 10 μM oligodT (Boehringer Mannheim, Mannheim, Germany). To amplify the entire coding region of GPIIIa by polymerase chain reaction (PCR), 4 overlapping sets of primers (nucleotides 56-698, 633-1341, 1116-1799, 1666-2415) were constructed based on the published cDNA.22For cDNA amplification of the fourth region forward primer no. 1 (1503-CCAGTGTGAGTGCTCAGAGGA-1524), reverse primer no. 2 (2415-GGCTGATAATGATCTGAGGATGACTG-2389), and nested primer no. 3 (1666-GTGACGACTTCTCCTGTGTCCGCTACAAGG-1695) were used. Then 5 μL cDNA was diluted with 10 times PCR buffer, 0.25 μM of primer no. 1 and no. 2, 175 μM of each dNTP, and 2.5 U TaqGold polymerase (Applied Biosystems, Weiterstadt, Germany) in a total volume of 50 μL. Amplification was performed on DNA thermal cycler 480 (Applied Biosystems) for 15 cycles. Each cycle consisted of denaturation at 96°C for 90 seconds, annealing at 52°C for 60 seconds, and extension at 72°C for 120 seconds. In the final cycle, the samples were kept at 72°C for 10 minutes and then chilled for 4°C. An aliquot of 1 μL diluted PCR products (1:100; 1:1000) was reamplified using primer no. 1 and no. 3 for 30 cycles under the same conditions with annealing temperature of 58°C. PCR products were analyzed on 1.6% SeaKem GTG agarose gel (Biozyme, Hameln, Germany). After purification by QIAquick Kit, PCR products were subcloned into theEcoRV cloning site of pGEM-5Zf (Promega Biotech, Madison, WI). Plasmid DNA from 16 positive clones was sequenced using Taq-FS Dye Terminator Cycle Sequencing Kit and were analyzed on ABI PRISM Genetic Analyzer 310 (Applied Biosystems).
Analysis of GPIIIa-specific DNA
To analyze the entire exon 10 of GPIIIa,23 5 μg genomic DNA was amplified using intronic sense primer no. 4 (5′-cagcgggtccaccttcct-3′) and antisense primer no. 5 (5′-cctgcctcccggctctct-3′) for 30 cycles. Each cycle consisted of denaturation at 95°C for 4 minutes, annealing at 61°C for 30 seconds, and extension at 72°C for 60 seconds. After purification by QIAquick Kit, 60 ng amplified DNA was directly sequenced as above.
Genotyping of HPA-1 and Leu40Arg dimorphisms
Genotyping of Oea alloantigen
DNA typing of Oea alloantigen was performed by sequence-specific PCR (PCR-SSP). In brief, 5 μg genomic DNA was added to 50 μL reaction mixture containing 10 mM Tris, 50 mM KCl, 2.75 mM MgCl2, 0.2 mM of each dNTP, 0.4 μM each of sense (1753-ACTGGACCGGCTACTACTGCAA-1774) and sequence-specific antisense primer (ctccagactccacactcacTTC/A-1931), 0.2 μM each of human growth factor (HGH) I (5′-CAGTGCCTTCCCAACCATTCCCTTA-3′) and HGH II (5′-ATCCACTCACGGATTTCTGTTGT GTTTC-3′), and 2.5 UTaqGold polymerase. After initial denaturation at 96°C for 10 minutes, amplification was performed in a DNA thermocycler (Hybaid, Heidelberg, Germany) for 35 cycles (denaturation at 94°C for 50 seconds, annealing 55°C for 30 seconds, and extension 72°C for 15 seconds). The PCR products were analyzed on 2.0% agarose gel electrophoresis using molecular marker V (Boehringer Mannheim) as DNA standard.
Nucleotide sequence analysis of nonhuman primates GPIIIa
DNA from nonhuman primates (prosimian, new world monkeys, old world monkeys, and Hominoidea) were kindly provided by Dr C. Roos (Institute for Genetics, University of Munich, Germany). For the genotyping of GPIIIa gene polymorphism DNA was amplified and analyzed by direct nucleotide sequencing as described above.
Generation of allele-specific GPIIIa constructs
Full-length human wild-type GPIIIa and GPIIb cDNAs in the mammalian expression vector pcDNA3/Neo, which encode Leu33GPIIIa isoform and Ser843 GPIIb isoform, respectively, were kindly provided by Dr P. Newman (Blood Research Institute, Milwaukee, WI). Allele-specific recombinant GPIIIa isoforms Pro33, Leu33ΔLys611, and Pro33ΔLys611 were produced from the wild-type GPIIIa cDNA by site-directed mutagenesis using Quick-Change Mutagenesis Kit (Strategene, Heidelberg, Germany).
For the construction of the expression vector encoding for Pro33 isoform PCR was performed using one mismatched (underlined) forward primer (5′-CTGATGAGGCCCTGCCTCCGGGCTCACCTCGCTGTG-3′) and reverse primer (5′-CACAGCGAGGTGAGCCCGGAGGCAGGGCCTCATCAG-3′) from base 178-213 of GPIIIa cDNA as previously described.21 Both Leu33 and Pro33 GPIIIa constructs were then deleted for 3 bases (nucleotides 1929-1931) by site-directed mutagenesis using forward primer (5′-CCAAGATGCCTG CACCTTTAAAGAATGTGTGGAGTG-3′) and reverse primer (5′-CACTCCAC ACATTCTTTAAAGGTGCAGGCATCTGG-3′) encompassing nucleotides 1910-1948 as described above. Full-length GPIIb in pcDNA3/Neo plasmid was digested with XbaI and EcoRI endonucleases (Biolabs, Frankfurt, Germany) and was shuttled into the pcDNA3.1/Zeo vector, which had been digested with the same enzymes.
After subcloning in Escherichia colibacteria, all constructs were validated by nucleotide sequence analysis for subsequent transfection studies.
Stable transfection of allele-specific constructs in CHO cells
The CHO cells were transfected with allele-specific GPIIIa and GPIIb expression vectors by the use of the reagent Lipofectin (Gibco, Karlsruhe, Germany) as previously described.21 In brief, 3 μg of each plasmid was mixed with 25 μL lipofectin in 2 mL OptiMEM Medium (Gibco) and then added to a subconfluent 10-cm plate of CHO cells (8 × 105 cells) for 12 hours. Medium (9 mL) was then added and the incubation was continued for 48 hours. After splitting, transfectants were selected with Geneticin (800 μg/mL, Gibco) and Zeocin (500 μg/mL, Invitrogen, Groningen, The Netherlands) for approximately 2 weeks. After subcloning, surface GPIIb-IIIa was analyzed by flow cytometry using mAbs AP3 and Gi5 specific for GPIIb and GPIIb-IIIa complex, respectively. To obtain a homogenous cell population, stable cell lines were cloned 3 times and different clones (3 cell lines for each transfection) were maintained in the same selection media.
Flow cytometry analysis
Stable transfectants were analyzed by flow cytometry as previously described.21 Cell suspensions of 200 μL (8 × 105 cells) were incubated with 20 μL mAb Gi5 (20 μg/mL), washed, and labeled with 40 μL fluorescein isothiocyanate (FITC)–conjugated rabbit anti–mouse IgG (1:80 dilution; Dako). Cell were resuspended in 500 μL PBS containing 0.5% bovine serum albumin (BSA) and 0.1% NaN3 and were analyzed by flow cytometry (FACS Calibur, Becton Dickinson). Binding of the LIBS mAb D3 in the absence of RGDW or RGEW peptide was assessed as described.26 Cells were incubated with 1 mM peptides for 5 minutes at room temperature prior to labeling with 20 μL mAb D3 (0.02 mg/mL).
PAC-1 binding was analyzed as described by Lyman et al.27Stable transfectant (2 × 107) in 1 mL Tyrode buffer (137 mM NaCl, 2.8 mM KCl, 12 mM NaHCO3, 10 mM Hepes, pH 7.4) was treated with 10 mM dithiothreitol (DTT) or buffer for 20 minutes at room temperature. Cells were then washed once and resuspended in 1 mL Tyrode buffer supplemented with 3.5 mg/mL BSA (TB). Aliquots of 100 μL untreated and DTT-treated transfectants in TB were stained with 20 μL FITC-labeled mAb PAC-1 (1 μg/mL) in the presence of 10 μL 10 mM MgCl2 and 1 mM CaCl2 for 30 minutes at room temperature. Cells were washed once and resuspended in 500 μL TB containing MgCl2 and CaCl2 for FACS analysis.
Cell adhesion assay
Adhesion of transfected CHO cells on fibrinogen-coated wells was performed as described by Wang and Newman.26Microtiter wells were coated with 100 μL PBS containing different concentrations of fibrinogen (Calbiochem, Schwalbach, Germany), 10 μg/mL mAb Gi5, or 1% BSA at 4°C overnight and blocked with 1% BSA in PBS at room temperature before use. Stable transfectants were labeled with 2 μM calcein-am (Mobitec, Göttingen, Germany) at 37°C for 30 minutes, washed, and resuspended in serum-free media. An aliquot of 100 μL cell suspension (2 × 105 cells) was added to each well and cells were allowed to adhere for 1 hour at 37°C. The wells were then washed 3 times with media containing 1% BSA. Finally, 200 μL wash media was added to each well and the plates were read in a microtiter plate fluorescence reader (Spectrafluor Plus, Tecan, Crailsheim, Germany) at excitation and emission wavelengths of 485 and 515 nm, respectively.
Tyrosine phosphorylation of pp125FAK
Tissue culture plates (100 mm) were coated overnight at 4°C with either fibrinogen (Calbiochem) or BSA (10 mg/mL; Serva, Heidelberg, Germany) and blocked with 1% BSA for 2 hours at room temperature. Transfectants in serum-free media were added and incubated at 37°C for 60 minutes. Adherent cells were lysed in RIPA buffer containing 1% Triton X-100, 150 mM NaCl, 10 mM Tris, 1 mM EDTA, 1 mM Na3VO4, 0.5% Nonidet P40, 1% sodium desoxycholate, 2 mM PMSF, and 10 μg/mL each aprotinin and leupeptin (Sigma). After centrifugation at 15 000g at 4°C for 30 minutes, protein concentration of the cell lysate was determined using the BCA protein assay (Perbio, Bonn, Germany). Proteins (500 μg) were incubated with 5 μL rabbit polyclonal anti-pp125FAK(Becton Dickinson) overnight and immunoprecipitated as previously described.21 Immunoprecipitates were transferred onto PVDF membrane and the tyrosine phosphorylation state was then detected with mAbs 77 (250 μg/mL) and PY20 (1 mg/mL) specific for pp125FAK and phosphotyrosine, respectively (dilutions 1:1000). Prior to incubation with anti-pp125FAK blots probed with antiphosphotyrosine were stripped for 30 minutes at 50°C in 62.5 mM Tris buffer, pH 6.7, containing 2% SDS and 100 mM β-mercaptoethanol. Labeled proteins were then visualized using peroxidase-labeled rabbit anti–mouse IgG (dilution 1:10 000; Dianova, Hamburg, Germany) and chemiluminescence substrate (ECL Plus; Amersham Pharmacia, Freiburg, Germany). The signals were scanned using Correl PhotoPaint software and the densitometric quantitation was performed using Kodax 1D Image. Phosphorylation of pp125FAK was determined from densitometric scans of pTyr mean intensity divided by pp125FAK mean intensity.
Results
Serologic identification and family studies
When maternal serum was tested in the MAIPA assay using mAb Gi5 specific for GPIIb/IIIa complex, a positive reaction was obtained with platelets from the father and grandfather, but not with autologous platelets and with platelets from unselected donors. In addition, the Oea serum did not react with platelets carrying known low-frequency antigens on the GPIIb or GPIIIa subunits. Analysis of maternal serum with mAb Gi9 and FMC25 against GPIa-IIa and GPIb-IX complex, respectively, yielded negative results (data not shown). In a population study, none of 600 unrelated blood donors was found to carry the Oea alloantigen (phenotype frequency < 0.0017).
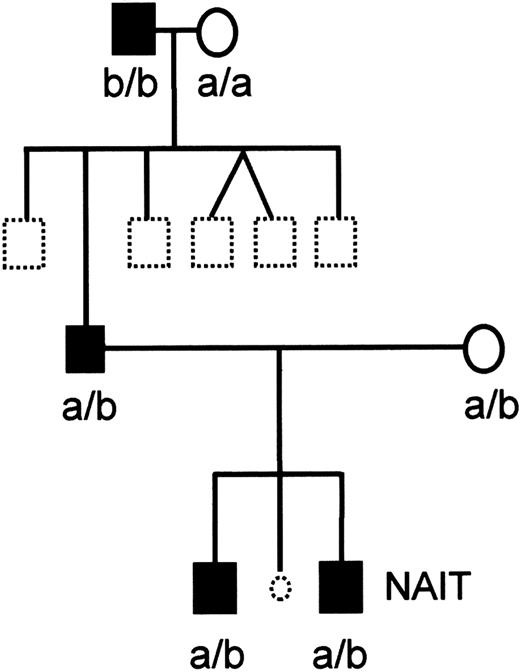
Figure 1 shows the pedigree of the family Oea. This segregation pattern demonstrated that the Oea antigen is inherited as a codominant allele. Interestingly, the Oea (+) grandfather was homozygous for the HPA-1b character.
Pedigree of the family Oe.
Black symbols represent Oea (+) and open symbols Oea (−) individuals. Dotted symbols represent individuals who were not typed. The child with NAIT is indicated. The HPA-1a and HPA-1b phenotypes are shown. Oe and HPA-1 alleles of the affected child and his brother were identified by genotyping.
Pedigree of the family Oe.
Black symbols represent Oea (+) and open symbols Oea (−) individuals. Dotted symbols represent individuals who were not typed. The child with NAIT is indicated. The HPA-1a and HPA-1b phenotypes are shown. Oe and HPA-1 alleles of the affected child and his brother were identified by genotyping.
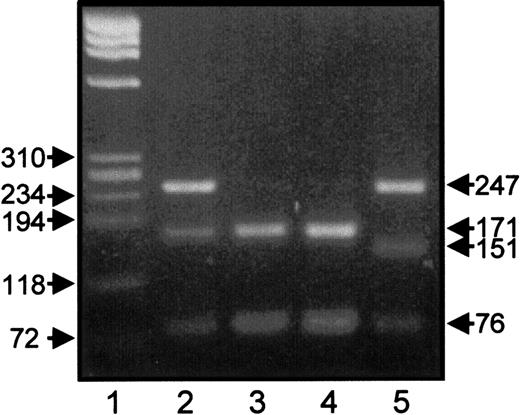
A rare Leu40Arg variant of the GPIIIa gene associated with the HPA-1b allelic isoform was found in the human gene pool.24 Because this mutation could theoretically be linked to the Oea alloantigen, we did HPA-1 genotyping of Oea (+) individuals by RFLP using MspI. As shown in Figure 2, HPA-1b individuals carrying the Arg40 (lane 5) could be clearly distinguished from HPA-1b/1b homozygous healthy blood donor (lane 4) and HPA1b, Oea (+) individuals (lanes 2 and 3) by the presence of a 151–base pair (bp) instead of 171-bp restriction fragment. This finding demonstrates that the Arg40 is not associated with the Oea alloantigen.
RFLP analysis of HPA-1 genotypes.
PCR products of GPIIIa gene of heterozygous HPA-1a/1b, Oea (+) father (lane 2), homozygous HPA-1b/b, Oea (+) grandfather (lane 3), homozygous HPA-1b/1b control individual (lane 4), and heterozygous HPA-1a/1b having Arg40 mutation (lane 5) were digested withMspI endonuclease and were analyzed on 2.2% agarose gel stained with ethidium bromide. Lane 1 represents DNA size standard (pBR322 HaeIII DNA fragments).
RFLP analysis of HPA-1 genotypes.
PCR products of GPIIIa gene of heterozygous HPA-1a/1b, Oea (+) father (lane 2), homozygous HPA-1b/b, Oea (+) grandfather (lane 3), homozygous HPA-1b/1b control individual (lane 4), and heterozygous HPA-1a/1b having Arg40 mutation (lane 5) were digested withMspI endonuclease and were analyzed on 2.2% agarose gel stained with ethidium bromide. Lane 1 represents DNA size standard (pBR322 HaeIII DNA fragments).
Immunochemical investigations
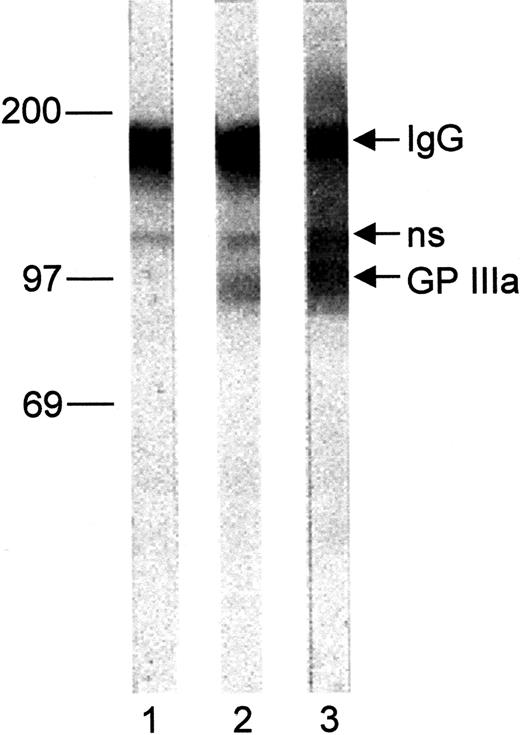
To further localize the Oea alloantigenic determinants, immunoblotting analysis was performed with platelet lysates derived from an Oea (+) individual (Figure3). Under nonreduced conditions, Oea alloantibodies recognize the GPIIIa subunit. However, the reactivity of this serum with GPIIIa was abolished after treatment with β-mercaptoethanol (data not shown).
Immunoblot of Oea (+) platelets.
Paternal platelet lysate was separated on 7.5% SDS-PAGE under nonreducing conditions and transferred onto nitrocellulose paper. After incubation with eluates from normal human serum (lane 1), anti-Oea (lane 2), and anti–HPA-1a (lane 3) bound IgG were detected with BCIP as substrate; ns indicates nonspecific binding.
Immunoblot of Oea (+) platelets.
Paternal platelet lysate was separated on 7.5% SDS-PAGE under nonreducing conditions and transferred onto nitrocellulose paper. After incubation with eluates from normal human serum (lane 1), anti-Oea (lane 2), and anti–HPA-1a (lane 3) bound IgG were detected with BCIP as substrate; ns indicates nonspecific binding.
In addition, we performed immunoprecipitation analysis with chymotrypsin-treated platelets. Labeled platelets of an Oea(+) individual were partially digested with chymotrypsin and were then subjected for immunoprecipitation. As shown in Figure4, anti–HPA-1a alloantibody recognized 72- and 66-kd proteolytic fragments of GPIIIa (lane 1), whereas Oea and HPA-4a alloantibodies (lanes 2 and 3) failed to react with both fragments. In contrast, undigested GPIIIa could be precipitated with all alloantibody specificities. These results indicated that the Oea alloantigenic determinant is located on the GPIIIa loop, which is cleaved after chymotrypsin treatment or resides on the remnant membrane- bound GPIIIa, and is destroyed by chymotrypsin treatment.
Immunoprecipitation analysis of chymotrypsin-treated platelets.
Paternal platelets (Oea [+], HPA-1a/1b, HPA-4a/4a) were surface labeled with biotin, digested with chymotrypsin, lysed, and precipitated with anti–HPA-1a (lane 1), anti-Oea (lane 2), anti-HPA-4a (lane 3), and normal human serum (lane 4). Immunoprecipitates were analyzed on 7.5% SDS-PAGE under nonreducing conditions, blotted, and visualized with chemiluminescence substrate.
Immunoprecipitation analysis of chymotrypsin-treated platelets.
Paternal platelets (Oea [+], HPA-1a/1b, HPA-4a/4a) were surface labeled with biotin, digested with chymotrypsin, lysed, and precipitated with anti–HPA-1a (lane 1), anti-Oea (lane 2), anti-HPA-4a (lane 3), and normal human serum (lane 4). Immunoprecipitates were analyzed on 7.5% SDS-PAGE under nonreducing conditions, blotted, and visualized with chemiluminescence substrate.
Genetic analysis
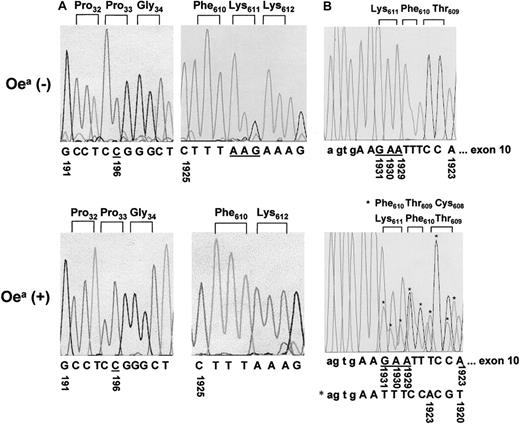
To elucidate the molecular basis underlying the Oeaalloantigens, we amplified the entire coding region of GPIIIa cDNA derived from an Oea (+) HPA-1b homozygous individual. PCR products were then subcloned and sequenced. Figure5A shows the sequence analysis of 2 GPIIIa regions (surrounding nucleotide 1925 and nucleotide 191). In the wild-type GPIIIa allele, the amino acids Phe610Lys611Lys612 are encoded by the nucleotides sequences TTT AAG AAA. In contrast, the mutant GPIIIa allele (5 of 16 clones) shows a deletion of AAG triplet encoding Lys611. Furthermore, both Oea (+) and (−) HPA-1b homozygous individuals carry the nucleotide C at position 196, which encodes Pro33 GPIIIa isoform and is responsible for the formation of the HPA-1b epitopes. These results are in accord with our serologic findings showing that Oea alloantigen is inherited with the HPA-1b allele (Figure 1).
Nucleotide sequence analyses.
(A) Nucleotide sequence analysis of amplified GPIIIa cDNA derived from an Oea (−) individual (HPA-1b/1b) and the Oea (+) (HPA-1b/1b) grandfather. PCR products encompassing bases 56-698 (left panel) and bases 1666-2415 (right panel) were subcloned into the plasmid vector pGEM-5Zf and sequenced. The missing of the triplet AAG (underlined) in Oea (+) individual results in ΔLys611. Both individuals carry the Pro33 GPIIIa form, which corresponds to their HPA-1b phenotypes. (B) Nucleotide sequence analysis of amplified GPIIIa DNA derived from Oea (+) and Oea (−) individuals. PCR products encompassing the entire exon 10 were sequenced directly. The mutated GPIIIa allele of Oea (+) individual is shown by the asterisks (top panel). The deletion of the triplet AAG (underlined) resulting in a shift of 3 bases was observed in Oea (+), but not in Oea (−) individual (bottom panel).
Nucleotide sequence analyses.
(A) Nucleotide sequence analysis of amplified GPIIIa cDNA derived from an Oea (−) individual (HPA-1b/1b) and the Oea (+) (HPA-1b/1b) grandfather. PCR products encompassing bases 56-698 (left panel) and bases 1666-2415 (right panel) were subcloned into the plasmid vector pGEM-5Zf and sequenced. The missing of the triplet AAG (underlined) in Oea (+) individual results in ΔLys611. Both individuals carry the Pro33 GPIIIa form, which corresponds to their HPA-1b phenotypes. (B) Nucleotide sequence analysis of amplified GPIIIa DNA derived from Oea (+) and Oea (−) individuals. PCR products encompassing the entire exon 10 were sequenced directly. The mutated GPIIIa allele of Oea (+) individual is shown by the asterisks (top panel). The deletion of the triplet AAG (underlined) resulting in a shift of 3 bases was observed in Oea (+), but not in Oea (−) individual (bottom panel).
Genotyping of Oea alloantigen
To analyze the genomic DNA, we performed direct nucleotide sequencing analysis from Oea-phenotyped individuals (Figure5B). In comparison to Oea (−), Oeaheterozygous individuals are striking by a shift of 3 bases due to the deletion of AAG triplet. Using this technique, we sequenced 2 Oea (+) individuals and 20 Oea (−) blood donors and the results were in full accordance with our phenotyping results.
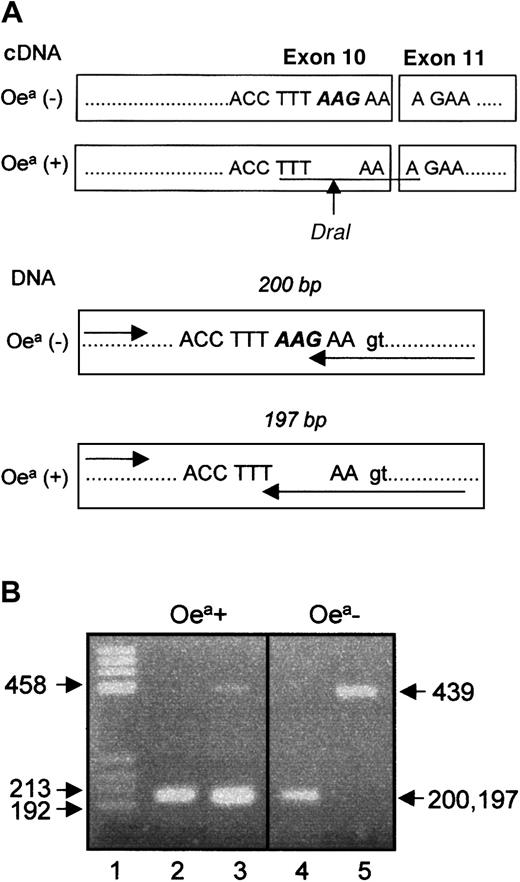
Figure 6 illustrates the location of ΔLys611 in GPIIIa cDNA and in genomic DNA. The AAG deletion encodes for the last amino acid on exon 10, which creates a recognition site for DraI restriction enzyme in Oea carriers. Thus, typing by PCR-RFLP technique could be performed with cDNA (data not shown). However, this technique could not be adapted for genomic DNA typing, because the introduction of the intronic nucleotide G in the mutant allele abolished the recognition site of DraI. Therefore, we developed the PCR-SSP approach (Figure 6). Both allele-specific primers amplified the 197- and 200-bp fragments of Oea heterozygous individuals. In contrast, DNA derived from Oea (−) blood donors could only be amplified with the wild-type primer. As internal control, amplification of the 439-bp fragment of the HGH gene was used. The genotypes of 22 individuals determined by PCR-SSP were identical with the phenotypes detected by MAIPA assay.
Location of ΔLys611 in GPIIIa cDNA and genomic DNA.
(A) Location of the AAG deletion in exon 10. The arrow indicates the cleavage site of DraI endonuclease in cDNA from Oea (+) individual. The recognition site of the enzyme is underlined. (B) Genotyping analysis of Oea alloantigen by PCR-SSP. The genotyping of Oea-phenotyped individuals (father and grandfather) using wild-type primer (lanes 2 and 4) or mutant primer (lanes 3 and 5) is shown. The 439-bp fragment represents the internal control. Note that reverse primer (arrow) specific for mutant (T) and wild-type allele (G) amplified products with 3 bases different in length (200 bp versus 197 bp).
Location of ΔLys611 in GPIIIa cDNA and genomic DNA.
(A) Location of the AAG deletion in exon 10. The arrow indicates the cleavage site of DraI endonuclease in cDNA from Oea (+) individual. The recognition site of the enzyme is underlined. (B) Genotyping analysis of Oea alloantigen by PCR-SSP. The genotyping of Oea-phenotyped individuals (father and grandfather) using wild-type primer (lanes 2 and 4) or mutant primer (lanes 3 and 5) is shown. The 439-bp fragment represents the internal control. Note that reverse primer (arrow) specific for mutant (T) and wild-type allele (G) amplified products with 3 bases different in length (200 bp versus 197 bp).
Analysis of recombinant GPIIIa allelic isoforms
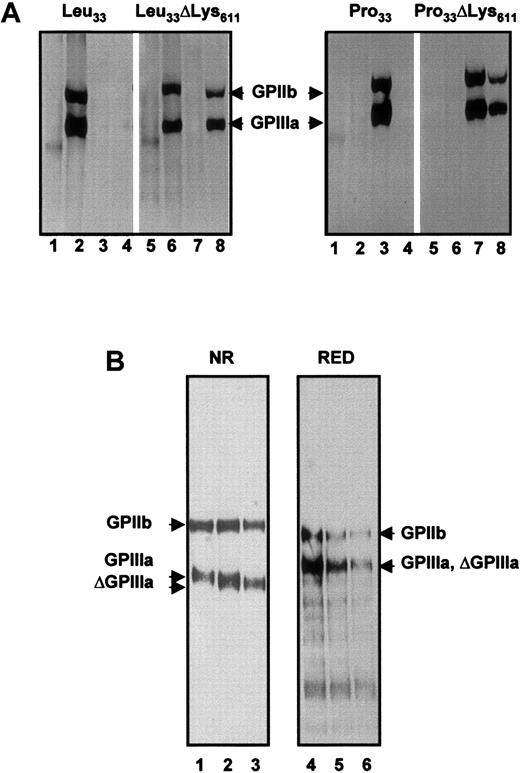
To demonstrate whether this deletion is directly responsible for the formation of Oea epitopes, we constructed allele-specific expression vectors. Site-directed mutagenesis was performed with the wild-type GPIIIa HPA-1a construct to introduce C-T mutation at position 33. In both HPA-1a and -1b constructs (Leu33 and Pro33) the codon at positions 1929-1931 (Leu33ΔLys611 and Pro33ΔLys611) was deleted. All 4 GPIIIa allele-specific constructs were cotransfected with GPIIb expression vector into CHO cells. Stable transfectants expressing allele-specific GPIIb-IIIa complex were established and labeled with biotin for immunoprecipitation analysis (Figure 7A).
Immunoprecipitation analysis of allele-specific recombinant GPIIIa isoforms.
(A) Recombinant isoforms of GPIIb-IIIa complex were produced in CHO cells transfected either with Leu33, Leu33ΔLys611, Pro33, or Pro33ΔLys611 constructs of GPIIIa. Biotinylated cell lysates were immunoprecipitated with AB serum (lanes 1 and 5), anti–HPA-1a (lanes 2 and 6), anti–HPA-1b (lanes 3 and 7), and anti-Oea alloantibodies (lanes 4 and 8). Immunoprecipitates were analyzed on 7.5% SDS-PAGE under nonreduced conditions. (B) Electrophoretic mobilities of Pro33 and Pro33 ΔLys611 GPIIIa isoforms. Biotin surface-labeled GPIIb-IIIa complex derived from CHO transfectants was immunoprecipitated with mAb Gi5 and analyzed on 7.5% SDS-PAGE under nonreduced (NR) and reduced (RED) conditions. Lanes 1 and 5, Pro33; lanes 2 and 4, a mixture of Pro33 and Pro33 ΔLys611; and lanes 3 and 6, Pro33 ΔLys611 GPIIIa isoforms.
Immunoprecipitation analysis of allele-specific recombinant GPIIIa isoforms.
(A) Recombinant isoforms of GPIIb-IIIa complex were produced in CHO cells transfected either with Leu33, Leu33ΔLys611, Pro33, or Pro33ΔLys611 constructs of GPIIIa. Biotinylated cell lysates were immunoprecipitated with AB serum (lanes 1 and 5), anti–HPA-1a (lanes 2 and 6), anti–HPA-1b (lanes 3 and 7), and anti-Oea alloantibodies (lanes 4 and 8). Immunoprecipitates were analyzed on 7.5% SDS-PAGE under nonreduced conditions. (B) Electrophoretic mobilities of Pro33 and Pro33 ΔLys611 GPIIIa isoforms. Biotin surface-labeled GPIIb-IIIa complex derived from CHO transfectants was immunoprecipitated with mAb Gi5 and analyzed on 7.5% SDS-PAGE under nonreduced (NR) and reduced (RED) conditions. Lanes 1 and 5, Pro33; lanes 2 and 4, a mixture of Pro33 and Pro33 ΔLys611; and lanes 3 and 6, Pro33 ΔLys611 GPIIIa isoforms.
As shown in the right panel, the Pro33Lys611 as well as the Pro33ΔLys611 GPIIIa isoforms could be precipitated by anti–HPA-1b alloantibodies (lanes 3 and 7). In contrast, anti-Oea alloantibodies did not recognize the Pro33Lys611 (lane 4), but only reacted with the Pro33ΔLys611 GPIIIa variant (lane 8). In the control experiments anti–HPA-1a (lanes 2 and 6) and AB serum from a healthy blood donor (lanes 1 and 5) did not precipitate any protein. In addition, immunoprecipitation analysis of the Leu33 GPIIIa isoforms (Figure 7A, left panel) showed that anti-Oea was also able to precipitate the Leu33ΔLys611isoform (lane 8). These results clearly demonstrate (1) that the deletion of Lys611 leads to the creation of Oeaepitopes and (2) this deletion has no influence on the formation of HPA-1a as well as HPA-1b alloantigenic determinants.
To analyze the influence of ΔLys611 on the structure of GPIIIa we compared the migration of GPIIIa subunits of Pro33Lys611 and Pro33ΔLys611 mutant under nonreduced and reduced conditions (Figure 7B). Under nonreduced conditions, the Lys611 isoform (lane 1) precipitated with mAb Gi5 migrated slightly slower than ΔLys611 isoform (lane 3). In the control experiment, mixture of both isoforms migrated as doublet (lane 2). Under reduced conditions, however, both GPIIIa, Lys611and ΔLys611 isoforms migrated with the same mobility. These results indicate that the molecular size polymorphism observed in the mutant GPIIIa is dependent on the disulfide bond structure of GPIIIa molecule. This is in accord with our immunoblotting experiment showing the sensitivity of Oea epitopes for disulfide reduction.
Adhesion properties of the Oea allelic isoforms of GPIIb-IIIa
To study the adhesion properties of Oea allelic isoform, clones of stable transfectants expressing high and similar amounts Pro33Lys611 or Pro33ΔLys611 form of GPIIb-IIIa were selected. Flow cytometry analysis using mab Gi5 specific for GPIIb-IIIa complex revealed equivalent levels of receptor expression on both cell lines (Figure 8A). To examine whether the Oea allelic form of GPIIIa could undergo conformational changes for ligand binding, we compared the binding of anti-LIBS mab D3 to both Lys611 or ΔLys611 transfectants in the presence or absence of RGDW peptide. Equivalent expressions of D3 epitopes were observed in both cell lines in the presence of RGDW peptide (Figure 8B). In the control experiment, no binding of mAb D3 could be detected in the presence of RGEW peptide.
Flow cytometry analysis of recombinant Oeaalloantigen.
(A) Binding of non-LIBS mAb Gi5 to stable transfectants expressing Pro33 (Oea [+]; black curve) and Pro33 ΔLys611 (Oea −; gray). (B) Binding of anti-LIBS mAb D3 to both stable transfectants in the presence of RGEW or RGDW peptides. (C) Binding of ligand-mimetic mAb PAC-1 to untreated and DTT-treated “activated” transfectants.
Flow cytometry analysis of recombinant Oeaalloantigen.
(A) Binding of non-LIBS mAb Gi5 to stable transfectants expressing Pro33 (Oea [+]; black curve) and Pro33 ΔLys611 (Oea −; gray). (B) Binding of anti-LIBS mAb D3 to both stable transfectants in the presence of RGEW or RGDW peptides. (C) Binding of ligand-mimetic mAb PAC-1 to untreated and DTT-treated “activated” transfectants.
To further determine if the ligand binding domains of the mutant GPIIb-IIIa was functionally intact we measured binding of the ligand mimetic mAb PAC-1 to DTT-treated “activated” transfectants. As shown in Figure 8C, ΔLys611 transfectants bound PAC-1 antibody in an equivalent activation-dependent manner as wild-type cells. This indicated that the ligand-binding domains were functional.
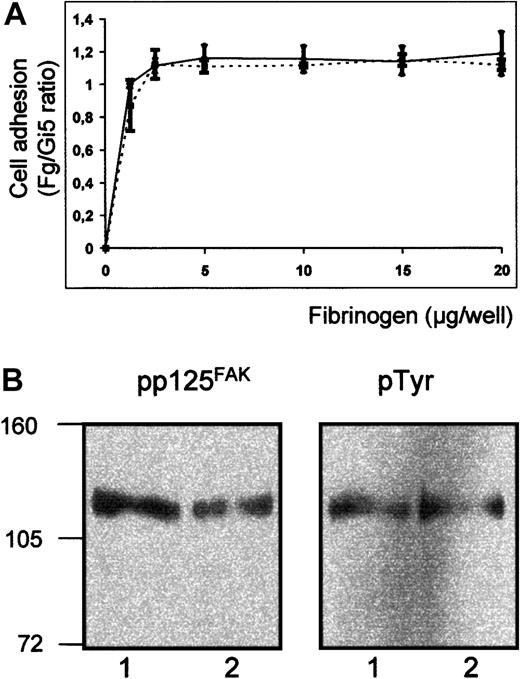
Furthermore, we compared the adhesion ability of both transfectants to immobilized fibrinogen. To exclude the influence of different expression level on the evaluation of cell adhesion, adhesion to immobilized fibrinogen was normalized to cell binding on Gi5. As shown in Figure 9A, Lys611 and ΔLys611 transfected cells showed an equivalent adhesion to immobilized fibrinogen. These results demonstrated that the ΔLys611 form of GPIIb-IIIa is able to undergo conformational change and binds fibrinogen in a manner similar to Lys611.
Adhesion ability of transfectants to immobilized fibrinogen.
(A) Binding of transfected CHO cells to immobilized fibrinogen. Transfected cells expressing Pro33 (solid line) and Pro33ΔLys611 (broken line) of GPIIIa were labeled with calcein-am. Labeled cells were allowed to adhere to microtiter wells coated with fibrinogen, BSA, or mAb Gi5 specific for GPIIb-IIIa complex. After washing, adherent cells were measured in fluorescence microtiter plate reader. Nonspecific cell adhesion on BSA was subtracted. Cell adhesion was normalized to cell binding on Gi5 (Fg/Gi5 ratio). (B) Tyrosine phosphorylation of pp125FAK following cell adhesion to immobilized fibrinogen. Stable transfectants expressing GPIIb-IIIa Pro33 (lanes 1) and GPIIb-IIIa Pro33Δ Lys611 (lanes 2) were allowed to bind fibrinogen-coated wells. Adherent cells were lysed and precipitated with rabbit polyclonal anti-pp125FAK. Immunoprecipitates were separated on 7.5% SDS-PAGE, blotted, and probed subsequently with mAbs against phosphotyrosine (pTyr) and pp125FAK and visualized using peroxidase-labeled secondary antibody and chemiluminescence substrate.
Adhesion ability of transfectants to immobilized fibrinogen.
(A) Binding of transfected CHO cells to immobilized fibrinogen. Transfected cells expressing Pro33 (solid line) and Pro33ΔLys611 (broken line) of GPIIIa were labeled with calcein-am. Labeled cells were allowed to adhere to microtiter wells coated with fibrinogen, BSA, or mAb Gi5 specific for GPIIb-IIIa complex. After washing, adherent cells were measured in fluorescence microtiter plate reader. Nonspecific cell adhesion on BSA was subtracted. Cell adhesion was normalized to cell binding on Gi5 (Fg/Gi5 ratio). (B) Tyrosine phosphorylation of pp125FAK following cell adhesion to immobilized fibrinogen. Stable transfectants expressing GPIIb-IIIa Pro33 (lanes 1) and GPIIb-IIIa Pro33Δ Lys611 (lanes 2) were allowed to bind fibrinogen-coated wells. Adherent cells were lysed and precipitated with rabbit polyclonal anti-pp125FAK. Immunoprecipitates were separated on 7.5% SDS-PAGE, blotted, and probed subsequently with mAbs against phosphotyrosine (pTyr) and pp125FAK and visualized using peroxidase-labeled secondary antibody and chemiluminescence substrate.
Effect of Lys611 deletion on signaling properties of GPIIb-IIIa complex
To examine whether ΔLys611 affects the outside-in signaling of GPIIb-IIIa, tyrosine phosphorylation of focal adhesion kinase pp125FAK was measured after adhesion of GPIIb-IIIa–transfected cells to fibrinogen (Figure 9B). Blots were incubated subsequently with antiphosphotyrosine and anti-pp125FAK. Equivalent phosphorylation of pp125FAK was observed in Pro33 and Pro33 ΔLys611 transfectants. Thus, the deletion of Lys, which is responsible for the formation of the Oea alloantigenic determinant, affects neither the adhesive properties of GPIIb-IIIa nor the outside-in signaling.
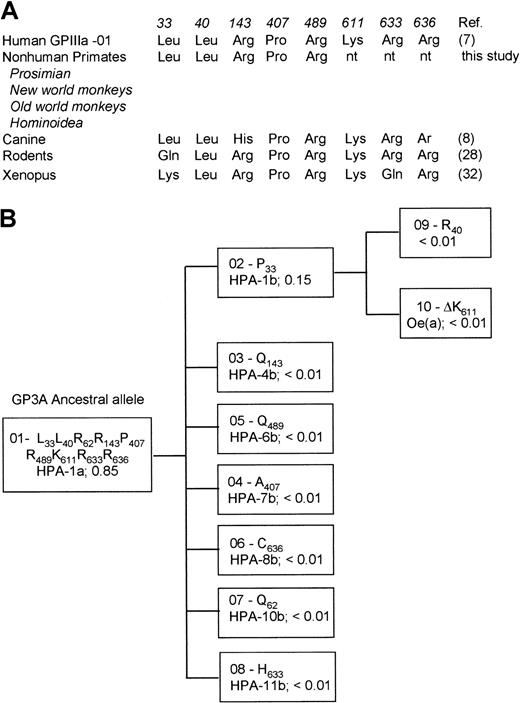
Evolution model for the generation of different GPIIIa allelic variants
To better understand the evolutionary relationship of GPIIIa alleles, we analyzed DNA derived from 13 nonhuman primates representing 4 different species ranging from prosimian (Varecia variegata), new world monkeys (Callimico goeldii, Saimiri sciureus), old world monkeys (Cercopithecus griseoviridis, Macaca mulatta, Macaca nemestrinus, Macaca fascicularis, Mandrilus sphinx), and Hominoidea (Hylobates syndactylus, Pongo abelii, Gorilla gorilla, Pan paniscus, Pan troglodytes). Amplified DNA encompassing the polymorphic regions 196, 217, 526, 1317, 1564, which encode Leu33Pro, Leu40Arg, Gln143Arg, Pro407Ala and Arg489Gln, respectively, were sequenced directly using PCR primers. All nonhuman primates revealed the amino acid residues Leu33, Leu40, Arg143, Pro407, and Arg489 (Figure 10A). This allelic isoform, referred to as GP3A*01 carrying the HPA-1a epitopes is high frequent distributed among humans.8 These data indicate that GP3A*01 represents the ancestral allele. Seven point mutations of this ancestral allele were found (Figure 10B). The first mutation led to formation of GP3A*02, which encodes GPIIIa Pro33 form carrying HPA-1b epitopes. A further mutational event (ΔLys611) occurred in this allele leading to the formation of Oea alloantigenic determinant.
Evolution model for generation of GPIIIa allelic variants.
(A) Comparison of selected regions of human GPIIIa with homologous regions on nonhuman primates and other species. The high-frequency allele of human GPIIIa (GP3A*01) as well as nonhuman primates carry the same amino acids at residues 33, 40 143, 407, and 489. (B) Evolutionary model of human platelet alloantigens on GPIIIa. The frequencies of HPAs in the white population are shown.
Evolution model for generation of GPIIIa allelic variants.
(A) Comparison of selected regions of human GPIIIa with homologous regions on nonhuman primates and other species. The high-frequency allele of human GPIIIa (GP3A*01) as well as nonhuman primates carry the same amino acids at residues 33, 40 143, 407, and 489. (B) Evolutionary model of human platelet alloantigens on GPIIIa. The frequencies of HPAs in the white population are shown.
Discussion
In this study, we present data on a new immunogenic GPIIIa variant, the Oea alloantigen, responsible for a typical case of NAIT in a white family. Analysis by the use of antigen capture assay, immunoprecipitation, and immunoblot allowed us to localize the Oea alloantigenic determinant on the platelet GPIIIa subunit. Nucleotide sequence analysis of GPIIIa cDNA in the Oea (+) family members (father and grandfather of the affected child) showed a deletion of one codon (AAG at positions 1929-1931). This deleted triplet encodes for the last amino acid Lys611 on exon 10. Our findings could be confirmed by direct nucleotide sequencing analysis of genomic DNA. Furthermore, full accordance between Oea phenotyping and genotyping was shown by allele-specific PCR analysis.
Stable expression of recombinant allele-specific GPIIIa in mammalian cells allowed confirmation that the single deletion of Lys611 was sufficient to induce the Oeadeterminant. In addition, we could demonstrate that this deletion did not impair the epitopes of HPA-1.
For the formation of the Oea alloantigenic determinants, 3-dimensional structures of GPIIIa appeared to be required, because reduction of disulfide bonds and chymotrypsin treatment abolished the presentation of Oea epitopes.
Treatment of platelets with chymotrypsin led to digestion of GPIIIa giving rise to 3 major fragments that represent amino acids 1-100, 101-321, and 322-762. The 66-kd fragment is composed by the amino terminal part (amino acids 1-100) and the carboxy terminal portion (amino acids 322-762), which are disulfide linked and anchored in the platelet membrane.28 29 Although the ΔLys611responsible for Oea epitopes is located in this part of the GPIIIa molecule, anti-Oea failed to react with the 66-kd fragment. This result indicated that the cleaved fragment (amino acids 101-321) took part in the formation of Oea epitopes.
Interestingly, the ΔLys611 GPIIIa isoform was found to migrate slightly faster than the wild-type GPIIIa isoform under nonreducing conditions. After disulfide reduction, however, both isoforms migrated with the same mobility, indicating that ΔLys611 altered the 3-dimensional structure of GPIIIa by impairment of disulfide bonds.
Four main structural domains in GPIIIa could be assigned; the N-terminal cysteine-rich domain (residues 1-62), the fibrinogen-binding domain (residues 101-422), the cysteine-rich proteinase-resistant core (residues 423-622), which is bound to the N-terminal domain by a single disulfide bond (Cys5-Cys435), and the C-terminal domain, comprising an extracellular subdomain (residues 623-692), a transmembrane (residues 693-721), and cytoplasmic subdomains (residues 722-762). Within the cysteine-rich proteinase-resistant core, 4 cysteine-rich repeats have been assigned.30 The ΔLys611 responsible for the formation of Oea alloantigenic determinants is located in the fourth cysteine-rich repeat encompassing cysteine residues 601, 604, 608, 614, 617, and 687, which are known to be paired among themselves. One could speculate that the ΔLys611 may impair the adjacent disulfide bond(s). Further investigations of the mutant ΔLys611 using proteomic approach will help to ascertain the GPIIIa disulfide pattern in the cysteine repeat region.
Another molecular weight polymorphism associated with a human platelet alloantigen has been observed in HPA-8 system.31 In HPA-8b individuals we found an Arg636Cys mutation in the cysteine-rich repeat, which led to the presence of a free sulfhydryl group in the mutant GPIIIa isoform. This mutation resulted in different glycosylation of GPIIIa, which appeared as molecular weight polymorphism.
Recently, several observations suggested that the cysteine-rich repeat of GPIIIa (β3 integrin) plays an important role in the regulation of αIIbβ3 functions. Sequence alignment of β3 integrins indicates that about 90% of noncysteine residues in the cysteine-rich repeats are conserved between mouse, rodent, canine, and human. In contrast, these noncysteine residues are poorly conserved among β1, β2, and β3 integrins.9,32 Wippler et al33 demonstrated that recombinant αIIbβ3 lacking the cysteine repeats of β3 showed a high-affinity binding to fibrinogen. Recently, Kashiwagi et al34 demonstrated that a point mutation (Thr562Asn) in the extracellular cysteine-rich repeat region is directly involved in integrin structural changes during inside-out signaling. This mutation resulted in a constitutive activation of αIIbβ3 and αVβ3 integrins leading to spontaneous binding of soluble fibrinogen.
To study the influence of ΔLys611 on GPIIb-IIIa receptor function, we examined ligand binding and postligand binding events of GPIIb-IIIa in CHO cells. Using LIBS antibody we could demonstrate that the ΔLys611 GPIIIa isoform was not constitutively active and was capable of undergoing conformational changes on ligand binding like the wild-type GPIIIa form. In addition, the ΔLys611GPIIIa could mediate downstream signaling events such as phosphorylation of pp125FAK. These data suggest that the Lys611 deletion has no effect on integrin function.
Ten different genetic variants of the GPIIIa molecule have been identified (Figure 10). The GP3A*01 allele encoding for the GPIIIa Leu33 isoform, which carries the HPA-1a alloantigenic determinants, is the most frequent allele with a gene frequency of nearly 85% within the white population. This allele differs from the second most common form of GP3A*02 (HPA-1b, frequency ∼15%) by a single amino acid substitution (Leu33Pro). Other alleles of GPIIIa (GP3A*03, *04…) are much less frequently represented and appear to have arisen from the high frequency allele GP3A*01 by independent point mutations, a scenario for the evolution of human platelet alloantigens that has been proposed by Newman and Nathalie.35 From the higher frequency of GP3A*01 in human and sequence comparison with rodents and Xenopus, the authors hypothesized that this variant comprises the ancestralGPIIIa gene. Unfortunately, the evolutionary relationships of the HPA-1 alloantigen system could not be directly examined, because these species carry neither leucine nor proline at position 33.32,36 Recently, Lipscomb et al9 reported that the deduced amino acid sequence of canine GPIIIa has leucine at position 33 and therefore corresponds to the high frequency human GP3A*01. In this study, we could demonstrate that GPIIIa of nonhuman primates also carry Leu33 supporting the model for the GPIIIa allelic generation as described above.
Recently, we have reported a Leu40Arg dimorphism on GPIIIa that was associated with HPA-1b, but so far no corresponding alloantibody has been detected.24 Interestingly, we observed that the Oea alloantigen also segregated with the HPA-1b allele.
By the genotyping analysis we could exclude a relation between Oea and the Leu40Arg dimorphism. Therefore, 2 independent mutations of the HPA-1b allele have occurred. The proportional distribution of rare mutations among the more frequent GP3A*01 and *02 alleles (7 versus 2) suggests that mutational events occurred randomly during evolution and no further selection took place (Figure 10).
Point mutations or a deletion (in this study) responsible for the formation of human platelet alloantigens on GPIIIa were found in different domains of the molecule: HPA-1 and HPA-10 in the amino terminal domain, HPA-4 in the ligand-binding domain, HPA-6 and Oea in the cysteine-rich repeat domain, and HPA-8 and HPA-11 in the extracellular C-terminal domain. To date, comparative functional analysis has been performed for the HPA-1 and HPA-4. Whereas the HPA-4 polymorphism does not seem to have an influence on GPIIb-IIIa function, some data on the functional relevance of different HPA-1 alleles have been reported.26 14 From these and our results, one could speculate that the alloantigenic polymorphism of GPIIIa does not have a major influence on integrin function.
The authors thank Dr K. Adam, Children Hospital, Weiden, Germany, who kindly referred this NAIT case to us. We are grateful to M. Ernst-Schlegel, M. Boehringer, S. Werth, and A. Wittchen for their technical assistance. We are grateful to Dr P. Newman for his support. Our gratitude is also extended to the families concerned for their cooperation in this study. This work is part of the doctoral theses of A. Rachman and B. Carl.
In agreement with the policy of ISBT/ISTH working party on nomenclature of platelet alloantigens, materials have been sent to another expert laboratory for confirmation. The existence of the new human platelet alloantigen Oea could be confirmed by Dr B. Curtis and Dr R. H. Aster (Blood Research Institute, Milwaukee, WI) by analyzing maternal serum, paternal platelets, and DNA.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Sentot Santoso, Institute for Clinical Immunology and Transfusion Medicine, Justus Liebig University Giessen, Langhansstr 7, 35385 Giessen, Germany; e-mail:sentot.santoso@immunologie.med.uni-giessen.de.

![Fig. 4. Immunoprecipitation analysis of chymotrypsin-treated platelets. / Paternal platelets (Oea [+], HPA-1a/1b, HPA-4a/4a) were surface labeled with biotin, digested with chymotrypsin, lysed, and precipitated with anti–HPA-1a (lane 1), anti-Oea (lane 2), anti-HPA-4a (lane 3), and normal human serum (lane 4). Immunoprecipitates were analyzed on 7.5% SDS-PAGE under nonreducing conditions, blotted, and visualized with chemiluminescence substrate.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/4/10.1182_blood.v99.4.1205/6/m_h80422165004.jpeg?Expires=1769273448&Signature=y6bZGMgem0MdQGnxcvuxkRvOVBoS-WSstPxCPN2ClDld~Yj5Uglav8RO7od1VnHnYCq8ox3lllFhuTS532LVoFd5L2cO5M0OtOiCwmGo-XvPBAYTrHdnhocW9ulgcNThZSYGck9JfUd-1fNeCxaiPaemgrtrm~~4B4FDJzXA9rZJXX4bIaspwOoWWvXQb-zwFTNmOaS335nkzr3LPEy86o2aDoSjw1aBv~smbfULoI-wjjYsrXm7FNO5KaXJrntj~jkwUV9DmIq04PX5jrtSXy~4JpC26V-wAZx9vhUSLXzoHunO~fmOZFtHRWUboAxGFd2B7ZfHw4aQ-Z3TcuUIHw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 8. Flow cytometry analysis of recombinant Oeaalloantigen. / (A) Binding of non-LIBS mAb Gi5 to stable transfectants expressing Pro33 (Oea [+]; black curve) and Pro33 ΔLys611 (Oea −; gray). (B) Binding of anti-LIBS mAb D3 to both stable transfectants in the presence of RGEW or RGDW peptides. (C) Binding of ligand-mimetic mAb PAC-1 to untreated and DTT-treated “activated” transfectants.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/4/10.1182_blood.v99.4.1205/6/m_h80422165008.jpeg?Expires=1769273448&Signature=JUDMt2Wt1PtwWEr2gPavoKHhXNNM2KErnzCSP46P0lJYyqQI2mq5YcvZQ5bPNhfXZJQW3Gj0dqu~hGtkvqyuz2UhJjZR4PTsGDGGsAQj7oT4LhoI09cDuPruNYD5415d9aKB1JMVoyPwGBp528FNlaYbcFe5hXeiD7vJsJL8~VVlB7TQrSUWxdzqRfwUcTly1wD80cDiXLgDOwa39a~BssHTUV7m4x5weUeXdtXuMoj~zXz5reY7ElmqQ1KgmzuRzDYzQtSH6~pH7rgb18wCBC34z3oU3UKIxc4bNmk6fFcmBbcmFvtsy-ry4SX~E1owcl0L55lrhxNGB5HcGecvjQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal