While studying Ikaros proteins in childhood acute myeloid leukemia (AML), Ikaros isoform 6 (Ik6) expression was detected in 7 of 10 cases of M4 and M5 leukemia, but in none of the remaining French-American-British subtypes (M2, 8 cases; M7, 6 cases). The spliced Ikaros isoforms 4 to 8 (Ik4-8) suppress the function of full-length Ik1 or Ik2 in a dominant-negative manner, owing to their reduced numbers of DNA binding sites. Thus, dominant-negative Ikaros isoforms may inhibit the normal transcriptional regulation of hematopoietic cell development. To clarify the function of Ik6 in developing blood cells, this isoform was transiently transfected into an Ik2+, interleukin-3 (IL-3)–dependent 32D murine myeloid precursor cell line and studied the expression of Bcl-2 family proteins in relation to in vitro cell growth, using a tetracycline-inducible TREx system. The possibility of aberrant cell regulation due to Ikaros functional changes was examined by cotransfecting both Ik2 and Ik6 into Ikaros/Aiolos/Helios triple-negative Cos-7 cells. The results demonstrated IL-3–independent growth by Ik6-transfected 32D clones coincident with up-regulation of the antiapoptotic protein Bcl-XL. Up-regulation of Bcl-XL, but not of other Bcl-2 family proteins, was associated with the suppression of functional Ik2 by Ik6 in a dominant-negative fashion. Thus, the pathogenesis of myelomonocytic/monocytic AML may involve aberrant regulation of apoptosis due to unscheduled expression of the Ik6 isoform.
Introduction
The Ikaros proteins constitute one of the most important transcription factor families in lymphoid cell differentiation and proliferation.1-3 Their structures are similar in mice and humans, characterized by motifs of 4 DNA-binding zinc-finger domains in the N terminus and 2 zinc-finger domains for dimerization in the C terminus.4-7 By alternative splicing, 8 kinds of isoforms (Ik1-8) have been identified, 2 of which, Ik1 and Ik2, are considered to be functional.4 5 Because Ik4-8 lack 2 or more N-terminal zinc-finger domains, any one of these isoforms can be regarded as a functionally reduced dimer by comparison to Ik1 or Ik2.
Studies of mutant mice heterozygous for the Ikaros gene indicated that loss of normal Ikaros function can contribute to the development of lymphoid malignancies8; moreover, dominant-negative Ikaros isoforms have been reported in cases of infant and childhood acute lymphoblastic leukemia (ALL),9-12 as well as blasterisis of Philadelphia chromosome–positive (Ph+) chronic myeloid leukemia (CML).13Although the precise mechanism is unknown, Ikaros proteins appear to regulate many factors controlling hematopoietic cell function, including differentiation, not only in the lymphoid lineage but in the myeloid lineage as well.14 Taken together, these reports implicate dominant-negative Ikaros isoforms in the pathogenesis of both lymphoid and myeloid leukemias.
Because of the paucity of data on Ikaros involvement in acute myeloid leukemia (AML), we studied the pattern of Ikaros isoform expression in 24 pediatric cases of this disease. Although the leukemic cells from most patients expressed only Ik1 and 2, or only Ik2 alone, Ik6 expression was detected in 7 of 10 patients with acute myelomonocytic or monocytic (French-American-British [FAB] M4/M5) leukemia, usually in the absence of other Ikaros isoforms. Further analyses, in which an Ik2+ interleukin-3 (IL-3)–dependent 32D murine cell line was transfected with Ik6 and Ikaros/Aiolos/Helios triple-negative Cos-7 cells with both Ik2 and Ik6, suggested the latter isoform contributes to leukemogenesis by suppressing Ik2 function, permitting up-regulation of the Bcl-XL antiapoptotic proteins.
Patients, materials, and methods
Patients and controls
Expression patterns of Ikaros isoforms were studied in bone marrow (n = 23) and peripheral blood (n = 1) samples from 24 pediatric patients with active AML. The clinical and laboratory features of the patients are summarized in Table1. Bone marrow (n = 2) and peripheral blood (n = 15) samples from healthy children and adults, obtained with informed consent, served as normal controls. Informed consent was provided according to the Declaration of Helsinki.
Presenting clinical data and Ikaros isoform expression in children with AML
| Case . | Age . | Sex . | WBC count onset (per ML) . | Percentage of blasts . | FAB . | Karyotype . | Positive immunologic markers . | Ikaros isoforms expressed* . |
|---|---|---|---|---|---|---|---|---|
| 1 | 5 y 6 mo | F | 5 600 | 58 | M2 | 45,X t(8;13)(q22;q34) | CD13, 56, HL-DR | Ik2 |
| 2 | 5 y 10 mo | F | 19 100 | 44 | M2 | 46,XX, t(8;21) | CD7, 19, 34, HL-DR | Ik2 |
| 3 | 13 y 6 mo | F | 19 400 | 84 | M2 | 46,XX, t(8;21) | CD2, 13, 14, 15, 33, 11b | Ik1, 2 |
| 4 | 6 y 6 mo | F | 9 500 | 40 | M2 | 46,XX, t(8;21) | CD7, 19, 33, 34 | Ik1, 2 |
| 5 | 9 y 1 mo | F | 15 400 | 36 | M2 | 46,XX, t(8;21) | CD13, 19, 33, 34, HL-DR | Ik1, 2 |
| 6 | 8 y | M | 10 100 | 67 | M2 | 45,X, −Y, t(8;21)(q22;q22) | CD15, 33, 34, HL-DR | Ik1, 2 |
| 7 | 7 y 4 mo | F | 6 200 | 26 | M2 | 45, X, −X, t(8;21) | CD19, 15, 34, HL-DR | Ik2 |
| 8 | 4 y | M | 7 300 | 29 | M2 | 46,XY, t(8;21)(q22;q22) | CD13, 33, 34, 11b | Ik1, 2 |
| 9 | 7 mo | F | 278 000 | 47 | M4 | 46,XX | CD13, 14, 15, 33, 11b | Ik2, 6 |
| 10 | 13 y 6 mo | F | 4 000 | 53 | M4 | 46,XX | CD7, 15, 33, 11b | Ik6 |
| 11 | 10 y 1 mo | M | 164 700 | 91 | M4 | 46,XY, inv(16)(p13;q22) | CD13, 15, 33, 34 | Ik6 |
| 12 | 10 y 1 mo | F | 174 700 | 81 | M4 | 46,XX,inv(9)(p11;q13), inv(6)(p13;q22) | CD2, 19, 33, 34, 11b | Ik6 |
| 13 | 14 y | M | 8 000 | 78 | M4 | 47,Y,add(X)(p22),add(3)(p25),−17, others | CD4, 7, 13, 15, 33, HL-DR | Ik2 |
| 14 | 10 y 1 mo | M | 95 900 | 93 | M4 | 46,XY | CD4, 15, 33, 34, 11b | Ik1, 2 |
| 15 | 4 y | F | 2 900 | 79 | M5a | 50,XXX, +6, +8, +18, del(11)(q21;q23) | CD5, 56 | Ik1, 2, 6 |
| 16 | 2 y 1 mo | M | 20 000 | NA | M5a | 48,XY, +8, +9, t(9;11)(p22;q23) | CD4, 15, 33 | Ik1, 2 |
| 17 | 14 y 2 mo | F | 16 800 | 64 | M5b | 47,XX, +21 | CD7, 13, 34, 38, HL-DR | Ik2, 6 |
| 18 | 13 y 6 mo | F | 900 | 7 | M5b | 47,XX, +8, t(9;11)(p22;q23) | NE | Ik6 |
| 19 | 11 mo | F | 25 000 | 50 | M7 | 46,XX | CD19, 33, 34, 41a, 41b, 42a | Ik1, 2 |
| 20 | 1 y 7 mo | M | 30 800 | 35 | M7 | 46,XY, t(6;22;17)(p23,q11,q12) | CD10, 11b | Ik1, 2 |
| 21 | 2 y 2 mo | M | 3 500 | 83 | M7 | 47,Y, −X, t(4;11)(q21,q23) | CD56, PPO | Ik1, 2 |
| 22 | 10 mo | M | 20 200 | 48 | M7 | 48, XY, +8, +19 | CD4, 33, 41a, 41b, 42a | Ik1, 2 |
| 23 | 1 y 9 mo | M | 7 800 | 40 | M7 | NE | CD2, 7, 13, 38, 11b | Ik1, 2 |
| 24 | 2 y | M | 15 800 | 38 | M7 | 46,XY, t(16;21) | CD13, 34, HL-DR, 11c | Ik1, 2 |
| Case . | Age . | Sex . | WBC count onset (per ML) . | Percentage of blasts . | FAB . | Karyotype . | Positive immunologic markers . | Ikaros isoforms expressed* . |
|---|---|---|---|---|---|---|---|---|
| 1 | 5 y 6 mo | F | 5 600 | 58 | M2 | 45,X t(8;13)(q22;q34) | CD13, 56, HL-DR | Ik2 |
| 2 | 5 y 10 mo | F | 19 100 | 44 | M2 | 46,XX, t(8;21) | CD7, 19, 34, HL-DR | Ik2 |
| 3 | 13 y 6 mo | F | 19 400 | 84 | M2 | 46,XX, t(8;21) | CD2, 13, 14, 15, 33, 11b | Ik1, 2 |
| 4 | 6 y 6 mo | F | 9 500 | 40 | M2 | 46,XX, t(8;21) | CD7, 19, 33, 34 | Ik1, 2 |
| 5 | 9 y 1 mo | F | 15 400 | 36 | M2 | 46,XX, t(8;21) | CD13, 19, 33, 34, HL-DR | Ik1, 2 |
| 6 | 8 y | M | 10 100 | 67 | M2 | 45,X, −Y, t(8;21)(q22;q22) | CD15, 33, 34, HL-DR | Ik1, 2 |
| 7 | 7 y 4 mo | F | 6 200 | 26 | M2 | 45, X, −X, t(8;21) | CD19, 15, 34, HL-DR | Ik2 |
| 8 | 4 y | M | 7 300 | 29 | M2 | 46,XY, t(8;21)(q22;q22) | CD13, 33, 34, 11b | Ik1, 2 |
| 9 | 7 mo | F | 278 000 | 47 | M4 | 46,XX | CD13, 14, 15, 33, 11b | Ik2, 6 |
| 10 | 13 y 6 mo | F | 4 000 | 53 | M4 | 46,XX | CD7, 15, 33, 11b | Ik6 |
| 11 | 10 y 1 mo | M | 164 700 | 91 | M4 | 46,XY, inv(16)(p13;q22) | CD13, 15, 33, 34 | Ik6 |
| 12 | 10 y 1 mo | F | 174 700 | 81 | M4 | 46,XX,inv(9)(p11;q13), inv(6)(p13;q22) | CD2, 19, 33, 34, 11b | Ik6 |
| 13 | 14 y | M | 8 000 | 78 | M4 | 47,Y,add(X)(p22),add(3)(p25),−17, others | CD4, 7, 13, 15, 33, HL-DR | Ik2 |
| 14 | 10 y 1 mo | M | 95 900 | 93 | M4 | 46,XY | CD4, 15, 33, 34, 11b | Ik1, 2 |
| 15 | 4 y | F | 2 900 | 79 | M5a | 50,XXX, +6, +8, +18, del(11)(q21;q23) | CD5, 56 | Ik1, 2, 6 |
| 16 | 2 y 1 mo | M | 20 000 | NA | M5a | 48,XY, +8, +9, t(9;11)(p22;q23) | CD4, 15, 33 | Ik1, 2 |
| 17 | 14 y 2 mo | F | 16 800 | 64 | M5b | 47,XX, +21 | CD7, 13, 34, 38, HL-DR | Ik2, 6 |
| 18 | 13 y 6 mo | F | 900 | 7 | M5b | 47,XX, +8, t(9;11)(p22;q23) | NE | Ik6 |
| 19 | 11 mo | F | 25 000 | 50 | M7 | 46,XX | CD19, 33, 34, 41a, 41b, 42a | Ik1, 2 |
| 20 | 1 y 7 mo | M | 30 800 | 35 | M7 | 46,XY, t(6;22;17)(p23,q11,q12) | CD10, 11b | Ik1, 2 |
| 21 | 2 y 2 mo | M | 3 500 | 83 | M7 | 47,Y, −X, t(4;11)(q21,q23) | CD56, PPO | Ik1, 2 |
| 22 | 10 mo | M | 20 200 | 48 | M7 | 48, XY, +8, +19 | CD4, 33, 41a, 41b, 42a | Ik1, 2 |
| 23 | 1 y 9 mo | M | 7 800 | 40 | M7 | NE | CD2, 7, 13, 38, 11b | Ik1, 2 |
| 24 | 2 y | M | 15 800 | 38 | M7 | 46,XY, t(16;21) | CD13, 34, HL-DR, 11c | Ik1, 2 |
NA indicates not available; NE, not examined.
Determined by RT-PCR.
Cells and culture
The 32D cells, a murine IL-3–dependent myeloid cell line, were cultured in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 10% 10T1/2 conditioning medium (source of IL-3; a generous gift from Dr Inaba, University of Hiroshima, Japan), and Cos-7 cells were cultured in RPMI 1640 and 10% FBS.
RNA extraction and reverse transcription–polymerase chain reaction
RNA was extracted with Trizol (Gibco BRL, Paisley, United Kingdom) reagent according to the manufacturer's specifications. The expression of Ikaros isoforms was examined by reverse transcription–polymerase chain reaction (RT-PCR) analysis with the following primers: F1 5′-ATGGATGCTGACGAGGGTCAAGAC-3′, and R1 5′-TTAGCTCATGTGGAAGCGGTGCTC-3′ for full-length Ikaros isoforms (Ik1-8). The primer sequences for nested PCR were Ex2f 5′-CCCCTGTAAGCGATACTCCAGATG-3′ and Ex7r 5′-GATGGCTTGGTCCATCACGTGGGA-3′.
Sequence analysis
Amplified PCR products were cloned and sequenced as previously described.15
Preparation of full-length Ik2 and Ik6 RT-PCR products
The full-length PCR product of Ik2 was obtained from normal bone marrow cells, whereas that of Ik6 came from the leukemic cells at acute crisis phase of Ph+ patients with CML.
Expression vectors
The amplified products (Ik2 and Ik6), confirmed by sequence analysis, were cloned in the following expression vectors: pRC/CMV for Ik2 (pRC/CMV/Ik2) and pcDNA6/TO for Ik6 (pcDNA6/TO/Ik6) (tetracycline-inducible TREx-system).
Transient transfection and regulation of Ik genes
The gene-encoding IK6 gene was transiently transfected into 32D cells by electroporation (Bio-Rad Gene Pulser, Bio-Rad Laboratories, Richmond, VA). The 32D/TREx/Ik6 cell lines were prepared by cotransfection first with pcDNA6/TR and then with pcDNA4/TO/Ik6, with the former regulating the expression of Ik6 ligated into the latter in the presence or absence of tetracycline. After 48 hours of pcDNA6/TR transfection, the cells were subjected to selection in an IL3-containing standard medium with Blastcydine (Invitrogen, Carlsbad, CA). Within 2 weeks, the surviving cells were subjected to electroporation with 50 μg pcDNA4/TO/Ik6 plasmids. After 48 hours, the Ik6-expressing cells were selected in an IL-3–containing medium with Zeocin (Invitrogen), and within 3 weeks, the surviving cells were analyzed.
Because Ikaros proteins are known to interact with other members of the Ikaros family, such as Aiolos and Helios, we chose the Cos-7 cell line, which does not express either Ikaros or Aiolos or Helios, for studies of apoptosis. After confirming the lack of expression of these proteins in Cos-7 cells, we transiently transfected the cells by a Fugene lipofection method using the TREx system, first with pRC/CMV/Ik2, and then, after selection in the presence of G418, with both pcDNA6/TR and pcDNA4/TO/Ik6.
As negative controls, we used cells transfected with empty vectors (32D/TREx/empty or Cos-7/TREx/empty).
Ikaros protein expression in 32D, 32D/TREx/Ik6, Cos-7, and Cos-7/pRC/CMV/Ik2/TREx/Ik6 clones
First, we confirmed that the 32D cells expressed Ik2 alone, whereas the Cos-7 cells did not express any Ikaros isoforms. Expressions of Ik2 and Ik6 in each of the established clones (32D/TREx/Ik6 and Cos-7/pRC/CMV/Ik2/TREx/Ik6) were determined by RT-PCR, which were further confirmed by the nucleotide sequences using TA ligation system.
Western blot analysis
Whole cell (1 × 106) lysates prepared in sample lysis buffer (150 mM, 1.0% Nonidet P-40, 5 mM EDTA, 50 mM Tris-HCl, pH 7.5) were analyzed by Western blotting, as follows. The samples were loaded onto a 7.5% Mini-tall sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel, and the size-fractionated proteins were transferred to a polymembrane (Millipore, Bedford, MA), which was blocked in phosphate-buffered saline (PBS) with 0.5% Triton-X 100 (PBS-T) and 5% milk for 1 hour at room temperature. To detect Ikaros expression, we mixed the specimens for reaction of all 8 isoforms with anti-Ikaros antibodies (1:2000 dilution; generous gift from Dr K. Georgopoulos) in PBS overnight, using an enhanced chemiluminescence system (Amersham Pharmacia Biotech, Uppsala, Sweden). The Bcl-2 family proteins (Bcl-2, Bcl-XL, Bad, Bak, Bax, and Mcl-1) and STAT5, one of the up-regulation factors of Bcl-XL, were studied by using monoclonal or polyclonal antibodies known to recognize those proteins.
Subcellular localization studies
The detection of subcellular localization of Ikaros proteins was determined by immunofluorescence and fluorescence-laser scanning microscopy as previously described.9
Apoptosis assay by annexin-V FACS analysis
Apoptosis in 32D/TREx/Ik6 clones and a control 32D/TREx/empty was assessed by annexin-V FACS analysis according to the manufacturer's instructions (Takara Shuzo, Shiga, Japan). Cells were cultured in IL-3–deprived culture medium with or without tetracycline for 36 hours. Apoptosis was then determined for each of the 3 clones at 12, 24, and 36 hours after deprivation of IL-3 with or without tetracycline.
Overexpression of Bcl-XL in 32D cells
Based on the IL-3–independent growth in 32D cells with Ik6 expression, 32D cells with overexpression of Bcl-XL were analyzed by using retrovirus infection with pLNCX vectors. The complementary DNA (cDNA) for mouse Bcl-XL was produced by performing RT-PCR on the total RNA of 32D cells. The integrity of the insert was confirmed by sequencing.
Detection of Bcl-XL expression in AML cells
Using clinical samples of M4 (n = 3), M5 (n = 2), other types of AML (n = 14), and normal controls (n = 2), RT-PCR analysis was performed for Bcl-XL detection. Data showed no differences in the expression of Bcl-XL among these clinical samples.
Results
Ik2 and Ik6 expression in AML cells
Using an RT-PCR method, we detected expression of both the Ik1 and Ik2 isoforms in 13 cases of childhood AML; Ik2 alone in 4; Ik1, 2, and 6 in 1; Ik2 and Ik6 in 2; and Ik6 alone in 4 (Table 1). Overall, Ik6 expression was noted in 7 (29.2%) of the 24 AML cases, comprising M4 (4 of 6) and M5 (3 of 4) leukemias but none of the remaining subtypes (Figure 1A). These findings were confirmed by Western blot analysis in cases for which sufficient numbers of cells were available (Figure 1B). We detected the functional Ik1 and Ik2 but not Ik6 in normal controls.
Pattern of Ikaros proteins expression in AML cases.
(A) In the nested RT-PCR analysis, M indicates the 100-base pair (bp) ladder marker; lanes 1 to 7 represent FAB M4, M5a, and M5b cases; lanes 8 to 10; M2 cases; lanes 11 to 13; M7 cases. N1 and N2 indicate normal controls. The sizes of the RT-PCR products were Ik1, 907 bp; Ik2, 646 bp; and Ik6, 220 bp. (B) In Western blot analysis, the arrows indicate the positions of Ikaros isoforms; molecular mass Ikaros isoform 6 standards are included on the left. Case numbers are the same as in Table 1.
Pattern of Ikaros proteins expression in AML cases.
(A) In the nested RT-PCR analysis, M indicates the 100-base pair (bp) ladder marker; lanes 1 to 7 represent FAB M4, M5a, and M5b cases; lanes 8 to 10; M2 cases; lanes 11 to 13; M7 cases. N1 and N2 indicate normal controls. The sizes of the RT-PCR products were Ik1, 907 bp; Ik2, 646 bp; and Ik6, 220 bp. (B) In Western blot analysis, the arrows indicate the positions of Ikaros isoforms; molecular mass Ikaros isoform 6 standards are included on the left. Case numbers are the same as in Table 1.
Growth of Ik6-transfected 32D clones
Three 32D/TREx/Ik6 clones (no. 7, 11, and 14) were obtained after cotransfection of 32D cells with pcDNA4/TR and pcDNA6/TO/Ik6. After selection in Blastcydine- and Zeocin-containing culture medium, cells from these 3 clones and a control 32D/TREx/empty were cultured in IL-3–deprived medium with or without tetracycline (final concentration, 1μg/mL) for 5 days and the viable cells were counted by trypan blue dye exclusion. All 3 clones were able to grow in the presence of tetracycline (Ik6 on) despite the absence of IL-3, but showed greatly reduced viability when tetracycline was withhold (Ik6 off; Figure 2A). The 32D/TREx/empty failed to grow in the absence of IL-3, whether or not tetracycline was added. These findings suggested that all three 32D/TREx/Ik6 clones acquired the IL-3 growth independence through the induction of Ik6. As shown in Figure 2B, Ik2 was consistently detected by Western blot at 0 and 24 hours, whereas Ik6 induction was seen only at 24 hours in the tetracycline-stimulated culture.
Cell growth following induction of Ik6.
Growth of cells from 32D/TREx/Ik6 clones (clone 7, 11, and 14) and 32D/TREx/empty. Numbers of viable cells were determined by trypan blue dye exclusion. All 3 Ik6 clones grew well in an IL-3–deprived culture medium in the presence of tetracycline (closed symbols; Ik6 on), but not in its absence (open symbols; Ik6 off). No growth of the empty clone was noted in either medium. (B) Western blot analysis before (0 hour) and after (24 hours) induction of Ik6 in cells from 32D/TREx/Ik6 clone 11. Note Ik6 induction in cells cultured for 24 hours in IL-3–deprived medium with tetracycline (on).
Cell growth following induction of Ik6.
Growth of cells from 32D/TREx/Ik6 clones (clone 7, 11, and 14) and 32D/TREx/empty. Numbers of viable cells were determined by trypan blue dye exclusion. All 3 Ik6 clones grew well in an IL-3–deprived culture medium in the presence of tetracycline (closed symbols; Ik6 on), but not in its absence (open symbols; Ik6 off). No growth of the empty clone was noted in either medium. (B) Western blot analysis before (0 hour) and after (24 hours) induction of Ik6 in cells from 32D/TREx/Ik6 clone 11. Note Ik6 induction in cells cultured for 24 hours in IL-3–deprived medium with tetracycline (on).
Bcl-2 family protein expression in 32D/TREx/Ik6 clones and Cos-7/pRC/CMV/Ik2/TREx/Ik6
Because of the recognized association between up-regulated cell growth and the loss of normal apoptotic controls, we investigated the effects of Ik6 induction on expression of Bcl-2, Bcl-XL, Bad, Bak, Bax, Mcl-1, and STAT5 in our three 32D/TREx/Ik6 clones and a control 32D/TREx/empty. Representative results (clone 11, Figure3) clearly demonstrated up-regulation of Bcl-XL at 24 hours after induction of Ik6, with levels of other Bcl-2 family proteins remaining unchanged. (Bad was not detected in 32D cells.) To determine if Bcl-XL up-regulation is related to the dominant-negative effect of Ik6 on Ik2, we examined Cos-7 cells expressing either Ik2 or Ik6 or both isoforms induced in the TREx system with or without tetracycline. The expression of Bcl-XL was up-regulated after induction of Ik6, whereas that of other Bcl-2 family proteins and STAT5 remained stable (Figure4). Levels of Bcl-2 family proteins were unchanged in 32D/TREx/empty cells cultured in IL-3–deprived medium with and without tetracycline, indicating that tetracycline by itself is not associated with the stimulation of Bcl-XL. Comparing of densitometric assay results (Figure 5) for both the 32D/TREx/Ik6 and Cos-7/pRC/CMV/Ik2/TREx/Ik6 clones confirmed the above qualitative findings. Thus, the Bcl-XL protein affords an attractive target through which Ik2 and Ik6 might regulate apoptosis in hematopoietic cells.
Western blot analysis of Bcl-2, Bcl-XL, and STAT5 expression in 32D/TREx/Ik6 clone 11 growing in IL-3–deprived medium with or without tetracycline.
Only Bcl-XL was up-regulated. Other Bcl-2 family proteins (Bak, Bax, and Mcl-1) were unaffected by Ik6 induction (data not shown). Bad, another Bcl-2 family protein, could not be detected in the 32D cell line. On indicates with tetracycline; off, without tetracycline.
Western blot analysis of Bcl-2, Bcl-XL, and STAT5 expression in 32D/TREx/Ik6 clone 11 growing in IL-3–deprived medium with or without tetracycline.
Only Bcl-XL was up-regulated. Other Bcl-2 family proteins (Bak, Bax, and Mcl-1) were unaffected by Ik6 induction (data not shown). Bad, another Bcl-2 family protein, could not be detected in the 32D cell line. On indicates with tetracycline; off, without tetracycline.
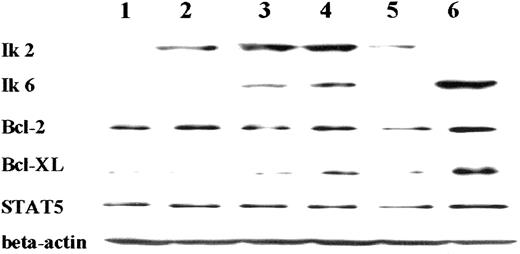
Western blot analysis of Ik2, Ik6, Bcl-2, Bcl-XL, and STAT5 expression in a Cos-7/pRC/CMV/Ik2/TREx/Ik6 clone.
Expression was tested before (lane 2) and at 12 hours (lane 3), 16 hours (lane 4), and 24 hours (lane 5) after Ik6 induction. Lane 1 (Cos-7/TREx/empty) served as the negative control for Ik2 and 6, whereas lane 6 (Cos-7/pRC/CMV/Ik6) was the positive control for Ik6. Bcl-XL was up-regulated after induction of Ik6 (lanes 3, 4, and 5).
Western blot analysis of Ik2, Ik6, Bcl-2, Bcl-XL, and STAT5 expression in a Cos-7/pRC/CMV/Ik2/TREx/Ik6 clone.
Expression was tested before (lane 2) and at 12 hours (lane 3), 16 hours (lane 4), and 24 hours (lane 5) after Ik6 induction. Lane 1 (Cos-7/TREx/empty) served as the negative control for Ik2 and 6, whereas lane 6 (Cos-7/pRC/CMV/Ik6) was the positive control for Ik6. Bcl-XL was up-regulated after induction of Ik6 (lanes 3, 4, and 5).
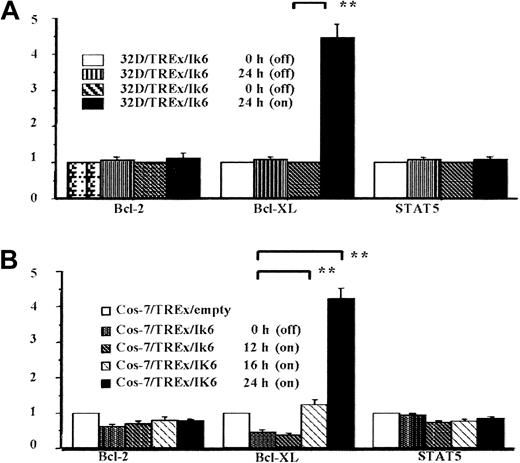
Semiquantification of Bcl-2, Bcl-XL, and STAT5 expression with a densitometric assay based on levels of β-actin expression.
(A) Summary of data for three 32D/TREx/Ik6 clones. Note the more than 4-fold increase in Bcl-XL expression at 24 hours of incubation in the presence of tetracycline (**P < .01). (B) Summary of data for 2 Cos-7/pRC/CMV/Ik2/TREx/Ik6 clones. The increases in Bcl-XL expression at 16 and 24 hours of incubation significantly exceeded the baseline value (0 hour; **P < .001 for both comparisons). Measurements are presented as mean with SEs (bars).
Semiquantification of Bcl-2, Bcl-XL, and STAT5 expression with a densitometric assay based on levels of β-actin expression.
(A) Summary of data for three 32D/TREx/Ik6 clones. Note the more than 4-fold increase in Bcl-XL expression at 24 hours of incubation in the presence of tetracycline (**P < .01). (B) Summary of data for 2 Cos-7/pRC/CMV/Ik2/TREx/Ik6 clones. The increases in Bcl-XL expression at 16 and 24 hours of incubation significantly exceeded the baseline value (0 hour; **P < .001 for both comparisons). Measurements are presented as mean with SEs (bars).
Subcellular localization of Ik2 and Ik6 proteins
The Ik2 protein expressed by the 32D/TREx/Ik6 clones was localized in chromatin when the cells were cultured without tetracycline. However, in the clones in which Ik6 was induced, both Ik2 and Ik6 proteins were localized in the cytoplasm. These results are consistent with previous observations (Figure6).
Annexin-V/fluorescein isothiocyanate FACS analysis of apoptotic cell death.
Representative data for 32D/TREx/Ik6 clone 11 are shown. In 24-hour culture in IL-3–deprived medium, apoptosis occurred in about 6.1% of the cells (A) when tetracycline was present, compared with 25.7% (B) when it was absent (P = .032). At 36 hours of culture, only about 20% of the cells cultured in the presence of tetracycline were necrotic or apoptotic (C), contrasted with about 60% of those growing in the absence of tetracycline (D) (P = .028). PI indicates propidium iodide.
Annexin-V/fluorescein isothiocyanate FACS analysis of apoptotic cell death.
Representative data for 32D/TREx/Ik6 clone 11 are shown. In 24-hour culture in IL-3–deprived medium, apoptosis occurred in about 6.1% of the cells (A) when tetracycline was present, compared with 25.7% (B) when it was absent (P = .032). At 36 hours of culture, only about 20% of the cells cultured in the presence of tetracycline were necrotic or apoptotic (C), contrasted with about 60% of those growing in the absence of tetracycline (D) (P = .028). PI indicates propidium iodide.
Apoptosis assay in 32D/TREx/Ik6 clones and in 32D cells with overexpression of Bcl-XL
As shown in Figure 7, significantly higher proportions of 32D/TREx/Ik6 cells underwent apoptosis in IL-3–deprived culture medium without tetracycline, as compared with findings in the presence of tetracycline at 24 and 36 hours of culture (P = .032 and .028, respectively). The growth of 32D cells with overexpression of Bcl-XL was inhibited under the IL-3–deprived culture condition; however, their growth was IL-3 dependent, not like that in 32D/TREx/IK6 cells (Figure 8). These results show that up-regulation of Bcl-XL led to the suppression of the apoptosis in 32D cells under the IL-3–deprived condition, but was not enough to show sufficient IL-3–independent growth.
Subcellular localization of Ik2 and Ik6 proteins.
Ik2 was localized in the chromatin structure of 32D cells expressing wild-type Ikaros proteins (A) and overexpressed IK2 (B), whereas both Ik2 and Ik6 were localized in the cytoplasm of 32D cells expressing Ik6 alone.
Subcellular localization of Ik2 and Ik6 proteins.
Ik2 was localized in the chromatin structure of 32D cells expressing wild-type Ikaros proteins (A) and overexpressed IK2 (B), whereas both Ik2 and Ik6 were localized in the cytoplasm of 32D cells expressing Ik6 alone.
Growth curve of 32D cells with and without Bcl-XL overexpression under the IL-3–deprived medium condition.
Growth of 32D cells with the overexpression of Bcl-XL was less inhibited, compared with that without the overexpression of Bcl-XL. However, their growth was IL-3 dependent. Y-axis, ratio of survive cells.
Growth curve of 32D cells with and without Bcl-XL overexpression under the IL-3–deprived medium condition.
Growth of 32D cells with the overexpression of Bcl-XL was less inhibited, compared with that without the overexpression of Bcl-XL. However, their growth was IL-3 dependent. Y-axis, ratio of survive cells.
Discussion
The lack of Ikaros proteins in the hematopoietic system causes an early and complete block in the production of B and T lymphocytes.6 Thus, such proteins must play a critical regulatory role in the differentiation of hematopoietic cells. Intensive study of Ikaros proteins in human leukemic cells has indicated a close correlation between the reduced function of Ik1 or Ik2 and the clonal expansion of hematopoietic cells leading to overt malignancies.8 In fact, Sun et al6,9-11detected several dominant-negative Ikaros isoforms (Ik4, 7, 8, and their mutants) in many cases of ALL, including those in infants, whereas other investigators have reported partially conflicting data.12-16 The frequency of Ik6 expression was particularly high in 2 Japanese studies, specifically, 9 of 17 cases of CML in lymphoid crisis and 8 of 14 cases of Ph+ALL.13 The available data strongly suggest that Ik6 may be involved in the commitment of hematopoietic progenitors to lymphoid lineage, providing a link to Ph+ leukemias. On the other hand, some myeloid lineage cells have been noted to express Ikaros isoforms Ik1, 2, and 6 in mature neutrophils and Ik6 in peripheral blood myeloid cells.5 Results of the present study extend these findings to childhood AML, where 7 of 10 cases of myelomonocytic or monocytic leukemia expressed Ik6. Among reports of STAT5 regulation of gene expression during myeloid differentiation, the one that interested us most showed an association between Bcl-XL expression and the differentiation and survival of monocytes/macrophages.17 Thus, by regulating the antiapoptotic Bcl-XL protein, Ik6 could play an exclusive role in monocyte/macrophage differentiation. In particular, its abnormal expression in primitive myeloid cells could up-regulate Bcl-XL, providing a mechanistic explanation for the Ik6+ M4/M5 leukemias reported here. Although Ikaros isoforms have been shown to participate in the differentiation and proliferation of hematopoietic cells,18-21 the precise mechanisms of this involvement remain unclear. Recently, Ik7 was suggested to down-regulate the levels of Flt3 receptor messenger RNA, indicating that this isoform might be involved in the control of the differentiation of both lymphoid and myeloid cell lineage.14 Our data strongly implicate regulation of an apoptotic pathway as a primary function of Ik6. In this regard, the Aiolos transcription factor, another member of the Ikaros family, has been reported to interact with Ikaros and to control T-cell death by regulating the expression and localization of Bcl-2.22,23 Aiolos was further shown to regulate B-cell activation and maturation to the effector state. Interestingly, in that study, leukemic CD34+ myeloid progenitors and promyelocytes, but not immature lymphoid progenitors, expressed Bcl-XL. Because B-cell malignancies are frequently observed in Aiolos mutant mice,24 these reports raise the interesting possibility that evasion of apoptotic cell death is a relatively common mechanism by which Ikaros proteins participate in leukemogenesis. In our study, all 3 of Ik6-expressing 32D/TREx/Ik6 clones grew exponentially in an IL-3–deprived culture medium in the presence of tetracycline. In this system, Ik6 appeared to prevent apoptotic cell death by regulating Bcl-XL expression, in a dominant-negative manner. Because the expression of STAT5 did not increase in these cells, we conclude that Ik6 acted independently on Ik2 to stimulate the Bcl-XL protein. The basis for Ik6 expression in M4/M5 but not other FAB subtypes of AML is unknown. In terms of the role of Bcl-XL expression in leukemogenesis, its overexpression in 32D cells affected their growth in experimental conditions, but clinically our study showed no findings specific to AML cells. Although requiring additional study, the associations described here suggested that Ikaros proteins may play an important role in monocyte/macrophage development by the antiapoptotic protein Bcl-XL. Aberrant expression of particular Ikaros isoforms, such as Ik6, could interfere with normal apoptotic pathways, providing a mechanism for Ikaros involvement in AML pathogenesis.
We thank Dr K. Georgopoulos and Dr N. Avitahl for anti-Ikaros antibody, and Yasuko Hashimoto for expert help in the preparation of this manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Tomohito Yagi, Department of Pediatrics, Kyoto Prefectural University of Medicine, 465 Kajiicho Hirokoji Kamigyoku, Kyoto, 602-8566, Japan; e-mail:tyagi@gan2.res.ncc.go.jp.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal