p14ARF, the alternative product from the humanINK4a/ARF locus, antagonizes Hdm2 and mediates p53 activation in response to oncogenic stimuli. An immunohistochemical study of p14ARF expression in 74 samples of aggressive B-cell lymphomas was performed, demonstrating an array of different abnormalities. A distinct nucleolar expression pattern was detected in nontumoral tissue and a subset of lymphomas (50/74). In contrast, a group of cases (8/74) showed absence of p14ARF expression, dependent either on promoter hypermethylation or gene loss. Additionally, 16 out of 74 cases displayed an abnormal nuclear p14ARF overexpression not confined to the nucleoli, as confirmed by confocal microscopy, and that was associated with high levels of p53 and Hdm2. A genetic study of these cases failed to show any alteration in the p14ARF gene, but revealed the presence of p53 mutations in over 50% of these cases. An increased growth fraction and a more aggressive clinical course, with a shortened survival time, also characterized the group of tumors with p14ARF nuclear overexpression. Moreover, this p14ARF expression pattern was more frequent in tumors displaying accumulated alterations in the p53, p16INK4a, and p27KIP1 tumor supressors. These observations, together with the consideration of the central role of p14ARF in cell cycle control, suggest that p14ARF abnormal nuclear overexpression is a sensor of malfunction of the major cell cycle regulatory pathways, and consequently a marker of a high tumor aggressivity.
Introduction
The 2 major tumor suppressor pathways, represented by the proteins p16INK4a-CDK4/6-Rb and p14ARF-Hdm2-p53,1 are inactivated in most human cancers. p53 is a transcription factor that induces cell cycle arrest and/or apoptosis in response to a variety of stimuli (eg, DNA damage, hyperproliferative signals).2 p53 is negatively regulated by Hdm2 through a multiple mechanism: Hdm2 binds to the transactivation domain of the p53 tetramer, inhibiting p53 transcriptional activity3,4; in addition, Hdm2 functions as an E3-ubiquitin ligase which targets p53 for nuclear export and proteasomal degradation.5-8Hdm2 is itself a p53-responsive gene, thus establishing a feedback loop through which p53 regulates its own activity and turnover.9
The atypical structure of the INK4a/ARF locus in 9p21 encodes 2 unrelated tumor suppressor proteins, p16INK4a and p14ARF (the human counterpart of murine p19ARF).10,11 These are specified by different first exons that are spliced to a common second exon translated in alternative reading frames, their expression being controlled by independent promoters. p16INK4a, a specific inhibitor of cyclin D–dependent kinases, contributes to G1 arrest by blocking Rb phosphorylation.1 On the other hand, p14ARFinterferes with all of the known functions of Hdm2 (eg, direct inhibition of p53-mediated transactivation,12,13 ubiquitin ligase activity,14 and nuclear export of p5315,16), possibly through induction of Hdm2 degradation,17 indirectly leading to an increase in the activity and stability of p53. p14ARF is induced by inappropriate hyperproliferative signals (such asmyc,18 E2F-1,19ras,20 E1A,21v-abl22) and mediates p53 activation in response to oncogenic stimuli. Specifically, responsiveness of the p14ARF promoter to E2F-1 makes p14ARF a nexus between the Rb and p53 pathways. p53 suppresses p14ARFexpression through a poorly understood mechanism, which generates an additional regulatory circuitry.13 23
p14ARF is a highly basic protein that localizes to the nucleolus.10,12,24 When induced, p14ARF binds to Hdm2, thereby allowing p53 to stabilize and accumulate in the nucleoplasm. Classically, this p14ARF-Hdm2 binding has been assumed to take place in the nucleolus,16 although antagonization of Hdm2 by p14ARF independently of nucleolar localization has recently been reported.25 In addition, it has been suggested that p14ARF-p53 direct binding without requirement for Hdm2 as a bridging molecule is also possible,23 although the functional implications of this interaction remain unknown.
Both human and murine p14ARF contact the central acidic domain of Hdm2 through 2 independent binding sites.26-28Additionally, nucleolar localization sequences (NrLS) have been mapped in exons 1β and 2 of p14ARF. These motifs are required for the localization of p14ARF to the nucleolus; mutations in the p14ARF NrLS have been described as impeding the correct localization of this protein, resulting in its nucleoplasmic accumulation and the consequent loss of its ability to stabilize p53.29
In human tumors, the p53 gene is inactivated by mutation in more than 50% of cases; in a high proportion of the rest, the p53 pathway would be expected to be disrupted by Hdm2 amplification or p14ARF loss. In some cancers, the frequency of p14ARF alteration is remarkably high; deletions affecting the 9p21 region (eg, in the cases of glioblastoma and astrocytoma30,31) and hypermethylation of CpG islands in the p14ARF promoter (eg, in the case of gastric cancer32) are the main inactivation mechanisms. Point mutations are infrequent and usually also affect p16INK4a. In other neoplasias, p14ARF loss appears to be a rarer event. Nevertheless, most of the information available is derived from analysis at the gene level, whereas little is known about the expression level and distribution of the protein in different tumors.
In aggressive B-cell non-Hodgkin lymphomas (NHLs), the frequency of p53 mutation is modest (∼20%),33,34 whereas amplification of the 12q14 region (where the Hdm2 gene is located) has not been detected.35 Therefore, our purpose was to investigate the expression pattern of the p14ARF protein and its subcellular localization, the possible presence of genetic or epigenetic alterations in p14ARF, and the relationship between the status of p14ARF and the major tumor suppressor pathways in a group of large B-cell lymphoma and Burkitt lymphoma.
Materials and methods
Tissue samples
We obtained 74 samples of tumor specimens from aggressive NHLs from the routine files of the Virgen de la Salud Hospital (Toledo, Spain) and the Spanish National Cancer Center (CNIO) Tumor Bank (Madrid, Spain) and diagnosed using the criteria of the Revised European-American Lymphoma (REAL) classification.36 The samples included 55 cases of diffuse large B-cell lymphoma (DLBCL), 7 cases of follicular lymphoma grade 3 (FL-3), and 12 cases of Burkitt lymphoma (BL). Frozen tissue (for performing molecular studies) and clinical follow-up information were available for 51 of these samples (36 DLBCL, 3 FL-3, and 12 BL).
Cell lines
The human lymphoid cell lines RAJI, NAMALWA, MOLT-4, GRANTA-519, KARPAS-422, WSU-NHL, RPMI 8226, and HuT 78 were obtained from ATCC (Manassas, VA). Cells were cultured in Dulbecco modified Eagle medium (DMEM) (GRANTA-519) or RPMI-1640 medium (all other cell lines) supplemented with 10% fetal bovine serum, 2 mM L-glutamine, penicillin, and streptomycin. Cells were kept in cell culture flasks in a humidified incubator at 37°C and 5% CO2.
For immunostaining, cells were harvested by centrifugation, washed with cold phosphate buffered saline (PBS), cytospun onto poly-L-lysine–coated slides, and fixed in ethanol/acetone 1:1.
Mutational analysis
Exons 5 to 8 of the p53 gene and exons 1β and 2 of the p14ARF gene were amplified from genomic DNA extracted from tissue samples and cell lines, using previously described primers and conditions.31,34,37 Polymerase chain reaction (PCR) products were purified using the Microcon PCR system (Millipore, Bedford, MA). Direct sequencing of purified PCR products was performed with an automated DNA Sequencer ABI PRISM 3700 Genetic Analyzer (Applied Biosystems, Weiterstadt, Germany). Results for p53 and p14ARF exon 2 mutations in tumor samples have been previously published.33 38
Allelic loss
Homozygous deletions affecting p14ARF in cell lines were confirmed by simultaneous amplification of p14ARF exon 2 and p53 exon 8 (multiplex PCR). Results for homozygous or hemizygous deletions in the 9p21 region in the series of NHLs have been published as part of previous studies concerning p16INK4astatus.33 38
Analysis of p14ARF promoter hypermethylation
Methylation-specific PCR (MSP)39 assays were performed to determine the methylation status of the CpG islands of the p14ARF promoter in tissue samples and cell lines. Briefly, 1 μg of denatured genomic DNA was modified by reaction with sodium bisulfite under conditions that convert unmethylated cytosines to uracils. Modified DNA was purified using the Wizard DNA Clean-Up system (Promega, Madison, WI). Modification was completed by NaOH 0.3 M treatment for 5 minutes at room temperature, followed by ethanol precipitation.
A quantity of 50 ng bisulfite modified DNA was amplified using p14ARF unmethylated-specific and methylated-specific primers.40 The Hodgkin disease–derived L-540 cell line was used as a positive control of p14ARFmethylation41; DNA from nontumoral samples was included as a negative control. Methylation of the p14ARF promoter was detected by the amplification of a 122-bp fragment with the methylated-specific primers.
Antibodies
The following primary antibodies were used for immunohistochemistry: goat polyclonal anti-p14ARF C-18 (Santa Cruz Biotechnology, Santa Cruz, CA); mouse monoclonal anti-Hdm2 Ab-1 2IF-2 (Oncogene, Darmstadt, Germany); mouse monoclonal anti-p53 DO-7 (Novocastra, Newcastle upon Tyne, United Kingdom). Proliferation index was evaluated by the expression of the nuclear antigen Ki67, detected with the MIB1 monoclonal antibody (Novocastra).
Immunohistochemistry
Immunohistochemical techniques were performed on paraffin-embedded tissue sections or cytospin preparations of the cell lines using an initial heat-induced antigen retrieval step (slides were heated in a pressure cooker for 3 minutes in a 0.01 M solution of sodium citrate prior to incubation with the antibodies).
After incubation with the primary antibodies, immunodetection was performed with biotinylated antimouse or antigoat secondary antibodies as appropriate, followed by peroxidase-conjugated streptavidine (DAKO, Carpinteria, CA) with diaminobenzidine chromogen as substrate. Immunostaining was performed with the Techmate 500 (DAKO) automatic immunostaining device. Incubation omitting the specific antibody was used as a control of the technique.
p53 and Hdm2 expression were scored semiquantitatively and expressed as the nearest tenth percentile. Ki67 expression was quantified by scoring up to 200 tumoral cells in representative areas.
Immunofluorescence and confocal microscopy
Double fluorescent immunolabeling was performed on 3-μm–thick tissue sections mounted on poly-L-lysine–coated slides. After antigen retrieval and simultaneous incubation with the 2 primary antibodies, slides were washed and incubated with the secondary antibodies Cy3-conjugated donkey anti–goat (Jackson Immunoresearch, Baltimore, MD) and Alexa 488–conjugated rabbit anti–mouse (Molecular Probes, Eugene, OR). Nuclei were stained with TO-PRO-3 (Molecular Probes). The slides were mounted with VectaShield (Vector, Burlingame, CA) and examined with a TCS NT laser scanning confocal microscopy system (Leica Microsystems, Wetzlar, Germany). The series of images was processed with the software package provided (Leica Microsystems) and Adobe Photoshop 5.5.
Statistical analysis
The relationship between p14ARF and other variables (p53, Hdm2, Ki67) was examined using the Kruskal-Wallis test. In order to assess the specific association between p14ARF and Ki67, a multivariate analysis was performed including those markers significantly related to Ki67 levels as revealed by the univariate analysis. Since neither Ki67 nor its log-transformed values were normally distributed, as assumed by standard multiple regression techniques, a median regression analysis44 was performed. This method compares the median instead of the mean of each category.
The prognostic value of p14ARF and all other markers was evaluated by standard survival analysis, using Kaplan-Meier and Cox regression. Hazard ratios were computed for each marker, adjusting for International Prognostic Index (IPI) and histology.
Statistical analyses were performed using the STATA and SPSS software packages.
Results
Analysis of p14ARF, Hdm2, and p53 expression
Cell lines.
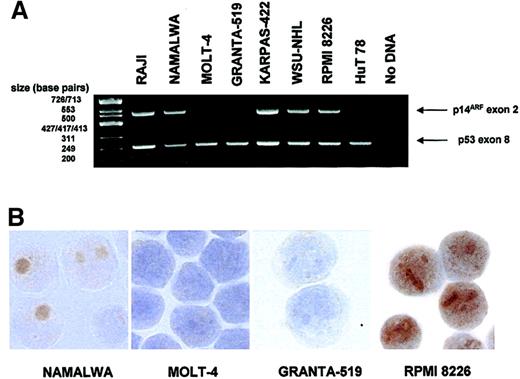
p14ARF expression was analyzed by immunostaining in several human lymphoid cell lines in which p53 and p14ARF gene status were also determined (Figure 1; Table 1).
p14ARF expression in lymphoid cell lines.
(A) Multiplex PCR demonstrating deletion of p14ARF exon 2 in some of the cell lines. p53 exon 8 is simultaneously amplified as a control. (B) Images showing absence of p14ARF staining in 2 cell lines with exon 2 deletion (MOLT-4, GRANTA-519) and nucleolar p14ARF expression in 2 nondeleted cell lines (NAMALWA, RPMI 8226). Note the nucleoplasmic staining for RPMI 8226 observed in addition to the nucleoli. Original magnification × 1000.
p14ARF expression in lymphoid cell lines.
(A) Multiplex PCR demonstrating deletion of p14ARF exon 2 in some of the cell lines. p53 exon 8 is simultaneously amplified as a control. (B) Images showing absence of p14ARF staining in 2 cell lines with exon 2 deletion (MOLT-4, GRANTA-519) and nucleolar p14ARF expression in 2 nondeleted cell lines (NAMALWA, RPMI 8226). Note the nucleoplasmic staining for RPMI 8226 observed in addition to the nucleoli. Original magnification × 1000.
p14ARF and p53 status in lymphoid cell lines
| Cell line . | p53 gene . | p14ARF gene . | p14ARF expression . |
|---|---|---|---|
| RAJI | R213Q (het) + Y234H (het) | WT | + |
| NAMALWA | R248Q | R120L (het) | + |
| MOLT-4 | R306STOP (het) | Del | − |
| GRANTA-519 | WT | Del | − |
| KARPAS-422 | WT | WT | + |
| WSU-NHL | R248Q | WT | + |
| RPMI 8226 | E285K | WT | + |
| HuT 78 | R196STOP | Del | − |
| Cell line . | p53 gene . | p14ARF gene . | p14ARF expression . |
|---|---|---|---|
| RAJI | R213Q (het) + Y234H (het) | WT | + |
| NAMALWA | R248Q | R120L (het) | + |
| MOLT-4 | R306STOP (het) | Del | − |
| GRANTA-519 | WT | Del | − |
| KARPAS-422 | WT | WT | + |
| WSU-NHL | R248Q | WT | + |
| RPMI 8226 | E285K | WT | + |
| HuT 78 | R196STOP | Del | − |
Del indicates homozygous deletion; WT, wild-type; het, heterozygous; −, negative; +, positive.
Inactivation of the p53 pathway was observed in the vast majority (7/8) of cell lines: 3 out of 8 lines had only p53 mutation; 1 out of 8 had only p14ARF deletion; 2 out of 8 featured both alterations simultaneously; 1 out of 8 had both p53 mutation and a heterozygous mutation within p14ARF exon 2 (R120L). Only in one of the cell lines were no alterations detected. None of the cell lines analyzed showed p14ARF promoter hypermethylation.
Loss of p14ARF expression was observed exclusively in the cell lines displaying p14ARF deletion. In the cell lines expressing p14ARF the signal was predominantly nucleolar, although additional nucleoplasmic staining was evident in the RPMI 8226 cell line (Figure 1).
Normal human tissues.
In nontumoral lymphoid tissue (tonsils and reactive lymphadenitis), reactive cells showed distinct nucleolar p14ARF immunostaining (Figure2A). Staining for Hdm2 (Figure 2B) and p53 (Figure 2C) revealed weak nucleoplasmic expression of both proteins in proliferating cells within the germinal centers. No Hdm2 nucleolar staining was observed in normal cells.
Immunohistochemical study of p14ARF, Hdm2, and p53 in normal and tumoral lymphoid tissue.
(A) Distinct nucleolar p14ARF staining, in nontumoral lymphoid tissue (a reactive lymphadenitis). (B) Hdm2 and (C) p53 in the same sample. (D) An NHL case with loss of p14ARF in tumoral cells (indicated by arrows); normal lymphocytes retain nucleolar p14ARF expression. Hdm2 (E) and p53 (F) in this case are also shown. (G) An NHL case showing nucleolar p14ARF in tumoral cells, with intermediate Hdm2 (H) and p53 (I) expression. (J) Nuclear p14ARF overexpression (note the nucleoli in small lymphocytes) associated with high levels of Hdm2 (K) and p53 overexpression (L). Original magnification × 1000.
Immunohistochemical study of p14ARF, Hdm2, and p53 in normal and tumoral lymphoid tissue.
(A) Distinct nucleolar p14ARF staining, in nontumoral lymphoid tissue (a reactive lymphadenitis). (B) Hdm2 and (C) p53 in the same sample. (D) An NHL case with loss of p14ARF in tumoral cells (indicated by arrows); normal lymphocytes retain nucleolar p14ARF expression. Hdm2 (E) and p53 (F) in this case are also shown. (G) An NHL case showing nucleolar p14ARF in tumoral cells, with intermediate Hdm2 (H) and p53 (I) expression. (J) Nuclear p14ARF overexpression (note the nucleoli in small lymphocytes) associated with high levels of Hdm2 (K) and p53 overexpression (L). Original magnification × 1000.
Aggressive B-cell lymphomas.
p14ARF expression was analyzed in a series of 74 cases of NHLs including 55 DLBCL, 7 FL-3, and 12 BL. In these samples, an internal control of the technique was provided by the nucleolar staining of small lymphocytes, macrophages, and some endothelial cells.
Concerning the p14ARF expression pattern in tumoral cells, the following situations were observed: (1) p14ARF loss in a subset of NHLs. A small number of cases (8/74; Table2) showed no appreciable p14ARF staining in large cells (Figure 2D), whereas nucleolar staining was observed in benign lymphocytes. The predominant phenotype in this subset of cases was characterized by a low level of expression of both Hdm2 (Figure 2E) and p53 (Figure 2F). Of these cases, 3 could be sequenced for p14ARF, but no mutations were found. In 1 of 3 cases (case 19), silencing of the p14ARF gene was associated with promoter hypermethylation coupled with an LOH (loss of heterozygosity), which could affect the unmethylated allele. A homozygous deletion within the 9p21 region was detected in another case (case 47). The p53 gene was wild-type in the 4 cases sequenced. (2) Nucleolar p14ARFexpression. Nucleolar p14ARF staining in tumoral cells was the most frequently observed expression pattern (50/74 cases). The intensity of staining was variable, ranging from weak to strong nucleolar staining in most large cells (Figure 2G). The staining for Hdm2 (Figure 2H) and p53 (Figure 2I) was always nucleoplasmic and of variable intensity, often with distinct nucleolar exclusion. Nucleolar accumulation of Hdm2 was never observed. No alterations in the p14ARF sequence (a nucleotide change in exon 2, detected in one case,33 does not affect the p14ARFprotein) or promoter hypermethylation were detected in any of the 42 cases analyzed in this group; 4 cases showed LOH in 9p21. p53 mutations were detected in a minority of cases (1 nonsense and 3 missense mutations out of 42 samples). (3) p14ARF nuclear overexpression. A subset of NHLs (16/74) showed an atypical p14ARF expression pattern characterized by intense nuclear staining that was not confined to the nucleolus (Figure 2J). Since mutations affecting the NrLS of p14ARF have been described as partially or totally impeding the correct localization of this protein,29 both exons of the p14ARF gene were sequenced in 9 of 16 cases. However, no alterations were detected. Allelic loss and promoter hypermethylation analyses, performed in the same samples, also yielded negative results. This group of cases (Table3) was characterized by high p53 and Hdm2 levels of expression (Figure 2K-L). When compared with the rest of the series, a statistically significant relationship was found between p14ARF nuclear overexpression and higher expression of both p53 and Hdm2 (Kruskal-Wallis test, P = .001 for p53 and P = .010 for Hdm2). p53 was either wild-type or mutated in this subset of cases, but the frequency of p53 mutation was significantly higher (5/9 sequenced cases) when compared with the rest of the series (Fisher exact test, P = .005). There were no significant differences in the distribution of the cases with p14ARF nuclear overexpression between the categories defined by histologic diagnosis (BL vs non-BL; Fisher exact test,P = .715) or IPI (Pearson chi square: 0.360,P = .948).
Cases with loss of p14ARF expression
| Case no. . | Diagnosis . | p53 (%) . | Hdm2 (%) . | Ki67 (%) . |
|---|---|---|---|---|
| 19 | DLBCL | 50 | 50 | 60 |
| 40 | DLBCL | 20 | 0 | 75 |
| 47 | BL | < 10 | < 10 | 90 |
| 51 | DLBCL | 10 | NA | 90 |
| 53 | DLBCL | < 10 | < 10 | NA |
| 59 | FL-3 | 30 | 40 | 70 |
| 60 | DLBCL | 10 | 10 | 65 |
| 64 | DLBCL | 0 | 0 | 30 |
| Case no. . | Diagnosis . | p53 (%) . | Hdm2 (%) . | Ki67 (%) . |
|---|---|---|---|---|
| 19 | DLBCL | 50 | 50 | 60 |
| 40 | DLBCL | 20 | 0 | 75 |
| 47 | BL | < 10 | < 10 | 90 |
| 51 | DLBCL | 10 | NA | 90 |
| 53 | DLBCL | < 10 | < 10 | NA |
| 59 | FL-3 | 30 | 40 | 70 |
| 60 | DLBCL | 10 | 10 | 65 |
| 64 | DLBCL | 0 | 0 | 30 |
DLBCL indicates diffuse large B-cell lymphoma; BL, Burkitt lymphoma; FL-3, follicular lymphoma grade 3; NA, not available.
Cases with nuclear p14ARF overexpression
| Case no. . | Diagnosis . | p53 gene . | p53 (%) . | Hdm2 (%) . | Ki67 (%) . |
|---|---|---|---|---|---|
| 3 | DLBCL | C135F | 100 | 10 | 100 |
| 6 | DLBCL | R158H | 60 | 30 | 80 |
| 13 | BL | WT | NA | 50 | 100 |
| 21 | BL | WT | 100 | 30 | 95 |
| 26 | DLBCL | G245S | 100 | 0 | 90 |
| 34 | BL | WT | 60 | 80 | 95 |
| 36 | DLBCL | G244D | 80 | 10 | 95 |
| 46 | DLBCL | WT | 60 | 80 | 95 |
| 48 | DLBCL | V157F | 100 | 80 | 70 |
| 52 | DLBCL | NA | NA | NA | NA |
| 54 | DLBCL | NA | 20 | 20 | NA |
| 63 | DLBCL | NA | 60 | 40 | 40 |
| 67 | DLBCL | NA | 20 | 30 | 85 |
| 69 | DLBCL | NA | 40 | 100 | 75 |
| 72 | DLBCL | NA | 60 | 100 | 85 |
| 73 | DLBCL | NA | 10 | 50 | 85 |
| Case no. . | Diagnosis . | p53 gene . | p53 (%) . | Hdm2 (%) . | Ki67 (%) . |
|---|---|---|---|---|---|
| 3 | DLBCL | C135F | 100 | 10 | 100 |
| 6 | DLBCL | R158H | 60 | 30 | 80 |
| 13 | BL | WT | NA | 50 | 100 |
| 21 | BL | WT | 100 | 30 | 95 |
| 26 | DLBCL | G245S | 100 | 0 | 90 |
| 34 | BL | WT | 60 | 80 | 95 |
| 36 | DLBCL | G244D | 80 | 10 | 95 |
| 46 | DLBCL | WT | 60 | 80 | 95 |
| 48 | DLBCL | V157F | 100 | 80 | 70 |
| 52 | DLBCL | NA | NA | NA | NA |
| 54 | DLBCL | NA | 20 | 20 | NA |
| 63 | DLBCL | NA | 60 | 40 | 40 |
| 67 | DLBCL | NA | 20 | 30 | 85 |
| 69 | DLBCL | NA | 40 | 100 | 75 |
| 72 | DLBCL | NA | 60 | 100 | 85 |
| 73 | DLBCL | NA | 10 | 50 | 85 |
DLBCL indicates diffuse large B-cell lymphoma; BL, Burkitt lymphoma; FL-3, follicular lymphoma grade 3; WT, wild type; NA, not available.
Analysis of subcellular localization
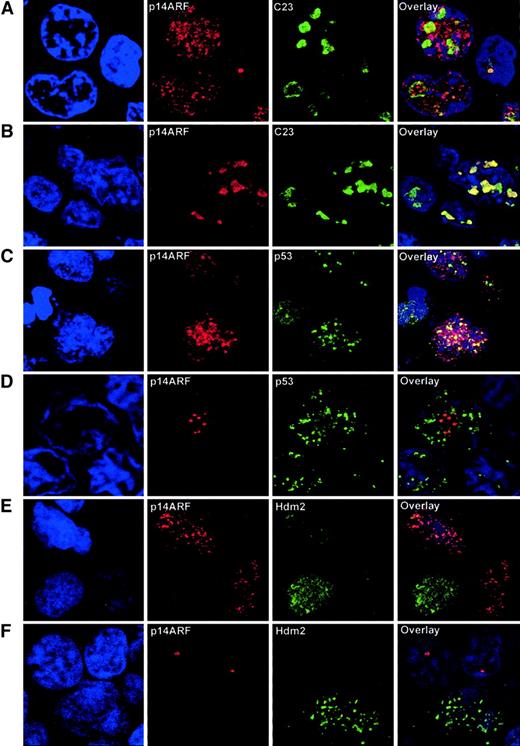
In order to characterize more precisely the subcellular localization of the p14ARF, Hdm2, and p53 proteins, 5 tumor samples with different patterns of expression of p14ARFwere analyzed by confocal microscopy. Double fluorescent immunolabeling was performed for p14ARF and either C23 (nucleolar marker), p53, or Hdm2.
Existence of a nucleoplasmic fraction of p14ARF was effectively observed in cases with abnormal p14ARF nuclear overexpression, as demonstrated by double labeling with C23 (Figure3A). Although nucleolar exclusion was not observed, part of the signal was clearly extranucleolar. Nucleoplasmic p14ARF was restricted to the large tumoral cells, whereas small lymphocytes showed distinct nucleolar p14ARFstaining. This pattern contrasts with the more typically observed situation in both normal and tumoral cells, in which p14ARFwas confined to the nucleolus (Figure 3B). C23 characteristic nucleolar staining demonstrates that the integrity of the nucleolus was always preserved, and anomalous localization of p14ARF was not a consequence of structural alterations of the nucleolus.
Confocal microscopy analysis of NHL samples.
(A) Double immunolabeling for p14ARF and the nucleolar marker C23 in a sample with p14ARF nuclear overexpression, showing that a significant fraction of the p14ARF signal is extranucleolar. Note the nucleolar p14ARF signal in the normal lymphocyte (right). (B) A sample with intense nucleolar accumulation of p14ARF, coincident with the C23 signal, is shown for comparison. (C) Double immunolabeling for p14ARFand p53 shows a partial colocalization of nucleoplasmic p14ARF and p53. (D) Lack of colocalization in a sample with nucleolar p14ARF, where the discrete granular p14ARF signal contrasts with the nucleoplasmic distribution of p53. (E) Lack of colocalization between nucleoplasmic p14ARF and Hdm2. (F) Hdm2 expression in a tumoral cell where p14ARF is absent; however, nucleolar p14ARF is expressed in adjacent lymphocytes lacking detectable Hdm2. Original magnification × 2000-3500.
Confocal microscopy analysis of NHL samples.
(A) Double immunolabeling for p14ARF and the nucleolar marker C23 in a sample with p14ARF nuclear overexpression, showing that a significant fraction of the p14ARF signal is extranucleolar. Note the nucleolar p14ARF signal in the normal lymphocyte (right). (B) A sample with intense nucleolar accumulation of p14ARF, coincident with the C23 signal, is shown for comparison. (C) Double immunolabeling for p14ARFand p53 shows a partial colocalization of nucleoplasmic p14ARF and p53. (D) Lack of colocalization in a sample with nucleolar p14ARF, where the discrete granular p14ARF signal contrasts with the nucleoplasmic distribution of p53. (E) Lack of colocalization between nucleoplasmic p14ARF and Hdm2. (F) Hdm2 expression in a tumoral cell where p14ARF is absent; however, nucleolar p14ARF is expressed in adjacent lymphocytes lacking detectable Hdm2. Original magnification × 2000-3500.
Double labeling for p14ARF-p53 revealed a partial colocalization of these proteins in the nucleoplasm, suggesting the existence of a fraction of nucleoplasmic p14ARF not bound to p53 (Figure 3C). This colocalization was absent in samples exhibiting p14ARF nucleolar localization (Figure 3D).
A striking pattern was observed for Hdm2, in that it was not found to colocalize with nucleoplasmic or nucleolar p14ARF; in fact, these signals usually appeared in alternative cells, and Hdm2 never accumulated in the nucleolus (Figure 3E-F).
Clinical and biologic significance of p14ARFnuclear overexpression
Since p14ARF has been reported as accumulating in response to oncogenic stimuli,18-22 we examined whether p14ARF nuclear overexpression had any correspondence with the biology of the tumor or clinical outcome of the patients.
To this end, Ki67 expression was quantified in 70 of 74 cases (range: 32%-100%, median: 82%) as a measure of proliferation index. Univariate analysis showed a strong association between p14ARF nuclear overexpression and higher Ki67 levels (Kruskal-Wallis test, P = .002). Histology (BL vs non-BL) and p53 and Hdm2 expression were also significantly related to Ki67 (Table 4). However, after performing a multivariate analysis that included the variables found to be significant in the univariate analysis, the only variable that retained statistical significance, in addition to histology, was p14ARF nuclear overexpression (P = .035).
Relationship between proliferation index provided by Ki67 expression and other variables
| Variable . | N . | Median of Ki67 expression (%) . | Univariate analysis . | Median regression . | |||
|---|---|---|---|---|---|---|---|
| P25-P75 (%) . | P . | Beta (%) . | 95% CI (%) . | P . | |||
| p14ARF | |||||||
| Nonnucleoplasmic | 56 | 77 | 60 -90 | ||||
| Nucleoplasmic | 14 | 91 | 86 -95 | .002 | 12 | 0.9-23.1 | .035 |
| IPI | |||||||
| 0 to 1 | 15 | 85 | 65 -95 | ||||
| 2 | 14 | 73 | 60 -95 | ||||
| 3 | 10 | 83 | 70 -95 | ||||
| 4 to 5 | 11 | 80 | 70 -90 | .955 | |||
| Histology | |||||||
| Non-Burkitt | 58 | 76 | 30 -87 | ||||
| Burkitt | 12 | 95 | 90 -99 | < .001 | 23 | 11.9-34.1 | < .001 |
| p53 expression | |||||||
| Less than 10% | 17 | 60 | 50 -90 | ||||
| 10% to 50% | 26 | 80 | 70 -90 | ||||
| At least 50% | 23 | 90 | 75 -95 | .022 | 10 | −1.4-21.4 | .086 |
| p53 mutation | |||||||
| No | 42 | 80 | 60 -95 | ||||
| Yes | 9 | 90 | 85 -90 | .226 | |||
| Hdm2 | |||||||
| Less than 10% | 18 | 65 | 60 -75 | ||||
| 10% to 40% | 21 | 87 | 75 -95 | ||||
| At least 40% | 24 | 86 | 70 -92 | .007 | 5 | −6.2-16.2 | .374 |
| Variable . | N . | Median of Ki67 expression (%) . | Univariate analysis . | Median regression . | |||
|---|---|---|---|---|---|---|---|
| P25-P75 (%) . | P . | Beta (%) . | 95% CI (%) . | P . | |||
| p14ARF | |||||||
| Nonnucleoplasmic | 56 | 77 | 60 -90 | ||||
| Nucleoplasmic | 14 | 91 | 86 -95 | .002 | 12 | 0.9-23.1 | .035 |
| IPI | |||||||
| 0 to 1 | 15 | 85 | 65 -95 | ||||
| 2 | 14 | 73 | 60 -95 | ||||
| 3 | 10 | 83 | 70 -95 | ||||
| 4 to 5 | 11 | 80 | 70 -90 | .955 | |||
| Histology | |||||||
| Non-Burkitt | 58 | 76 | 30 -87 | ||||
| Burkitt | 12 | 95 | 90 -99 | < .001 | 23 | 11.9-34.1 | < .001 |
| p53 expression | |||||||
| Less than 10% | 17 | 60 | 50 -90 | ||||
| 10% to 50% | 26 | 80 | 70 -90 | ||||
| At least 50% | 23 | 90 | 75 -95 | .022 | 10 | −1.4-21.4 | .086 |
| p53 mutation | |||||||
| No | 42 | 80 | 60 -95 | ||||
| Yes | 9 | 90 | 85 -90 | .226 | |||
| Hdm2 | |||||||
| Less than 10% | 18 | 65 | 60 -75 | ||||
| 10% to 40% | 21 | 87 | 75 -95 | ||||
| At least 40% | 24 | 86 | 70 -92 | .007 | 5 | −6.2-16.2 | .374 |
N indicates number of patients; CI, confidence interval; Beta, change in the median value of Ki67 compared with the reference category.
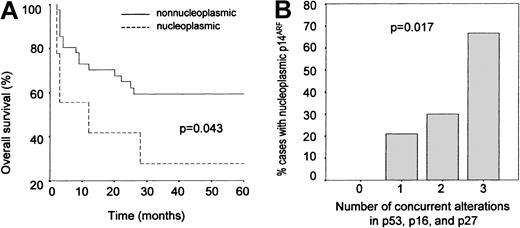
To test this possible association between p14ARF nuclear overexpression and tumor aggressivity further, an overall survival analysis was performed for the subset of cases (51/74) for which clinical follow-up information during at least 60 months was available. A worse prognosis was observed for the cases with nuclear p14ARF when compared with the rest of the series (P = .060, crude analysis) (Table 5); this relationship proved to be statistically significant after adjusting the results by IPI and histology (P = .043; Figure4A). Since the small group of cases in which p14ARF expression was not detected may include aggressive tumors in which p14ARF overexpression is impeded by alterations at the gene level, the cases lacking p14ARFexpression were excluded from the analysis and overall survival of the patients overexpressing p14ARF was compared with that of the cases showing a nucleolar expression of the protein; again, differences were found to be significant (P = .037).
Relationship between p14ARF nuclear overexpression and overall survival
| Variable . | N . | Deaths . | % Survival (60 months) . | Crude analysis . | Adjusted by IPI and histology . | ||||
|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | ||||
| p14ARF | |||||||||
| Nonnucleoplasmic | 41 | 16 | 59 | 1.00 | 1.00 | ||||
| Nucleoplasmic | 9 | 6 | 28 | 2.47 | 0.96-6.32 | .060 | 2.65 | 1.03-6.83 | .043 |
| IPI | |||||||||
| 0 to 1 | 15 | 1 | 93 | 1.00 | 1.00 | ||||
| 2 | 14 | 7 | 43 | 12.17 | 1.49-99.3 | .020 | 11.65 | 1.43-95.1 | .022 |
| 3 | 10 | 6 | 34 | 13.88 | 1.67-116 | .015 | 12.69 | 1.51-106 | .019 |
| 4 to 5 | 11 | 8 | 27 | 20.40 | 2.53-164 | .005 | 19.12 | 2.36-155 | .006 |
| Histology | |||||||||
| Non-Burkitt | 38 | 19 | 48 | 1.00 | 1.00 | ||||
| Burkitt | 12 | 3 | 73 | 0.43 | 0.13-1.47 | .180 | 0.56 | 0.17-1.90 | .352 |
| Variable . | N . | Deaths . | % Survival (60 months) . | Crude analysis . | Adjusted by IPI and histology . | ||||
|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | ||||
| p14ARF | |||||||||
| Nonnucleoplasmic | 41 | 16 | 59 | 1.00 | 1.00 | ||||
| Nucleoplasmic | 9 | 6 | 28 | 2.47 | 0.96-6.32 | .060 | 2.65 | 1.03-6.83 | .043 |
| IPI | |||||||||
| 0 to 1 | 15 | 1 | 93 | 1.00 | 1.00 | ||||
| 2 | 14 | 7 | 43 | 12.17 | 1.49-99.3 | .020 | 11.65 | 1.43-95.1 | .022 |
| 3 | 10 | 6 | 34 | 13.88 | 1.67-116 | .015 | 12.69 | 1.51-106 | .019 |
| 4 to 5 | 11 | 8 | 27 | 20.40 | 2.53-164 | .005 | 19.12 | 2.36-155 | .006 |
| Histology | |||||||||
| Non-Burkitt | 38 | 19 | 48 | 1.00 | 1.00 | ||||
| Burkitt | 12 | 3 | 73 | 0.43 | 0.13-1.47 | .180 | 0.56 | 0.17-1.90 | .352 |
N indicates number of patients; HR, hazard ratio; CI, confidence interval.
p14ARF overexpression as a marker of aggresivity.
(A) Kaplan-Meier analysis: overall survival curves showing a shorter survival in cases with p14ARF nuclear overexpression compared with the rest of the series. (B) Relative frequency of abnormal p14ARF overexpression as a function of cell cycle status. Cases are grouped according to the number of simultaneous alterations in major tumor suppressors (p53, p16INK4a, p27KIP1). Nuclear p14ARF overexpression becomes more frequent as the number of alterations increases.
p14ARF overexpression as a marker of aggresivity.
(A) Kaplan-Meier analysis: overall survival curves showing a shorter survival in cases with p14ARF nuclear overexpression compared with the rest of the series. (B) Relative frequency of abnormal p14ARF overexpression as a function of cell cycle status. Cases are grouped according to the number of simultaneous alterations in major tumor suppressors (p53, p16INK4a, p27KIP1). Nuclear p14ARF overexpression becomes more frequent as the number of alterations increases.
p14ARF overexpression was also a negative predictor of survival when only DLBCLs were considered (P = .047, crude analysis); however, the reduced number of BL cases prevented us from performing informative statistical analyses for this histologic class as a separate group. When cases were grouped according to IPI and overall survival was analyzed separately for each group, a similar association between p14ARF nuclear overexpression and prognosis was found for the category of medium IPI (2-3) (P = .055); analyses were not considered informative for the groups of low (0-1) or high (4-5) IPI due to the reduced number of events in the first case and the small number of patients with p14ARF nuclear overexpression (2 patients) in the second.
In a previous report33 we analyzed different alterations in tumor suppressor pathways in a group of NHLs that included 51 of the samples described here. For these cases, information was available concerning p53 mutations, p16INK4a inactivation by promoter hypermethylation, deletion, or mutation, and p27KIP1overexpression, presumably reflecting its inactivation by CDK4-cyclin D3 binding.45
First, the relationship between p14ARF nuclear overexpression and each of these individual alterations was analyzed. As mentioned above, there was a strong association between p53 mutation and p14ARF nuclear overexpression (P = .005). Presence of nuclear p14ARF was also more frequent in tumors overexpressing p27KIP1, although this did not reach statistical significance (Fisher exact test, P = .118).
An association between p16INK4a alterations and p14ARF overexpression was not found when all the possible mechanisms of inactivation of p16INK4a were considered as a whole. However, this may be due to the fact that LOHs or homozygous deletions at 9p21 are likely to affect both genes simultaneously, preventing p14ARF from being overexpressed. Effectively, when LOHs were excluded and only p16INK4a promoter hypermethylation—plus a single mutation that did not affect p14ARF—was considered, an almost significant association was obtained between these alterations and higher frequency of p14ARF nuclear overexpression (Fisher exact test,P = .053). It was also observed that hypermethylation of the p14ARF and p16INK4a promoters were independent events, as could be inferred from their remarkably different incidence in this group of lymphomas (17/51 cases displayed p16INK4a hypermethylation whereas only 1/51 cases had p14ARF hypermethylation).
Finally, we analyzed the frequency of p14ARF abnormal overexpression as a function of the number of accumulated alterations in p53, p16INK4a, and p27KIP1. As shown in Figure 4B, the proportion of cases with anomalous p14ARFexpression increases with the number of cell cycle defects, this relationship being statistically significant (Pearson chi square: 10.233, P = .017). Interestingly, p14ARFnuclear overexpression was not observed in any of the 19 cases where no alterations in the p53 and Rb pathways were found.
Discussion
Information concerning regulation of the p14ARF-Hdm2-p53 pathway is mainly derived from in vitro studies and animal models, whereas a comprehensive analysis of its significance in human tumors has been hampered by technical considerations such as the lack of appropriate antibodies for detecting the p14ARF protein. We have chosen NHL as a model, since it features tumors with a high growth fraction in which p53 mutations are present in only a small proportion of cases, and therefore may be useful for revealing the complexity of molecular alterations taking place in this pathway.
Unlike what has been found in other neoplasias, molecular alterations resulting in loss of p14ARF expression are rare in NHLs. Hypermethylation of p14ARF promoter and deletions within the 9p21 region, the 2 main mechanisms for p14ARFsilencing, have been detected in our series of cases only exceptionally. This situation sharply contrasts with that observed in lymphoid cell lines, in which cancellation of the p53 pathway by alternative—and occasionally simultaneous—inactivation of p14ARF or p53 is a very frequent finding (Table 1). Tumors lacking detectable p14ARF are characterized by low levels of p53 and Hdm2 proteins, which is consistent with the proposed role of p14ARF as an inhibitor of Hdm2; thus, in the absence of p14ARF, active Hdm2 promotes a rapid p53 degradation, which in turn leads to a low level of p53-induced Hdm2 expression.
In contrast with the exclusively nucleolar localization of p14ARF observed in normal cells and most in vitro models, abnormal p14ARF nucleoplasmic accumulation has been found in a significant number of aggressive NHLs. It does not seem likely that this is a consequence of nonspecific staining, as a distinct nucleolar signal has been obtained with the same antibody and under the same conditions in nontumoral tissue, many NHL samples, and several lymphoid cell lines. Moreover, no staining (either nucleolar or nucleoplasmic) has been detected in cell lines with p14ARFsilencing by homozygous deletion (Figure 1). p14ARF-C23 double immunolabeling has demonstrated that our observations are not an artifact related to loss of nucleolar integrity. We have also ruled out the possibility that nucleolar distribution is impeded by mutations affecting the p14ARF NrLS, since the p14ARFgene has been found to be wild-type in all the cases that have been sequenced.
The significance of this findings is enhanced by the observation that p14ARF atypical overexpression defines a group of lymphomas characterized by higher aggresivity. A strong association exists between p14ARF nuclear overexpression and higher proliferation index, which remains significant even in a multivariate analysis including relevant variables such as p53, Hdm2, and histologic diagnosis. A similar correlation existed at the prognostic level, since cases with abnormal p14ARF overexpression were also characterized by a shorter overall survival.
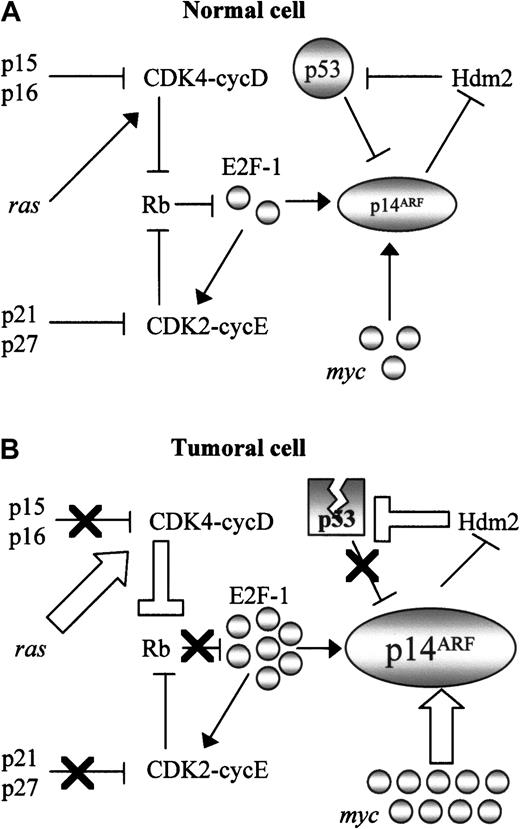
An explanation for these findings can be found in the consideration of the central role of p14ARF in cell cycle control, as a nexus between the major tumor suppressor pathways. Thus, the p14ARF promoter has an E2F-1 binding site which “senses” oncogenic stimuli transduced through the Rb pathway19; p14ARF is also induced by typical oncogenes (myc, ras, viral genes), and negatively regulated by p53 (Figure 5A). With the exception of alterations affecting p14ARF itself, which we have shown to be very rare in NHLs, virtually every cancer-related defect in these pathways (Figure 5B) should result in p14ARF upregulation: alterations of the Rb pathway such as p16INK4a inactivation or cyclin D overexpression, deregulation of oncogenes (eg, myc in BL), and p53 inactivation by mutation or by Hdm2 overexpression (resulting in disruption of the p53-p14ARF negative feedback loop). Therefore p14ARF should integrate all these stimuli, its level of expression being a measure of the accumulation of alterations in different points of the cell cycle, and consequently a marker of tumor aggressivity. Consistent with this hypothesis, p14ARFnuclear overexpression is a more frequent finding in tumors displaying simultaneous inactivation of several major tumor suppressors (p53, p16INK4a, p27KIP1), in addition to correlate with some of these alterations, taken individually. The postulation of an overexpressed nuclear p14ARF as a surrogate of a highly deregulated cell cycle is consistent with the higher aggressivity (as measured by proliferation index and worse prognosis) observed in this group of lymphomas.
Central role of p14ARF in cell cycle regulation.
The central role of p14ARF in the control of cell cycle in normal cells (A) converts it into a marker of inactivation of multiple cell cycle regulatory pathways (B). Nuclear overexpression as a consequence of multiple molecular alterations involving Rb pathway (inactivation of the Rb pathway generates active E2F-1, which induces transcription of the p14ARF gene); myc, and viral oncogenes such as E1A or v-abl, which also induce p14ARF; and alterations in the p53-Hdm2 pathway resulting in disruption of the p53-mediated negative regulation of p14ARF. Thick arrows indicate events derived from oncogene activation; black crosses represent inactivation of tumor suppressor genes.
Central role of p14ARF in cell cycle regulation.
The central role of p14ARF in the control of cell cycle in normal cells (A) converts it into a marker of inactivation of multiple cell cycle regulatory pathways (B). Nuclear overexpression as a consequence of multiple molecular alterations involving Rb pathway (inactivation of the Rb pathway generates active E2F-1, which induces transcription of the p14ARF gene); myc, and viral oncogenes such as E1A or v-abl, which also induce p14ARF; and alterations in the p53-Hdm2 pathway resulting in disruption of the p53-mediated negative regulation of p14ARF. Thick arrows indicate events derived from oncogene activation; black crosses represent inactivation of tumor suppressor genes.
Even if p14ARF overexpression is a consequence of cell cycle malfunction, the atypical nuclear localization of the protein remains an intringuing finding. Results of confocal microscopy suggest that p14ARF nucleoplasmic accumulation is only partially dependent on p53 binding and probably independent of Hdm2 binding. A recent report has suggested that the predominant p14ARFnucleolar accumulation is accompanied by an usually undetectable nucleoplasmic fraction which could be responsible for p53 activation.25 Consistent with this model, it would be expected that under conditions of massive p14ARF induction the nucleoplasmic fraction would become detectable, as could be the case for a group of aggressive lymphomas.
Our findings may help to resolve the controversy concerning Hdm2 and p14ARF subcellular localization, both in normal and tumoral cells. Thus, the presence of Hdm2 and p14ARF seems to be mutually exclusive, as shown by the lack of nucleolar Hdm2 staining in cells expressing nucleolar p14ARF, and the absence of Hdm2 in cells overexpressing nuclear p14ARF. This suggests that p14ARF-Hdm2 complexes, if they exist, should have a short half-life, dependent on a rapid Hdm2 degradation induced after p14ARF binding.
We are indebted to A. I. Sáez for her invaluable help with the statistical analysis, and to I. Fernández and M. J. Acuña for their expertise and excellent technical assistance with molecular and immunohistochemical assays, respectively. We also thank Dr Juan Carlos Martı́nez-Montero for his kind collaboration in the initial stages of the immunohistochemical analysis.
Supported by grants from the Fondo de Investigaciones Sanitarias (FIS 98/993), Ministerio de Sanidad y Consumo, Comisión Interministerial de Ciencia y Tecnologı́a (1FD97-0431), Comunidad Autónoma de Madrid (08.1/0028/2000 1) and Ministerio de Ciencia y Tecnologı́a (SAF2001-0060), Spain. A.S.-A. is supported by a grant from the Spanish National Cancer Center.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Margarita Sánchez-Beato, Molecular Pathology Program, Centro Nacional de Investigaciones Oncológicas Carlos III (CNIO), Ctra Majadahonda-Pozuelo, Km 2, 28220 Majadahonda, Madrid, Spain; e-mail: msbeato@cnio.es.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal