The CCAAT/enhancer binding protein α (C/EBPα) protein is essential for proper lung and liver function and granulocytic and adipose tissue differentation. It was hypothesized that abnormalties in C/EBPα function contribute to the development of malignancies in a variety of tissues. To test this, genomic DNA from 408 patient samples and 5 cell lines representing 11 different cancers was screened for mutations in the C/EBPα gene. Two silent polymorphisms termed P1 and P2 were present at frequencies of 13.5% and 2.2%, respectively. Of the12 mutations detected in 10 patients, silent changes were identified in one nonsmall cell lung cancer, one prostate cancer, and one acute myelogenous leukemia (AML) subtype M4. The 9 remaining mutations were detected in 1 of 92 (1.1%) myelodysplastic syndrome (MDS) samples and 6 of 78 (7.7%) AML (AML-M2 and AML-M4) samples. Some mutations truncated the predicted protein with loss of the DNA-binding (basic region) and dimerization (leucine zipper [ZIP]) domains by either deletions or nonsense codons. Also, inframe deletions or insertions in the fork region located between the leucine zipper and basic region, or within the leucine zipper, disrupted the α-helical phase of the bZIP domain. The inframe deletion and insertion mutations abrogated the transcriptional activation function of C/EBPα on the granulocyte colony-stimulating factor receptor promoter. These mutants localized properly to the nucleus, but were unable to bind to the C/EBP site in the promoter and did not possess dominant-negative activity. The mutations in the MDS patient and one AML-M2 patient were biallelic, indicating a loss of C/EBPα function. These results suggest that mutation of C/EBPα is involved in specific subtypes of AML and in MDS, but may occur rarely in other types of leukemias or nonhematologic malignancies.
Introduction
The CCAAT/enhancer binding protein α (C/EBPα) belongs to a family of proteins that possess a bipartite DNA-binding domain composed of a positively charged basic (b) region that contacts the DNA and a leucine zipper (ZIP) in the C terminus that mediates dimerization.1 The less-conserved N terminus contains regulatory and transactivation domains.2-5C/EBPα is expressed in a number of tissues, most prominently in the highly differentiated cells of the liver, white and brown adipose, lung, and myeloid-lineage cells.6-9 It has also been detected in the adrenal gland, skin, pancreas, prostate, differentiated enterocytes in the intestine, and, during follicular development, the ovary.9-12
C/EBPα is proposed to be a regulator of energy metabolism and transcriptionally activates the promoters of energy-related genes such as GLUT4 and PEPCK in hepatocytes and adipocytes.13-15 In myeloid cells, C/EBPα transcriptionally activates the promoters of the myeloid-specific receptors for the growth factors macrophage colony-stimulating factor, granulocyte colony-stimulating factor (G-CSF) and granulocyte-macrophage colony-stimulating factor.16-18 Studies demonstrate that C/EBPα is critical for the process of terminal differentiation of adipocytes. C/EBPα is upregulated in adipocyte differentiation, and blocking its expression halts differentiation of preadipocytes into adipocytes, while overexpression induces differentiation and inhibits proliferation.19-23 Also, overexpression of C/EBPα induces differentiation of myeloid leukemia cell lines and inhibits the proliferation of a number of cell lines and tumor cells.24-27 The inhibition of proliferation is partly due to the ability of C/EBPα to activate transcription and induce posttranscriptional stabilization of the cyclin-dependent kinase inhibitor p21 (WAF-1).28 29 These studies suggest a central role for C/EBPα in the regulation of cell proliferation and differentiation.
Targeted inactivation of C/EBPα in mice demonstrates its importance in the proper development and function of liver, adipose, lung, and hematopoietic tissues.8,30,31 Within 8 hours after birth, the mice die of impaired glucose metabolism, and adipose metabolism is altered with a failure of adipocytes to accumulate lipids.30,31 The lung shows hyperproliferation of type II pneumocytes and abnormal alveolar structure, and histopathology of the liver displays a structure resembling regenerative changes or hepatocellular carcinoma.30-32 The null mice also display impaired neutrophil development intrinsic to the hematopoietic system resulting from a blockade in differentiation and the absence of expression and signaling of the G-CSF and interleukin-6 receptors.8 33
The importance of C/EBPα in cell growth and differentiation suggests it may play the role of a tumor suppressor. A number of studies support this hypothesis. Expression of C/EBPα was transcriptionally downregulated in preneoplastic hepatic nodules and further decreased in hepatocellular carcinomas in rat liver.34 Primary hepatocytes from newborn C/EBPα-null mice showed significantly higher proliferative rates than wildtype mice, while cell lines from null mice exhibited rapid growth and accumulation of chromosomal abnormalities.35 These cell lines were capable of forming nodules when inoculated into nude mice. In squamous cell carcinomas, the expression of C/EBPα was greatly diminished.36Because of the early lethality of the C/EBPα-null mice, determination of whether these mice are susceptible to the development of tumors is not possible. The heterozygous (+/−) mice were not observed to suffer from an increased rate of tumor development.31 In this study, we tested the hypothesis that C/EBPα may function as a tumor suppressor that is mutated during tumorigenesis. We report the findings from a screen of 408 patient samples representing 11 different cancers.
Materials and methods
Samples
Primary samples (408) from 11 types of human malignancies were studied. These are summarized in Table1.The following cell lines were examined: HL60, Kasumi-1 [AML-M2, t(8;21)], Kasumi-5, KG-1, and NB-4 (AML-M3). The soft-tissue sarcoma samples were generously provided by Jonathan Said (Department of Pathology, UCLA School of Medicine). Genomic DNA was extracted as described.37
Summary of patient samples examined for C/EBPα mutations
| Tumor type . | No. . | P1* . | P2† . | Mutations . |
|---|---|---|---|---|
| AML | 78 | 10 | — | 8‡ |
| MDS | 92 | 14 | — | 2‡ |
| ALL | 23 | — | 1 | — |
| NHL | 34 | — | — | — |
| Lung (nonsmall cell) | 36 | 6 | — | 1 |
| Osteosarcomas | 20 | — | — | — |
| Other sarcomas | 52 | 13 | 4 | — |
| Breast | 22 | 1 | 2 | — |
| Prostate | 33 | 5 | 2 | 1 |
| Glioblastoma | 12 | 4 | — | — |
| Hepatoma | 6 | 2 | — | — |
| Total | 408 | 55 | 9 | 12 |
| Tumor type . | No. . | P1* . | P2† . | Mutations . |
|---|---|---|---|---|
| AML | 78 | 10 | — | 8‡ |
| MDS | 92 | 14 | — | 2‡ |
| ALL | 23 | — | 1 | — |
| NHL | 34 | — | — | — |
| Lung (nonsmall cell) | 36 | 6 | — | 1 |
| Osteosarcomas | 20 | — | — | — |
| Other sarcomas | 52 | 13 | 4 | — |
| Breast | 22 | 1 | 2 | — |
| Prostate | 33 | 5 | 2 | 1 |
| Glioblastoma | 12 | 4 | — | — |
| Hepatoma | 6 | 2 | — | — |
| Total | 408 | 55 | 9 | 12 |
A minus sign (−) indicates that none of the samples contained either a mutation or polymorphism.
P1 indicates polymorphism 1; P2, polymorphism 2; AML, acute myelogenous leukemia; MDS, myelodysplastic syndrome; ALL, acute lymphoblastic leukemia; and NHL, non-Hodgkin lymphoma.
836T > G.
836T > G, 839C > G, 902G > T.
One patient sample contained 2 mutant alleles.
Polymerase chain reaction–single-strand conformation polymorphism analysis
The primers used to amplify the C/EBPα genomic locus are described in Table 2 and represented graphically in Figure 1. The nucleotide numbering throughout this study is based on the published sequence available from EMBL/GenBank/DDBJ under accession number NM_004364.1. The primers were used in the following combinations to amplify 4 overlapping regions (regions 1 through 4) of the genomic DNA: (1) 1F plus 1R, 306 base pairs (bp); (2) 2F plus 2R, 309 bp; 3F plus 3R, 366 bp; and (4) 4F plus 4R, 266 bp. Platinum Taq DNA polymerase (Gibco/BRL, Bethesda, MD) with 4% dimethyl sulfoxide was used to amplify the fragments from regions 1, 2, and 4. To amplify region 3, Failsafe Taq DNA polymerase (Epicentre Technologies, Madison, WI) and buffer K (supplied by the manufacturer) were used. The polymerase chain reaction (PCR) conditions were as follows: 94°C, 3 minutes; 35 cycles 95°C, 30 seconds; either 66°C (region 1) or 64°C (regions 2, 3, and 4), 30 seconds; and 72°C, 1 minute. Shifted bands were excised from the dried gel and eluted in double-distilled H2O, reamplified, and gel purified for sequencing. The products were ligated into a pCR2.1-TA cloning vector (Invitrogen, Carlsbad, CA) as described by the manufacturer. Products were sequenced in both directions with an ABI Prism Dye Terminator Cycle Sequencing Ready Reaction kit (Perkin-Elmer, Foster City, CA) with the use of the region 1 through 4 PCR–single-strand conformation polymorphism (PCR-SSCP) primers or primer-binding sites available in the plasmid (T7 and M13R) for the cloned fragments. The sequencing reactions were analyzed by an ABI 377 sequencing machine.
Primers used for amplification of C/EBPα by polymerase chain reaction
| Primer name . | Nucleotide sequence . | Nucleotide position* . |
|---|---|---|
| 1F | 5′-cgccatgccgggagaactct-3′ | 121 -140 |
| 1R | 5′-gccttggccttctcctgctg-3′ | 408 -427 |
| 2F | 5′-gacctgttccagcacagccg-3′ | 387 -406 |
| 2R | 5′-gcggctggtaagggaagagg-3′ | 677 -696 |
| 3F | 5′-cgcgaggaggatgaagccaa-3′ | 639 -658 |
| 3R | 5′-cccggtactcgttgctgttcttgtc-3′ | 981 -1005 |
| 4F | 5′-gggcaaggccaagaagtcgg-3′ | 959 -978 |
| 4R | 5′-cctcacgcgcagttgcccat-3′ | 1206 -1225 |
| Primer name . | Nucleotide sequence . | Nucleotide position* . |
|---|---|---|
| 1F | 5′-cgccatgccgggagaactct-3′ | 121 -140 |
| 1R | 5′-gccttggccttctcctgctg-3′ | 408 -427 |
| 2F | 5′-gacctgttccagcacagccg-3′ | 387 -406 |
| 2R | 5′-gcggctggtaagggaagagg-3′ | 677 -696 |
| 3F | 5′-cgcgaggaggatgaagccaa-3′ | 639 -658 |
| 3R | 5′-cccggtactcgttgctgttcttgtc-3′ | 981 -1005 |
| 4F | 5′-gggcaaggccaagaagtcgg-3′ | 959 -978 |
| 4R | 5′-cctcacgcgcagttgcccat-3′ | 1206 -1225 |
Numbering based on published sequence available from EMBL/GenBank/DDBJ under accession no. NM_004364.1.
A schematic diagram of the C/EBPα gene locus.
The gene does not contain introns; therefore, the coding region is contained within one exon represented as an open box. The domains discussed in the text are indicated by the shaded boxes. The position of the primers used for PCR-SSCP analysis for each region (1 through 4) are indicated by the arrows above (forward or sense primers) and below (reverse or antisense primers) the schematic. Abbreviations: ADM, activation domain modules; BR, basic region; and L-ZIP, leucine zipper.
A schematic diagram of the C/EBPα gene locus.
The gene does not contain introns; therefore, the coding region is contained within one exon represented as an open box. The domains discussed in the text are indicated by the shaded boxes. The position of the primers used for PCR-SSCP analysis for each region (1 through 4) are indicated by the arrows above (forward or sense primers) and below (reverse or antisense primers) the schematic. Abbreviations: ADM, activation domain modules; BR, basic region; and L-ZIP, leucine zipper.
Expression vectors
The entire coding region (nucleotide [nt] 132-1230) of wildtype or mutant C/EBPα was amplified with primers ATG-FIX (5′-ggagaactctaactccaccatggagtcgg-3′) and 4R under the previously described PCR conditions for region 4. The C-nucleotide at the −3 position from the ATG was changed to an A-nucleotide to create a perfect Kozak consensus sequence.38 The fragments were gel-purified and cloned into the TA-cloning vector pCR2.1 (Invitrogen). Sequencing was performed to verify the integrity of the insert. The inserts were subcloned into the MIG retrovirus expression vector (kindly provided by Dan Tenen, Harvard Medical School, Boston, MA) with the use of EcoRI39 and the pCMV-Sport1 vector (Gibco/BRL). The orientation was determined by means of the BglII and NotI restriction enzymes.
Cell lines, transfections, and analysis of protein expression
NIH3T3 cells were maintained in Dulbecco modified Eagle medium supplemented with 10% bovine serum. For cellular localization, cells were plated at 70% confluency on a glass coverslip in a 35-mm dish. The cells were transfected with 3 μg MIG, MIG-C/EBPα mutant, or pCMV-C/EBPα wildtype plus pEGFPc with the use of 15 μL GenePORTER, as described by the manufacturer (Gene Therapy Systems, San Diego, CA). At 24 hours after transfection, cells were fixed in 10% formalin for 1 hour, washed twice in phosphate-bufferend saline (PBS), and permeablized in PBS with 0.1% Triton X-100 for 5 minutes. The cells were incubated with rabbit anti-C/EBPα antibody (SC-61) diluted to 0.01 μg/mL (Santa Cruz Biotechnology, CA) for 1 hour, washed twice with PBS, and incubated with a 1:200 dilution of tetrarhodamine isothiocyanate–conjugated goat antirabbit immunoglobulin G (Sigma, St Louis). The coverslips were mounted with Gel/Mount (Biomeda, Foster City, CA) and examined by fluorescent microscopy. Total cell protein was prepared from NIH3T3 cells transfected with pCMV, pCMV-C/EBPα42 wildtype (WT) or mutant expression vectors and lysed in 0.5% NP-40 buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 0.5% NP-40). Western blot analysis was performed as described previously.40 The blots were incubated overnight with 0.2 μg/mL of either rabbit anti-C/EBPα antiserum (Santa Cruz Biotechology) diluted in PBS containing 5% powdered milk.41 The primary antibodies were detected with either donkey antirabbit–horseradish peroxidase (HRP) or antigoat-HRP (1:5000). The complexes were developed with SuperSignal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL), as described by the manufacturer, and detected by autoradiography.
Transcriptional activation assays
For transcriptional activation assays, NIH3T3 cells were plated in a 12-well dish at 50% to 70% confluency. For each triplicate, the plasmids were prepared as a master mix of pG-CSF receptor–luciferase, pRLSV40, a renilla luciferase expression vector (Promega, Madison, WI), C/EBPα expression vector, and empty vector, for a final total of 3 μg DNA. The combinations and amounts of expression vectors are indicated in the figure legends. The plasmids in 0.5 mL Opti-MEM (Gibco/BRL) were mixed with 15 μL GenePorter in 0.5 mL Opti-MEM and incubated 45 minutes; then, 0.33 mL were aliquoted to each well of the 12-well plate (1 μg DNA per well). The reporter plasmid pG-CSF receptor–luciferase was generously provided by Dan Tenen.16 At 24 hours after transfection, cells were harvested in passive lysis buffer, and luciferase activity was measured by means of a dual luciferase assay (Promega).
Electrophoretic mobility shift assays
A double-stranded oligonucleotide containing the C/EBP site (bp −57 to −38; 5′-aaggtgttgcaatccccagc-3′) of the human G-CSF receptor promoter was end-labeled with γ32P adenosine triphosphate by means of T4 polynucleotide kinase as described by the manufacturer (Gibco/BRL).16 The labeled oligonucleotide (1 ng per reaction) was mixed with equal volumes of total cell extract from NIH3T3 cells transfected with expression vectors for the different C/EBPα proteins and buffer B (20% glycerol, 20 mM Hepes [pH 7.9], 50 mM NaCl, 2 mM MgCl2, and 1 mM dithiothreitol). Poly (dI-dC) (Amersham Pharmacia Biotech, Piscataway, NJ) and bovine serum albumin (Sigma) were added to 50 μg/mL and 300 μg/mL final concentrations, respectively. Reactions were incubated at room temperature for 30 minutes and analyzed by gel electrophoresis through a 4% polyacrylamide gel with the use of a Tris-glycine buffer (50 mM Tris, 400 mM glycine, 1 mM EDTA, with pH adjusted to 8.5). For supershifts, 0.2 μg antibody was added to the reaction 30 minutes prior to addition of the probe. Gels were exposed to Kodak XO-Mat film (Rochester, NY).
Results
Identification of C/EBPα polymorphisms
PCR-SSCP analysis using the region 3 primers revealed 3 variant patterns for the entire collection of samples (data not shown). Sequence analysis indicated that 1 pattern was from the wildtype sequence while the other 2 were due to nucleotide changes that were silent and would not affect the predicted amino acid sequence. The frequency of these changes suggested the existence of polymorphisms (Table 1). Polymorphism 1 (P1) (55 of 408 or 13.5%) consisted of an 836T>G transversion, and polymorphism 2 (P2) (9 of 408 or 2.2%) consisted of transversions at 3 different sites in the same allele (836T>G, 839C>G, and 902G>T) (Table 1). To verify that these changes were polymorphisms, we examined 30 matched tumor and normal samples from nonsmall cell lung cancers by PCR-SSCP (data not shown). For each tumor with a shift, the corresponding shift was present in the matched normal sample, indicating the existence of a polymorphism. P1 was present in 10.6% (24 of 227) of hematologic and 17% (31 of 181) of nonhematologic cancers (Table 1). In contrast, P2 was present in 0.4% (1 of 227) of hematologic and 4.4% (8 of 181) of nonhematologic cancers (Table 1).
Absence of C/EBPα mutations in nonhematologic malignancies and their presence in acute myelogenous leukemia and acute myelogenous leukemia
A summary table of the mutations described below indicates the type of cancer, the genotype of each patient, the mutation, and its effect on the amino acid sequence of the predicted protein (Table3). We examined 181 nonhematologic human tumors and found 2 mutations: a 551G>A for one prostate cancer and a 1087G>A in a nonsmall cell lung tumor (Table 3). These silent changes occurred in the third position of the codon and were not predicted to alter the amino acid sequence.
Summary of C/EBPα mutations detected in patient samples
| Patient sample by cancer type . | ID no. . | Karyotype . | Region . | Mutation . | Protein . | Function . |
|---|---|---|---|---|---|---|
| AML-M2 | K-6 | ND | 4 | 1063G > C | Arg305Pro | ND |
| AML-M2 | J6 | ND | 4 | 3-bp duplication of nt 1083-1085 | Inframe duplication of Lys312 | ND |
| AML-M2 | J3 | ND | 4 | 15-bp duplication of nt 1083-1097 | Inframe duplication of Lys-Val-Leu-Glu-Leu | Loss of DNA binding and transcriptional activation |
| AML-M2 | J2 | ND | 4 | 1001C > A | Nonsense codon at Tyr284 | ND |
| AML-M2 | F3820 | 46XY | 1 | 1-bp deletion of C381 | Frameshift at Phe77 | ND |
| AML-M2 | F3820 | 46XY | 4 | 24-bp duplication of nt 1062-1085 | Inframe duplication of Arg-Asn-ValGlu-Thr-Gln-Gln-Lys | Loss of DNA binding and transcriptional activation |
| AML-M4 | F3901 | 46XX d(9q22) | 4 | 3-bp deletion of nt 1083-1085 | Inframe deletion of Lys312 | Loss of DNA binding and transcriptional activation |
| AML-M4 | F4431 | 46XX | 2 | 551G > A | Silent | ND |
| MDS (RAEB-t) | F3881 | 46XY | 2 | 37-bp duplication of nt 483-519 | Frameshift at Ala134 | ND |
| MDS (RAEB-t) | F3881 | 46XY | 4 | 1015G > T | Nonsense codon at Glu290 | ND |
| Prostate | 202 | ND | 2 | 551G > A | Silent | ND |
| Lung (squamous) | 188 | ND | 4 | 1087G > A | Silent | ND |
| Patient sample by cancer type . | ID no. . | Karyotype . | Region . | Mutation . | Protein . | Function . |
|---|---|---|---|---|---|---|
| AML-M2 | K-6 | ND | 4 | 1063G > C | Arg305Pro | ND |
| AML-M2 | J6 | ND | 4 | 3-bp duplication of nt 1083-1085 | Inframe duplication of Lys312 | ND |
| AML-M2 | J3 | ND | 4 | 15-bp duplication of nt 1083-1097 | Inframe duplication of Lys-Val-Leu-Glu-Leu | Loss of DNA binding and transcriptional activation |
| AML-M2 | J2 | ND | 4 | 1001C > A | Nonsense codon at Tyr284 | ND |
| AML-M2 | F3820 | 46XY | 1 | 1-bp deletion of C381 | Frameshift at Phe77 | ND |
| AML-M2 | F3820 | 46XY | 4 | 24-bp duplication of nt 1062-1085 | Inframe duplication of Arg-Asn-ValGlu-Thr-Gln-Gln-Lys | Loss of DNA binding and transcriptional activation |
| AML-M4 | F3901 | 46XX d(9q22) | 4 | 3-bp deletion of nt 1083-1085 | Inframe deletion of Lys312 | Loss of DNA binding and transcriptional activation |
| AML-M4 | F4431 | 46XX | 2 | 551G > A | Silent | ND |
| MDS (RAEB-t) | F3881 | 46XY | 2 | 37-bp duplication of nt 483-519 | Frameshift at Ala134 | ND |
| MDS (RAEB-t) | F3881 | 46XY | 4 | 1015G > T | Nonsense codon at Glu290 | ND |
| Prostate | 202 | ND | 2 | 551G > A | Silent | ND |
| Lung (squamous) | 188 | ND | 4 | 1087G > A | Silent | ND |
RAEB-t indicates refractory anemia with excess of blasts in transformation; ND, not determined; bp, base pair; and nt, nucleotide. For other abbreviations, see Table 1.
Of 227 hematologic cancers, a total of 10 mutations were identified among 1 acute myelogenous leukemia (MDS) and 7 acute myelogenous leukemia (AML) patient samples (Table 3). The MDS sample, a refractory anemia with excess of blasts in transformation (RAEB-t), possessed biallelic mutations. Abnormally migrating bands for regions 2 and 4 (Figure 2A, lane 3) were observed. Sequence analysis revealed that one allele acquired a 37-bp duplication (nt 483 to 419) in region 2 that was predicted to result in a frameshift and truncation of the protein (Figure3). The other allele acquired a nonsense mutation (1015G>T) that would introduce a stop codon in region 4. Both of these mutations are predicted to encode C/EBPα proteins that lack the bZIP domain (Table 3). Also, biallelic mutations were detected in the AML-M2 sample F3820 (Table 3). Abnormally migrating bands for regions 1 and 4 were observed (Figure 2B, lane 3). Sequence analysis revealed that one allele acquired a deletion of the nucleotide C381 in region 1 that would produce a frameshift at amino acid Phe77 (Table 3). This allele would potentially generate a messenger RNA capable of synthesizing a shortened 30-kd form of C/EBPα, but not the 42-kd C/EBPα (Table 3). The second allele acquired a 24-bp duplication in region 4 (Figure 3) that would introduce an 8-amino acid inframe duplication within the first leucine zipper conserved among the bZIP family (Figure 4; Table 3).
PCR-SSCP analysis of the C/EBPα gene locus.
(A) Abnormally migrating bands in lane 3 of both panels for the MDS (RAEB-t) patient F3881. Region 2 is a 37-bp duplication and region 4 is a 1015G>T. The other lanes are derived from genomic DNA samples of other MDS patients and display bands with normal mobility. (B) Abnormally migrating bands in lane 3 of both panels for the AML-M2 patient F3820. Region 1 is a 1-bp deletion of C381, and region 4 is a 24-bp duplication of nt 1062 through 1085. The other lanes are derived from genomic DNA samples of other AML patients and display bands with normal mobility. (C) Abnormally migrating bands for region 4 are detected in lanes 1 (patient F3901), 10 (patient J2), 11 (patient J3), and 14 (patient J6). Lane 1 is a 3-bp deletion of nt 1083 through 1085; lane 10 is a transversion of 1001C>A; lane 11 is 15-bp duplication of nt 1083 through 1097; and lane 14 is a 3-bp duplication of nt 1083 through 1085. The other lanes are derived from genomic DNA samples of other AML patients and display bands of normal migration. The arrows at the left and right of each panel indicate the positions of the shifted bands.
PCR-SSCP analysis of the C/EBPα gene locus.
(A) Abnormally migrating bands in lane 3 of both panels for the MDS (RAEB-t) patient F3881. Region 2 is a 37-bp duplication and region 4 is a 1015G>T. The other lanes are derived from genomic DNA samples of other MDS patients and display bands with normal mobility. (B) Abnormally migrating bands in lane 3 of both panels for the AML-M2 patient F3820. Region 1 is a 1-bp deletion of C381, and region 4 is a 24-bp duplication of nt 1062 through 1085. The other lanes are derived from genomic DNA samples of other AML patients and display bands with normal mobility. (C) Abnormally migrating bands for region 4 are detected in lanes 1 (patient F3901), 10 (patient J2), 11 (patient J3), and 14 (patient J6). Lane 1 is a 3-bp deletion of nt 1083 through 1085; lane 10 is a transversion of 1001C>A; lane 11 is 15-bp duplication of nt 1083 through 1097; and lane 14 is a 3-bp duplication of nt 1083 through 1085. The other lanes are derived from genomic DNA samples of other AML patients and display bands of normal migration. The arrows at the left and right of each panel indicate the positions of the shifted bands.
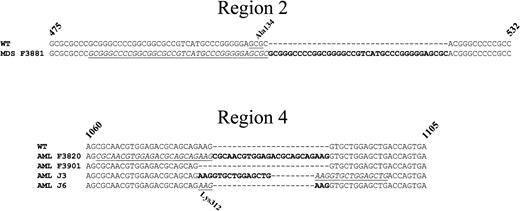
Sequences of the C/EBPα duplication and deletion mutants found in the MDS and AML patients.
Panel A represents an alignment of a portion of region 2 of the WT sequence of C/EBPα with the sequence from the MDS patient F3881. Panel B represents an alignment of a portion of region 4 of the WT sequence with the AML patient samples F3820 (24-bp duplication), F3901 (3-bp deletion), J3 (15-bp duplication), and J6 (3-bp duplication). The duplications are in bold text, and the duplicated regions are indicated by underlined italics. The codons for the alanine at position 134 (Ala134) in region 2 and the lysine at position 312 (Lys312) in region 4 are underlined and indicated above and below the nucleotide sequence, respectively.
Sequences of the C/EBPα duplication and deletion mutants found in the MDS and AML patients.
Panel A represents an alignment of a portion of region 2 of the WT sequence of C/EBPα with the sequence from the MDS patient F3881. Panel B represents an alignment of a portion of region 4 of the WT sequence with the AML patient samples F3820 (24-bp duplication), F3901 (3-bp deletion), J3 (15-bp duplication), and J6 (3-bp duplication). The duplications are in bold text, and the duplicated regions are indicated by underlined italics. The codons for the alanine at position 134 (Ala134) in region 2 and the lysine at position 312 (Lys312) in region 4 are underlined and indicated above and below the nucleotide sequence, respectively.
Predicted amino acid sequence comparison of AML region-4 mutations with WT C/EBPα and the family members C/EBPβ, C/EBPδ, and C/EBPε.
The predicted amino acid sequences from residues 272 through 354 for the AML region-4 mutants were aligned with that of WT human C/EBPα, C/EBPβ, C/EBPδ, and C/EBPε. The consensus sequence was derived by Vinson et al1 for 11 bZIP family members. The consensus basic (B) residues and leucine residues in each amino acid sequence are highlighted in bold text above the consensus sequence. The first conserved leucine is a threonine for C/EBPs. The duplicated amino acid residues for samples F3820, J3, and J6 are indicated by bold text. The Arg305Pro change in sample K-6 is indicated by the shaded box. The basic, fork, and leucine zipper regions are indicated above and below the alignments. The fork region is an invariant 7–amino acid residue spacer region between the first leucine in the zipper and the conserved basic region.1 The alignments were performed by means of AlignX (Vector NTI Suite v.6.0; Informax, Bethesda, MD).
Predicted amino acid sequence comparison of AML region-4 mutations with WT C/EBPα and the family members C/EBPβ, C/EBPδ, and C/EBPε.
The predicted amino acid sequences from residues 272 through 354 for the AML region-4 mutants were aligned with that of WT human C/EBPα, C/EBPβ, C/EBPδ, and C/EBPε. The consensus sequence was derived by Vinson et al1 for 11 bZIP family members. The consensus basic (B) residues and leucine residues in each amino acid sequence are highlighted in bold text above the consensus sequence. The first conserved leucine is a threonine for C/EBPs. The duplicated amino acid residues for samples F3820, J3, and J6 are indicated by bold text. The Arg305Pro change in sample K-6 is indicated by the shaded box. The basic, fork, and leucine zipper regions are indicated above and below the alignments. The fork region is an invariant 7–amino acid residue spacer region between the first leucine in the zipper and the conserved basic region.1 The alignments were performed by means of AlignX (Vector NTI Suite v.6.0; Informax, Bethesda, MD).
The AML patient samples F3901 (M4), J6 (M2), and J3 (M2) acquired a 3-bp deletion, 3-bp duplication, and 15-bp duplication, respectively, in region 4 (Figure 2C, lanes 1, 11, 14; Figure 3; Table3). These would generate either an inframe deletion (in sample F3901) or a duplication of 1 (J6) or 5 (J3) amino acids in the first leucine zipper (Figure 4). The AML sample J2 possessed a 1001C>A nonsense mutation that would replace the Tyr284 with a termination codon (Figure2C, lane 10; Table 3). The AML-M2 sample K-6 showed an abnormally migrating band in region 4 by SSCP (data not shown), and sequence analysis revealed a 1063G>C transversion. This missense mutation created an amino acid residue change of Arg305Pro in the fork region between the basic and the leucine zipper regions (Table 3; Figure 4). The AML-M4 sample F4431 possessed the same silent mutation 551G>A in region 2 as described for the prostate cancer (Table 3). Each of the AML-M2 samples lacked the t(8;21) translocation that produces an AML-ETO fusion (Table 3). From the PCR-SSCP analysis, it is difficult to conclude whether these samples possess a wildtype allele because potential contamination by normal cells with the subsequent amplification of the wildtype allele by PCR may occur. However, sample J6 lacks a wildtype pattern upon shorter exposure of the autoradiograph, suggesting it possesses only the mutant allele (Figure2C, lane 14, and data not shown). Also, in a cell line derived from sample K-6, a wildtype and mutant allele were identified, indicating that the mutation is heterozygous (H. Asou et al, submitted September 2001).
In addition to patient samples, 5 AML cell lines were examined for C/EBPα mutations. These were HL60, Kasumi-1 [M2, t(8;21)], Kasumi-5, KG-1, and NB-4 (M3). All cell lines were normal.
Wildtype and mutant C/EBPα proteins localize in the nucleus
Four of 7 mutations present in our samples involved inframe deletion or duplication of odd numbers of amino acid residues within the first 2 conserved leucines of the zipper domain (Figure 4). The remaining mutations would terminate or shift the translation reading frame and produce proteins lacking the bZIP domain. To determine the impact of the inframe alterations on the function of the C/EBPα protein, we cloned expressible complementary DNAs for the 3-bp deletion and the 15- and 24-bp duplications into the retrovirus vector MIG that allows coexpression of green fluorescent protein (GFP) with the gene of interest. Each vector was transiently transfected into NIH3T3 cells, and the nuclear localization was determined by immunofluorescent microscopy (Figure 5). Both the wildtype and mutant proteins localized in the nucleus. These results indicate that the mutations do not impair the protein localization of C/EBPα to its normal cellular compartment.
Locus of WT and mutant C/EBPα.
WT and mutant forms of C/EBPα localize in the nucleus. NIH3T3 cells were transfected with the MIG retrovirus constructs expressing the mutant samples F3901, J3, and F3820. The negative control cells were transfected with empty MIG, and the positive control cells were cotransfected with pCMV-C/EBPα42 and pEGFPc. The MIG retrovirus constructs express GFP along with the gene of interest, thereby allowing identification of transfected cells. All GFP+ cells, except for the empty vector control (MIG), showed nuclear staining for wildtype or mutant C/EBPα whereas all GFP− cells were negative for C/EBPα expression.
Locus of WT and mutant C/EBPα.
WT and mutant forms of C/EBPα localize in the nucleus. NIH3T3 cells were transfected with the MIG retrovirus constructs expressing the mutant samples F3901, J3, and F3820. The negative control cells were transfected with empty MIG, and the positive control cells were cotransfected with pCMV-C/EBPα42 and pEGFPc. The MIG retrovirus constructs express GFP along with the gene of interest, thereby allowing identification of transfected cells. All GFP+ cells, except for the empty vector control (MIG), showed nuclear staining for wildtype or mutant C/EBPα whereas all GFP− cells were negative for C/EBPα expression.
Deficient transcriptional activation by mutant C/EBPα proteins
The ability of these mutant proteins to activate transcription from the G-CSF receptor promoter hooked up to a luciferase reporter gene was tested (Figure 6). The pCMV-Sport1 expression vector was used to express the wildtype and mutant proteins in these experiments (Figure 6A). The wildtype C/EBPα protein activated the transcription 15-fold over the empty expression vector alone (Figure 6B). Each of the mutant proteins activated the reporter only 2-fold over the empty expression vector, indicating a greater than 80% reduction in transactivation function (Figure6B).
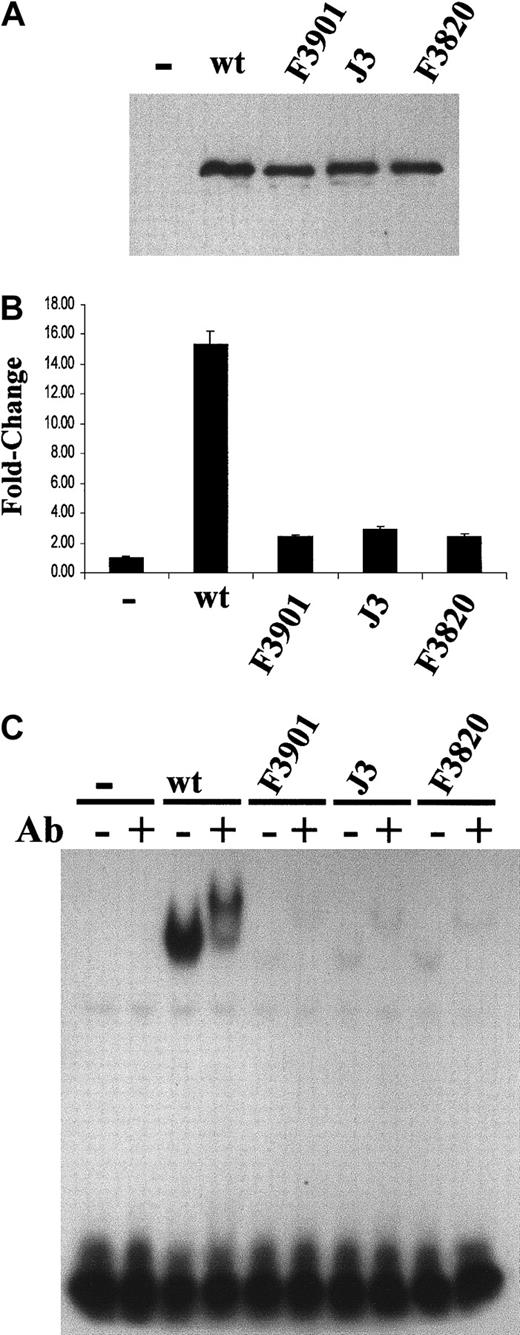
Transcriptional and DNA-binding activity in C/EBPα mutants.
C/EBPα mutants demonstrate severely impaired transcriptional and DNA-binding activity. (A) Western blot analysis of WT and mutant (F3901, J3, and F3820) C/EBPα proteins expressed in NIH3T3 cells from the pCMV-Sport1 vector. Similar expression levels for each protein were noted. (B) The pG-CSF receptor promoter–luciferase reporter construct (1 μg) was cotransfected with either empty (−), WT (50 ng), or mutant expression vector (100 ng). Relative firefly luciferase activity was measured and normalized to renilla luciferase activity. The fold change is indicated on the y-axis. The graph presents data from a duplicate experiment performed in triplicate. (C) Electrophoretic mobility shift assays (EMSAs) were performed with the use of the total cell lysates in panel A. An end-labled double-stranded oligonucleotide representing the C/EBP site in the G-CSF receptor promoter was incubated with the lysate in the absence (−) or presence (+) of 0.2 μg rabbit anti-C/EBPα antibody (Ab).
Transcriptional and DNA-binding activity in C/EBPα mutants.
C/EBPα mutants demonstrate severely impaired transcriptional and DNA-binding activity. (A) Western blot analysis of WT and mutant (F3901, J3, and F3820) C/EBPα proteins expressed in NIH3T3 cells from the pCMV-Sport1 vector. Similar expression levels for each protein were noted. (B) The pG-CSF receptor promoter–luciferase reporter construct (1 μg) was cotransfected with either empty (−), WT (50 ng), or mutant expression vector (100 ng). Relative firefly luciferase activity was measured and normalized to renilla luciferase activity. The fold change is indicated on the y-axis. The graph presents data from a duplicate experiment performed in triplicate. (C) Electrophoretic mobility shift assays (EMSAs) were performed with the use of the total cell lysates in panel A. An end-labled double-stranded oligonucleotide representing the C/EBP site in the G-CSF receptor promoter was incubated with the lysate in the absence (−) or presence (+) of 0.2 μg rabbit anti-C/EBPα antibody (Ab).
Deficiency in DNA binding of mutant C/EBPα proteins
One possible explanation for the loss of transactivation potential is the inability of the mutant proteins to bind their cognate site in the G-CSF receptor promoter. The DNA-binding activity of the mutant proteins was tested by electrophoretic mobility shift assay (EMSA) with the use of a double-stranded oligonucleotide containing the C/EBP site from the G-CSF receptor promoter and the total cell lysates from transiently transfected cells (Figure 6A). Although the expression of the wildtype and mutant proteins was similar, the wildtype C/EBPα showed significantly higher binding activity than the mutants (Figure6C, lanes 1, 3, 5, 7, 9). The bound DNA/protein complexes were supershifted with rabbit anti-C/EBPα antibody, verifying that the binding activity belonged to C/EBPα (Figure 6C, lanes 2, 4, 6, 8, 10). The mutants revealed a greater than 90% reduction in binding compared with the wildtype C/EBPα. These results indicate the mutations abrogated the DNA-binding function of C/EBPα.
Mutant forms of C/EBPα do not inhibit transcriptional activation by wildtype C/EBPα
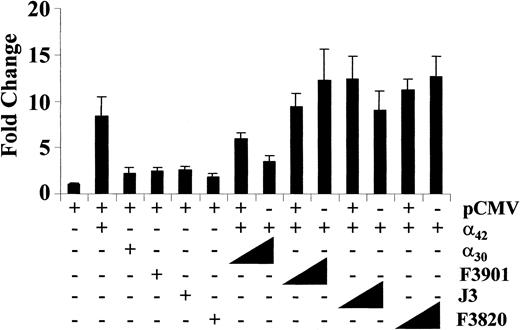
To determine if any of the mutant proteins could interfere with the transactivation function of wildtype C/EBPα, the wildtype protein was coexpressed with increasing amounts of each mutant (Figure7). The mutants were unable to activate transcription or interfere with the ability of the wildtype C/EBPα to activate transcription. These results suggest that the mutant C/EBPα proteins lost their transactivation potential and were unable to impair wildtype C/EBPα transcriptional activation of the G-CSF receptor promoter. As a positive control, the same transfections were performed with the 30-kd form of C/EBPα. This shortened isoform of C/EBPα attenuates transcriptional activation by C/EBPα42.42 It is potentially expressed in cells from patient F3820 from the other mutant allele (C381 deletion, Table 3). As expected, a dose-dependent reduction in C/EBPα42 transactivation was observed (Figure7).
Effect of C/EBPα mutants on transcriptional activation by wildtype C/EBPα.
C/EBPα mutants lack a dominant-negative effect on transcriptional activation by wildtype C/EBPα. NIH3T3 cells were transfected with empty expression vector (400 ng) or pCMV-C/EBPα42 (50 ng) with C/EBPα30 (100 ng) or with mutant samples F3901, J3, and F3820 (100 ng). To test for a dominant-negative effect, the cells were cotransfected with pCMV-C/EBPα42 (50 ng) and increasing amounts of the mutants (100 and 300 ng). Luciferase activities were measured and normalized and fold changes determined (y-axis).
Effect of C/EBPα mutants on transcriptional activation by wildtype C/EBPα.
C/EBPα mutants lack a dominant-negative effect on transcriptional activation by wildtype C/EBPα. NIH3T3 cells were transfected with empty expression vector (400 ng) or pCMV-C/EBPα42 (50 ng) with C/EBPα30 (100 ng) or with mutant samples F3901, J3, and F3820 (100 ng). To test for a dominant-negative effect, the cells were cotransfected with pCMV-C/EBPα42 (50 ng) and increasing amounts of the mutants (100 and 300 ng). Luciferase activities were measured and normalized and fold changes determined (y-axis).
Discussion
The growth-inhibitory and differentiation-promoting functions of C/EBPα make it an attractive candidate for a tumor suppressor. We tested this by screening the C/EBPα locus in 408 patient samples from 11 different malignancies. The only functionally significant mutations were found in 1 MDS and 6 AML (M2 and M4) patients (Table 3). These findings extend those of Pabst et al,43 who recently reported dominant-negative mutations in C/EBPα for AML-M1 and AML-M2 lacking the t(8;21) in a screen of 137 AML patient samples. We did not detect alterations in tumors derived from nonhematopoietic tissues that express C/EBPα. If abrogation of C/EBPα function is important for the development of these tumors, then it may occur via another mechanism.
The mutations in this report fall into 4 classes in regard to their effect on the predicted protein: (1) termination of translation by introducing a nonsense codon (samples J2 and F3881); (2) alteration of amino acid sequence by introducing a missense codon (K-6); (3) frameshift by either deletion or duplication of nucleotides and an eventual termination (F3820 and F3881); and (4) inframe deletion or duplication that removes or inserts additional amino acid residues (F3901, F3820, J3, and J6). The nonsense mutations in samples J2 (AML) and F3881 (MDS) would introduce termination codons before the bZIP domain. This would create polypeptides that are unable to localize to the nucleus, dimerize, and bind to DNA (Table 3).5,44 The mutation of Arg305Pro in patient K-6 occurs in the fork region of bZIP domain (Figure 4; Table 3). The inframe deletion and insertion mutations of patients F3901, F3820, J3, and J6 occur within the first conserved leucine finger (Figure 4; Table 3). Previous structure/function studies indicate that these mutations would abrogate C/EBPα function.1 45
Comparison of the predicted amino acid sequences of 11 bZIP family members reveals conservation between the basic and leucine zipper regions and the exact spatial register or phasing between these regions, referred to as the fork (Figure 4).1 The phasing is important for a continued α-helical structure that progresses from the zipper into the basic region.1,45,46 The basic region starts exactly 7 amino acid residues amino-terminal to the first leucine zipper.1 When the phasing between these regions is altered by insertion or deletion of 2–, 4–, 5–, or 6–amino acid residues, sequence-specific DNA-binding is eliminated although dimerization is reported to still occur via the leucine zippers.45,46 In contrast, a wide variety of mutants with insertions of an integral number of α-helical turns (7–amino acid residues) were functional.46 The inframe deletion and insertions identified in this study involve 1–, 5–, and 8–amino acid residues (Table 3; Figure 4). These are predicted to disrupt the conserved phasing of the bZIP domain. Consistent with this prediction, we demonstrated that these mutants were unable to activate transcription from the G-CSF receptor promoter because the proteins do not bind the C/EBP site in the promoter (Figure 6).
We hypothesized that these mutant forms would function in a dominant-negative fashion since they may heterodimerize with the wildtype as suggested by prior studies with similar mutations.45,46 These mutants did not attenuate transcriptional activation by wildtype C/EBPα (Figure 7). This may be due to a lack of heterodimerization between the wildtype and mutant forms of C/EBPα. The ability of these mutants to homodimerize and heterodimerize is currently under investigation. The data from this study are consistent with a model in which the mutations disrupt the dimerization interface formed by the leucine zipper and the proteins are unable to homodimerize or heterodimerize and bind to DNA. Of note, the previous studies were performed by cross-linking bacterially expressed and purified proteins whereas we expressed our mutant proteins in cell culture.45 46
The absence of helical-destabilizing residues, such as proline and glycine, in the bZIP domain in all family members is consistent with the hypothesis that phasing is critical for the bZIP domain.1 The Arg305Pro mutation found in K-6 is predicted to introduce a break in the α-helical structure of the fork region and disrupt the phasing of the bZIP domain (Table 3; Figure 4). Neither a homodimer nor a heterodimer formed with the wildtype polypeptide would bind to DNA. Preliminary studies indicate this mutant is unable to activate transcription, but the DNA-binding, dimerization, and dominant-negative properties are presently being investigated (data not shown).
Further studies are needed to determine how the different mutations contribute to the development of leukemia. For 2 patients, F3881 and F3820, the mutations were biallelic, and patient J6 appears to possess only the mutant allele, suggesting that loss of C/EBPα function is involved in leukemogenesis. Interestingly, patient F3820 carried a potentially dominant-negative mutation that would produce the p30 form of C/EBPα. This was the primary mutation reported by Pabst et al.43 Because the C/EBP family members heterodimerize with other bZIP family members, this dominant-negative function could extend beyond inhibition of wildtype C/EBPα to other C/EBP or CREB/ATF proteins. Our remaining patient samples with mutations appear to be heterozygous for the mutation, but we cannot rule out contamination of our samples by normal cells. This would result in the wildtype allele pattern and sequence in our SSCP and sequencing analyses. If the patients are heterozygous for the mutation, then the decreased expression of wildtype C/EBPα may be sufficient to contribute to leukemogenesis (haploinsufficiency). Alternatively, the mutants may have gained functions not yet identified in this study. Recent reports indicate that C/EBPα exerts effects inhibiting growth and gene expression independent of DNA-binding.47,48 The C/EBPα protein interacts with the p21 and CDK2 proteins, resulting in decreased CDK2 activity and inhibition of cell proliferation.47 The C/EBPα protein disrupts E2F complexes in several cell lines, and this correlates with C/EBPα-mediated growth arrest.49,50 Interaction of C/EBPα with E2F appears to be the mechanism by which it downregulates c-myc expression during granulocytic differentiation.48These protein-protein interactions appear to involve several regions of the C/EBPα protein, including the bZIP domain.47 Perhaps the duplication and deletion mutations in the bZIP domain may affect these interactions, thereby contributing to leukemogenesis.
The absence of mutations in the nonhematologic malignancies was surprising. In tissues such as liver and adipose, the lack of mutations could be due to the small size of our collections of hepatomas6 and liposarcomas.2 Further screening of these tumors may be of interest. In the case of our fairly extensive collection, one reason for the lack of mutations may be that other mechanisms are blocking C/EBPα function. A number of studies indicate that various oncogenic proteins appear to target the C/EBP family of proteins and abrogate their function. For example, some human myxoid liposarcomas have chromosomal translocations involving either translocation liposarcoma–C/EBP homologous protein (CHOP) or EWS (Ewing sarcoma)-CHOP.51-53 The fusion protein prevents adipocyte differentiation by directly interfering with C/EBPβ function that may regulate the levels of C/EBPα that in turn regulates the differentiation state of the cells.54 Muller et al55 reported that the E7 oncoprotein of human papillomavirus 16 overides C/EBPα-mediated proliferation arrest. Pabst et al43 reported in t(8;21) myeloid leukemias (AML-M2) that it appears the AML-ETO fusion protein suppresses C/EBPα expression indirectly by inhibiting positive autoregulation of the C/EBPα promoter. Lodie et al56 reported that the PML/RARα protein product resulting from the acute promyelocytic leukemia translocation t(15;17) physically interacts with C/EBPα. This interaction interferes with DNA binding and transcriptional activation by C/EBPα and, in turn, blocks granulocytic differentiation.56
In summary, we identified mutations of C/EBPα in AMLs of the M2 and M4 subtypes and an individual suffering from MDS (RAEB-t) that represents an early stage of leukemia. This report expands upon the identification of mutations in AML-M1 and AML-M2 samples.43 In addition, a wide range of other leukemias and solid tumor samples lacked C/EBPα mutations. Of interest, the previous report identified the majority of mutations resulting in the overexpression of the dominant-negative p30 isoform of C/EBPα; in contrast, a majority of our mutations affected the bZIP domain and did not appear to possess dominant-negative activity. The use of conditional, tissue-specific knockout murine models and overexpression of the identified mutant proteins will further clarify the role of C/EBPα in leukemogenesis.
We are grateful to Jonathan Said for generously providing soft-tissue sarcoma samples for analysis. We thank Alexey Chumakov for helpful discussions and advice.
Supported by National Institutes of Health grant CA26038-20, Joesph Troy Leukemia Fund, Horn Foundation, Lymphoma Research Foundation of America, and C. & H. Koeffler Fund. A.F.G. is a recipient of a Lymphoma Research Foundation of America Fellowship. W.K.H. is a recipient of a fellowship (HO2207/1-1) from the Deutsche Forschungsgemeinschaft. H.P.K. holds the Mark Goodson endowed chair for Cancer Research and is a member of the Jonsson Cancer Center.
A.F.G., W.-K. H., and S. K. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Adrian F. Gombart, Cedars-Sinai Medical Center, Division of Hematology/Oncology, UCLA School of Medicine, Davis Bldg 5065, 8700 Beverly Blvd, Los Angeles, CA 90048; e-mail:gombarta@csmc.edu.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal