In vitro studies suggest that all-trans retinoic acid (ATRA) synergizes with erythropoietin (EPO) for the stimulation of hematopoiesis in patients with myelodysplastic syndrome (MDS). A clinical trial was performed to evaluate whether a combination of these agents was effective in relieving the cytopenias associated with MDS. Twenty-seven patients with low- or intermediate-risk MDS were enrolled in a 12-week study. ATRA was administered orally at the dose of 80 mg/m2 per day in 2 divided doses for 7 consecutive days every other week. Recombinant human EPO was given subcutaneously 3 times a week. The EPO dose was initiated at 150 U/kg and was increased to 300 U/kg if after 6 weeks there was no or there was suboptimal erythroid response. Patients who responded to therapy were continued on ATRA and EPO at the same doses for 6 additional months (extension phase). Further treatment was given to patients with a continued response. Clinically significant erythroid responses with increases of hemoglobin levels of at least 1 g/dL or reduction of transfusion needs were seen in 13 (48%) patients, with 4 showing improved responses after dose escalation of EPO. Ten (37%) patients displayed continued responses during 6 months of extended treatment, and 7 (26%) are still responsive after a follow-up period of 13 months. Neutrophil responses were observed in 5 of 12 patients with neutropenia, and platelet responses were observed in 6 of 9 patients with thrombocytopenia. Three patients displayed trilineage responses that were sustained during continuation therapy. Side effects were observed in all patients but were of mild entity and did not require discontinuation of therapy. It is concluded that the combination ATRA + EPO is an effective and well-tolerated treatment for patients with low- and intermediate-risk MDS. The optimal ATRA and EPO schedule and the role of maintenance treatment remain to be determined and warrant further investigation.
Introduction
Myelodysplastic syndrome (MDS) is a heterogeneous group of clonal hematopoietic stem cell disorders characterized by peripheral cytopenias, functional defects, and a variable propensity for leukemic transformation. No current treatment has been shown to be consistently effective in producing sustained improvement in hematopoiesis in these patients (with the possible exception of allogeneic bone marrow transplantation in selected younger patients), and supportive care with blood products and administration of antibiotics remains the mainstay of therapy.
In recent years, experimental therapeutic approaches have been focused on the use of hematopoietic growth factors and biologic response modifiers that may act by decreasing the apoptosis rate and enhancing the differentiation of preleukemic progenitor cells or by stimulating the growth of residual normal hematopoietic clones. Several trials have been designed to evaluate the efficacy of recombinant human erythropoietin (EPO) in the enhancement of erythropoiesis in MDS, demonstrating an overall response rate of approximately 20%.1 Numerous studies have also explored the use of retinoids, but their results are difficult to interpret because of the differences in agents, doses, and schedules and the use of additional agents.2
All-trans retinoic acid (ATRA) has been reported to have poor activity when used as a single agent,3-5 but in vitro studies support a role for its use in combination with EPO to reduce apoptosis in patients with MDS.6,7 It is noteworthy that the declining efficacy of ATRA during long-term treatment has been associated with a reduction of ATRA plasma levels.8Although the mechanisms responsible for this phenomenon are unknown, those hypothesized include malabsorption, induction of cytochrome P450 activity by ATRA, or elevation of cellular ATRA-binding protein levels, resulting in increased plasma clearance into nontarget tissues.8 Potential methods to overcome this phenomenon include blocking oxidation by administration of a P450 inhibitor or using an intermittent dosing schedule.9 An intermittent schedule of ATRA administration has a potential advantage over a continuous one. Studies performed in patients with acute promyelocytic leukemia9 and chronic myelogenous leukemia10indicate that a period of time without drug administration allows a return to baseline plasma clearance levels and a down-regulation of cellular ATRA-binding protein, which results in higher ATRA plasma concentrations and possibly less cytoplasmic binding of the drug. In this report we detail the results of a phase 2 clinical trial in which we evaluated the biologic effects, tolerance, and safety of the combination EPO + intermittent ATRA in patients with MDS.
Patients and methods
Patients
Twenty-seven patients with low- or intermediate-risk MDS according to the International Prognostic Scoring System criteria11 were entered into the study after informed consent had been obtained. These were consecutive eligible patients from a series of 41 patients with low- or intermediate-risk MDS. All patients had histologically confirmed MDS; bone marrow aspirates were classified according to the French-American-British (FAB) criteria.12 Pretreatment clinical and hematologic characteristics of the patients are shown in Table1. Median time from diagnosis to initiation of growth factor therapy was 23 months (range, 8-56 months). At enrollment, 21 patients were transfusion dependent; the median was 2.5 U (range, 2-4 U) packed red blood cell (RBC) transfusions per month for 3 months before the study. Transfusions were usually given when the hemoglobin concentration was less than 8 g/dL, though occasionally we adopted different thresholds in individual patients in accordance with their compliance with low hemoglobin levels. In addition, sometimes transfusions were delayed because of a shortage of blood products, which occurred frequently during the holidays. None of the patients had previously undergone cytotoxic therapy for MDS.
Summary of pretreatment characteristics
| No. patients | 27 |
| Men | 13 |
| Women | 14 |
| Age (y) | |
| Median | 68 |
| Range | 52-78 |
| FAB subtype | |
| RA | 19 |
| RARS | 3 |
| RAEB | 5 |
| Karyotype* | |
| Good | 12 |
| Intermediate | 6 |
| Poor | 1 |
| IPSS† | |
| Low risk | 5 |
| Intermediate-1 risk | 13 |
| Intermediate-2 risk | 1 |
| Not applicable | 8 |
| Hemoglobin levels | |
| Median | 8.1 g/dL |
| Range | 6.1-9.5 g/dL |
| Absolute neutrophil count | |
| Median | 1.9 × 109/L |
| Range | 0.5-4.1 × 109/L |
| Platelet count | |
| Median | 145 × 109/L |
| Range | 32-298 × 109/L |
| Serum erythropoietin (n = 21) | |
| Median | 368.5 mIU/mL |
| Range | 80-1482 mIU/mL |
| Serum ferritin | |
| Median | 654 ng/mL |
| Range | 157-1348 ng/mL |
| MDS duration before rhEPO treatment | |
| Median | 23 months |
| Range | 8-56 months |
| No. patients | 27 |
| Men | 13 |
| Women | 14 |
| Age (y) | |
| Median | 68 |
| Range | 52-78 |
| FAB subtype | |
| RA | 19 |
| RARS | 3 |
| RAEB | 5 |
| Karyotype* | |
| Good | 12 |
| Intermediate | 6 |
| Poor | 1 |
| IPSS† | |
| Low risk | 5 |
| Intermediate-1 risk | 13 |
| Intermediate-2 risk | 1 |
| Not applicable | 8 |
| Hemoglobin levels | |
| Median | 8.1 g/dL |
| Range | 6.1-9.5 g/dL |
| Absolute neutrophil count | |
| Median | 1.9 × 109/L |
| Range | 0.5-4.1 × 109/L |
| Platelet count | |
| Median | 145 × 109/L |
| Range | 32-298 × 109/L |
| Serum erythropoietin (n = 21) | |
| Median | 368.5 mIU/mL |
| Range | 80-1482 mIU/mL |
| Serum ferritin | |
| Median | 654 ng/mL |
| Range | 157-1348 ng/mL |
| MDS duration before rhEPO treatment | |
| Median | 23 months |
| Range | 8-56 months |
Good indicates normal (Y, del[5q], del[20q]). Poor indicates complex (≥ 3 abnormalities) or chromosome 7 anomalies. Intermediate indicates other abnormalities.
International Prognostic Scoring System.11
Eligibility criteria were as follows: primary MDS with less than 10% blasts on bone marrow examination; performance status of 2 according to the Eastern Cooperative Oncology Group (ECOG) scale13; hemoglobin levels lower than 10 g/dL; normal renal and hepatic function; normal iron, vitamin B12, and folate levels, clinically significant heart and central nervous system disease, uncontrolled hypertension, florid infections, or other malignancies. On study entry, patients gave signed, institutional review board–approved informed consent.
Study design
Therapy consisted of a 12-week schedule of ATRA and EPO. ATRA (Vesanoid; Roche, Milan, Italy) was administered orally at a dose of 80 mg/m2 per day in 2 divided doses for 7 consecutive days every other week. Recombinant human EPO (Eprex; Janssen-Cilag, Milan, Italy) was given subcutaneously 3 times a week. The EPO dose was initiated at 150 U/kg and was increased to 300 U/kg if after 6 weeks there was no or there was suboptimal erythroid response. Patients who responded to therapy were continued on ATRA and EPO at the same doses for 6 more months (extension phase). Further treatment was given to patients who had continued responses. Patients were questioned weekly concerning possible adverse events. Treatment was discontinued at the patient's request if severe side effects or transformation to AML occurred.
Response criteria
Responses were categorized according to recently published criteria by an international working group.14 Regarding the erythroid lineage, a major response (MaR) was considered a rise in untransfused hemoglobin concentrations of at least 2 g/dL or a 100% decrease in RBC transfusion requirements during the treatment period. A minor response (MiR) was defined as an increase in untransfused hemoglobin values of 1 to 2 g/dL or a 50% or greater decrease in RBC transfusion requirements. No response was defined as a response less than a minor response. Regarding platelets, for patients with pretreatment platelet counts less than 100 × 109/L, MaR was defined as an absolute increase of 30 × 109/L or more, and MiR was a 50% increase in platelet count with a net increase greater than 10 × 109/L but not exceeding 30 × 109/L. Regarding neutrophils, for patients with pretreatment absolute neutrophil counts (ANCs) less than 1.5 × 109/L, MaR was considered at least a 100% increase or an absolute increase of 0.5 × 109/L, whichever was greater; MiR required a 100% or greater increment in ANC, but absolute increase required less than 0.5 × 109/L.
Study parameters and monitoring of patients
Patient evaluation before entry included complete history and physical examination. All patients underwent chest roentgenography and electrocardiography. Baseline laboratory evaluation included a complete blood cell count with reticulocytes, serum EPO, serum ferritin, vitamin B12 and folate levels, routine serum chemistry, coagulation tests, and urinalysis. Vital signs and complete blood cell counts were monitored once a week. Serum EPO levels were determined using a commercially available enzyme-linked immunoassay (Quantikine IVD Erythropoietin; R&D Systems, Minneapolis, MN). Bone marrow aspirates and biopsy specimens were taken at diagnosis and at the end of the study (aspirates) or when clinically required. Karyotyping was carried out with standard techniques15 at study entry and, in responders, at the end of treatment.
Erythroid progenitor cell assay
Heparinized blood samples were collected at baseline, at 12 weeks, and, in responders, during the extension phase. Erythroid blast-forming units (BFU-E) were assayed in viscous medium using a modification of the method of Iscove and colleagues,16 as previously described. Briefly, 2 × 105 peripheral blood mononuclear cells were plated in triplicate in 35-mm Petri dishes with 1-mL aliquots of 0.9% methylcellulose viscous Iscoves modified Dulbecco medium (Gibco, Grand Island, NY) supplemented with 30% human AB serum, 10% bovine serum albumin (Fraction V; Sigma, St Louis, MO), 1 × 10−4 M 2-mercaptoethanol (Sigma), and 2 U EPO (Ortho Diagnostic Systems, Raritan, NJ). After incubation for 14 days at 37°C in a humidified atmosphere supplemented with 5% CO2, the cultures were scored for BFU-E (defined as bursts of colonies consisting of hemoglobinated cells) with an inverted microscope.
Measurement of apoptosis
Apoptosis was measured by flow cytometry with a FACScan instrument (Becton Dickinson, Mountain View, CA). Mononuclear cell fractions of bone marrow samples were separated after Ficoll-Hypaque gradient centrifugation and washed twice with phosphate-buffered saline. Cells (1 × 106) were then incubated with phycoerythrin-conjugated anti-CD34 mAb (anti–HPCA-2, IgG1; Becton Dickinson) for 10 minutes at room temperature in the dark and were washed twice with phosphate-buffered saline. Pelleted cells were resuspended in 100 μL binding buffer (10 mM HEPES/NaOH, pH 7.4, 140 mM NaCl, 2.5 mM CaCl2; Bender Medsystems, Boehringer Ingelheim, Ridgefield, CT) and were incubated with 2 μL fluorescein isothiocyanate–conjugated annexin V (Bender Medsystems; Boehringer Ingelheim) for 10 minutes at room temperature in the dark. Afterward, cells were resuspended in 400 μL binding buffer before flow cytometric analysis. Analysis was based on gating of subpopulations of CD34+ cells by forward scatter versus side scatter and by side scatter versus fluorescence-2. Negative controls included peripheral blood mononuclear cells incubated with neither CD34-PE mAb nor annexin V–FITC and cells incubated with CD34-PE mAb only. Bone marrow from 10 healthy donors was used as reference.
Statistical analysis
Statistical evaluation was performed with the STATISTICA for Windows (StatSoft, Tulsa, OK) software package on an IBM-compatible computer. Mann-Whitney U test was used to compare continuous variables between responders and nonresponders. Wilcoxon matched-pairs test was used to compare repeated measurements in the same patients. Fisher exact test was used to evaluate the relationship between 2 dichotomous variables. Correlations of variables with other variables were calculated by Spearman rank correlation coefficient.P < .05 was designated as statistically significant; allP values were 2-tailed.
Results
Response to treatment
All patients completed the 12-week study and were evaluated for toxicity and response. Changes in blood cell counts and transfusion requirements before and after treatment are reported in Table2. According to defined response criteria, on week 12 of treatment the erythroid response rate was 48% (95% confidence interval [CI], 0.28-0.68). The response was major in 5 patients, minor in 8, and absent in 14. Of the 5 patients who attained MaR, 4 exhibited an optimal response at the dose of 150 IU/kg and were maintained with that dose, whereas the fifth achieved an MaR only at the higher dose. Five of the 8 patients with MiR had signs of response after the first 6 weeks of treatment but did not benefit from the higher EPO dose; the other 3 patients showed a response only after challenge with EPO at 300 IU/kg. Five patients (patients 1, 5, 6, 11, and 13) exhibited responses based solely on a decrease in transfusion requirements. Hemoglobin levels at which their transfusions were ordered before and after treatment initiation were: patient 1, 7.5 g/dL and 7.3 g/dL; patient 5, 7.8 g/dL and 7.4 g/dL; patient 6, 7.3 g/dL and 7.9 g/dL; patient 11, 8.0 g/dL and 8.2 g/dL; patient 13, 7.6 and 7.7 g/dL.
Clinical and hematologic characteristics of MDS patients administered EPO plus ATRA
| Patient no. . | Age (y) . | Sex . | FAB subtype . | MDS duration (mo) . | Baseline serum EPO (mIU/mL) . | RBC transfusions . | Hb (g/dL)* . | Erythroid response . | ANC (× 109/L)* . | Neutrophil response . | Platelets (× 109/qL)* . | Platelet response . | Karyotype . | IPSS score value . | IPSS risk group . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before . | After . | Before . | At 6 wks . | At 12 wks . | Before . | At 12 wks . | Before . | At 12 wks . | ||||||||||||
| 1 | 61 | M | RA | 25 | 513 | 3 | 1 | 7.9 | 7.3 | 8.2 | MiR | 2.5 | 2.7 | NA | 93 | 158 | MaR | Good | 0.5 | Int-1 |
| 2 | 64 | M | RA | 47 | 673 | 2 | 2 | 8.4 | 7.9 | 8.5 | NR | 1.7 | 1.6 | NA | 145 | 175 | NA | Good | 0 | Low |
| 3 | 57 | F | RA | 12 | 281 | 0 | 0 | 8.5 | 10.9 | 11.3 | MaR | 2.6 | 4.4 | NA | 214 | 266 | NA | ND | NA | NA |
| 4 | 52 | M | RA | 15 | 374 | 0 | 0 | 8.6 | 8.3 | 8.0 | NR | 1.3 | 1.2 | NR | 131 | 115 | NA | Good | 0.5 | Int-1 |
| 5 | 62 | M | RA | 38 | 586 | 4 | 2 | 7.4 | 8.1 | 7.9 | MiR | 0.5 | 1.2 | MaR | 54 | 121 | MaR | Good | 0.5 | Int-1 |
| 6 | 75 | F | RA | 56 | ND | 2 | 1 | 7.7 | 7.8 | 8.6 | MiR | 3.3 | 2.9 | NA | 168 | 238 | NA | Int | 0.5 | Int-1 |
| 7 | 78 | M | RARS | 23 | 938 | 3 | 2 | 6.1 | 6.4 | 6.6 | NR | 1.9 | 2.1 | NA | 151 | 191 | NA | ND | NA | NA |
| 8 | 77 | F | RA | 18 | 1115 | 2 | 0 | 7.2 | 8.5 | 8.3 | MaR | 2.4 | 2.2 | NA | 220 | 246 | NA | Good | 0 | Low |
| 9 | 56 | M | RA | 44 | 231 | 2 | 2 | 8.1 | 8.7 | 7.8 | NR | 0.9 | 1.2 | NR | 84 | 75 | NR | Int | 1 | Int-1 |
| 10 | 73 | F | RAEB | 33 | 125 | 0 | 0 | 9.5 | 9.3 | 10.1 | NR | 1.4 | 1.5 | NR | 136 | 163 | NA | Int | 1.5 | Int-1 |
| 11 | 71 | M | RA | 51 | 80 | 2 | 1 | 8.6 | 8.7 | 9.5 | MiR | 3.2 | 3.5 | NA | 117 | 191 | NA | ND | NA | NA |
| 12 | 68 | F | RA | 55 | 1482 | 4 | 4 | 6.3 | 6.1 | 6.6 | NR | 1.2 | 1.5 | NR | 298 | 263 | NA | ND | NA | NA |
| 13 | 54 | F | RAEB | 21 | 363 | 2 | 1 | 8.5 | 8.1 | 8.8 | MiR | 1.9 | 2.4 | NA | 201 | 152 | NA | Int | 1 | Int-1 |
| 14 | 72 | M | RARS | 9 | ND | 3 | 3 | 7.4 | 8.3 | 8.1 | NR | 2.1 | 3.2 | NA | 85 | 77 | NR | ND | NA | NA |
| 15 | 62 | F | RA | 15 | 269 | 0 | 0 | 9.3 | 11.4 | 11.6 | MaR | 3.7 | 4.1 | NA | 104 | 123 | NA | ND | NA | NA |
| 16 | 69 | M | RAEB | 16 | 181 | 0 | 0 | 8.6 | 9.1 | 9.7 | MiR | 1.3 | 3.5 | MaR | 32 | 53 | MiR | Poor | 2 | Int-2 |
| 17 | 76 | F | RA | 52 | ND | 2 | 2 | 8.3 | 9.0 | 8.7 | NR | 1.0 | 1.8 | MaR | 102 | 92 | NA | Good | 0.5 | Int-1 |
| 18 | 70 | F | RA | 47 | 135 | 3 | 0 | 7.6 | 9.6 | 10.9 | MaR | 3.1 | 4.3 | NA | 74 | 166 | MaR | ND | NA | NA |
| 19 | 64 | M | RA | 18 | 560 | 3 | 2 | 8.1 | 8.6 | 8.4 | NR | 2.3 | 2.1 | NA | 126 | 295 | NA | Good | 0 | Low |
| 20 | 77 | F | RARS | 31 | 175 | 0 | 0 | 9.1 | 10.0 | 9.8 | NR | 4.1 | 4.4 | NA | 229 | 247 | NA | Good | 0 | Low |
| 21 | 68 | F | RA | 27 | ND | 3 | 3 | 7.9 | 7.5 | 8.1 | NR | 2.0 | 2.6 | NA | 141 | 173 | NA | Int | 0.5 | Int-1 |
| 22 | 66 | F | RAEB | 19 | 658 | 2 | 1 | 6.7 | 8.0 | 7.8 | MiR | 1.1 | 2.7 | MaR | 158 | 179 | NA | Good | 1 | Int-1 |
| 23 | 61 | M | RA | 34 | 165 | 2 | 0 | 8.1 | 9.3 | 9.5 | MiR | 1.4 | 3.1 | MaR | 86 | 137 | MaR | Int | 1 | Int-1 |
| 24 | 70 | F | RA | 13 | 273 | 0 | 0 | 8.5 | 8.2 | 8.7 | NR | 2.3 | 2.0 | NA | 147 | 128 | NA | Good | 0 | Low |
| 25 | 63 | F | RA | 8 | ND | 3 | 2 | 6.9 | 7.3 | 8.0 | NR | 0.9 | 0.7 | NR | 131 | 126 | NA | Good | 0.5 | Int-1 |
| 26 | 72 | M | RA | 22 | 838 | 2 | 0 | 7.1 | 8.6 | 10.4 | MaR | 1.3 | 1.6 | NR | 93 | 145 | MaR | Good | 0.5 | Int-1 |
| 27 | 74 | M | RAEB | 9 | 715 | 3 | 3 | 6.8 | 7.3 | 7.5 | NR | 1.0 | 0.8 | NR | 67 | 78 | NR | ND | NA | NA |
| Patient no. . | Age (y) . | Sex . | FAB subtype . | MDS duration (mo) . | Baseline serum EPO (mIU/mL) . | RBC transfusions . | Hb (g/dL)* . | Erythroid response . | ANC (× 109/L)* . | Neutrophil response . | Platelets (× 109/qL)* . | Platelet response . | Karyotype . | IPSS score value . | IPSS risk group . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before . | After . | Before . | At 6 wks . | At 12 wks . | Before . | At 12 wks . | Before . | At 12 wks . | ||||||||||||
| 1 | 61 | M | RA | 25 | 513 | 3 | 1 | 7.9 | 7.3 | 8.2 | MiR | 2.5 | 2.7 | NA | 93 | 158 | MaR | Good | 0.5 | Int-1 |
| 2 | 64 | M | RA | 47 | 673 | 2 | 2 | 8.4 | 7.9 | 8.5 | NR | 1.7 | 1.6 | NA | 145 | 175 | NA | Good | 0 | Low |
| 3 | 57 | F | RA | 12 | 281 | 0 | 0 | 8.5 | 10.9 | 11.3 | MaR | 2.6 | 4.4 | NA | 214 | 266 | NA | ND | NA | NA |
| 4 | 52 | M | RA | 15 | 374 | 0 | 0 | 8.6 | 8.3 | 8.0 | NR | 1.3 | 1.2 | NR | 131 | 115 | NA | Good | 0.5 | Int-1 |
| 5 | 62 | M | RA | 38 | 586 | 4 | 2 | 7.4 | 8.1 | 7.9 | MiR | 0.5 | 1.2 | MaR | 54 | 121 | MaR | Good | 0.5 | Int-1 |
| 6 | 75 | F | RA | 56 | ND | 2 | 1 | 7.7 | 7.8 | 8.6 | MiR | 3.3 | 2.9 | NA | 168 | 238 | NA | Int | 0.5 | Int-1 |
| 7 | 78 | M | RARS | 23 | 938 | 3 | 2 | 6.1 | 6.4 | 6.6 | NR | 1.9 | 2.1 | NA | 151 | 191 | NA | ND | NA | NA |
| 8 | 77 | F | RA | 18 | 1115 | 2 | 0 | 7.2 | 8.5 | 8.3 | MaR | 2.4 | 2.2 | NA | 220 | 246 | NA | Good | 0 | Low |
| 9 | 56 | M | RA | 44 | 231 | 2 | 2 | 8.1 | 8.7 | 7.8 | NR | 0.9 | 1.2 | NR | 84 | 75 | NR | Int | 1 | Int-1 |
| 10 | 73 | F | RAEB | 33 | 125 | 0 | 0 | 9.5 | 9.3 | 10.1 | NR | 1.4 | 1.5 | NR | 136 | 163 | NA | Int | 1.5 | Int-1 |
| 11 | 71 | M | RA | 51 | 80 | 2 | 1 | 8.6 | 8.7 | 9.5 | MiR | 3.2 | 3.5 | NA | 117 | 191 | NA | ND | NA | NA |
| 12 | 68 | F | RA | 55 | 1482 | 4 | 4 | 6.3 | 6.1 | 6.6 | NR | 1.2 | 1.5 | NR | 298 | 263 | NA | ND | NA | NA |
| 13 | 54 | F | RAEB | 21 | 363 | 2 | 1 | 8.5 | 8.1 | 8.8 | MiR | 1.9 | 2.4 | NA | 201 | 152 | NA | Int | 1 | Int-1 |
| 14 | 72 | M | RARS | 9 | ND | 3 | 3 | 7.4 | 8.3 | 8.1 | NR | 2.1 | 3.2 | NA | 85 | 77 | NR | ND | NA | NA |
| 15 | 62 | F | RA | 15 | 269 | 0 | 0 | 9.3 | 11.4 | 11.6 | MaR | 3.7 | 4.1 | NA | 104 | 123 | NA | ND | NA | NA |
| 16 | 69 | M | RAEB | 16 | 181 | 0 | 0 | 8.6 | 9.1 | 9.7 | MiR | 1.3 | 3.5 | MaR | 32 | 53 | MiR | Poor | 2 | Int-2 |
| 17 | 76 | F | RA | 52 | ND | 2 | 2 | 8.3 | 9.0 | 8.7 | NR | 1.0 | 1.8 | MaR | 102 | 92 | NA | Good | 0.5 | Int-1 |
| 18 | 70 | F | RA | 47 | 135 | 3 | 0 | 7.6 | 9.6 | 10.9 | MaR | 3.1 | 4.3 | NA | 74 | 166 | MaR | ND | NA | NA |
| 19 | 64 | M | RA | 18 | 560 | 3 | 2 | 8.1 | 8.6 | 8.4 | NR | 2.3 | 2.1 | NA | 126 | 295 | NA | Good | 0 | Low |
| 20 | 77 | F | RARS | 31 | 175 | 0 | 0 | 9.1 | 10.0 | 9.8 | NR | 4.1 | 4.4 | NA | 229 | 247 | NA | Good | 0 | Low |
| 21 | 68 | F | RA | 27 | ND | 3 | 3 | 7.9 | 7.5 | 8.1 | NR | 2.0 | 2.6 | NA | 141 | 173 | NA | Int | 0.5 | Int-1 |
| 22 | 66 | F | RAEB | 19 | 658 | 2 | 1 | 6.7 | 8.0 | 7.8 | MiR | 1.1 | 2.7 | MaR | 158 | 179 | NA | Good | 1 | Int-1 |
| 23 | 61 | M | RA | 34 | 165 | 2 | 0 | 8.1 | 9.3 | 9.5 | MiR | 1.4 | 3.1 | MaR | 86 | 137 | MaR | Int | 1 | Int-1 |
| 24 | 70 | F | RA | 13 | 273 | 0 | 0 | 8.5 | 8.2 | 8.7 | NR | 2.3 | 2.0 | NA | 147 | 128 | NA | Good | 0 | Low |
| 25 | 63 | F | RA | 8 | ND | 3 | 2 | 6.9 | 7.3 | 8.0 | NR | 0.9 | 0.7 | NR | 131 | 126 | NA | Good | 0.5 | Int-1 |
| 26 | 72 | M | RA | 22 | 838 | 2 | 0 | 7.1 | 8.6 | 10.4 | MaR | 1.3 | 1.6 | NR | 93 | 145 | MaR | Good | 0.5 | Int-1 |
| 27 | 74 | M | RAEB | 9 | 715 | 3 | 3 | 6.8 | 7.3 | 7.5 | NR | 1.0 | 0.8 | NR | 67 | 78 | NR | ND | NA | NA |
NA indicates not applicable; ND, not done; Int, intermediate.
Blood counts before and after treatment were calculated by averaging the results of 3 counts taken in a 2-week period.
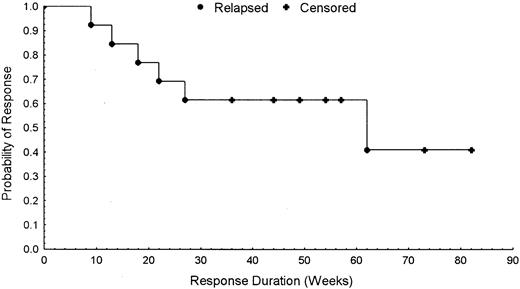
In responders, the increase in hemoglobin concentration was associated with a significant increase in reticulocyte counts. Mean reticulocyte count was 20 531/μL (95% CI, 13 326-27 736/μL) before treatment versus 50 423/μL (95% CI, 35 459-65 387/μL) on week 12 of treatment (P = .001475). Ten of the 13 patients administered ATRA + EPO during the extension phase had continued response. Although we had initially planned to stop treatment after 6 months, the good compliance to combination therapy and the requests from the patients themselves induced us to prolong treatment. Thus far, with a median follow-up of 15 months, 7 patients are still responsive. Figure 1 illustrates a Kaplan-Meier plot of probability of response versus time for the 13 patients with erythroid responses.
Response versus time.
Kaplan-Meier plot of probability of response versus time for the 13 patients with erythroid responses.
Response versus time.
Kaplan-Meier plot of probability of response versus time for the 13 patients with erythroid responses.
Five of the 12 patients with pretreatment neutropenia had increases in neutrophil count of 0.5 × 109/L or greater (range of ANC increase, 0.7 to 2.2 × 109/L), for a response rate of 42% (95% CI, 0.09-0.74). Ten other patients who did not have neutropenia before treatment showed an increase in neutrophil count ranging from 0.2 to 1.8 × 109/L. The neutrophil response began to appear after 2 weeks of treatment and was continued throughout treatment, though we observed that the ANC tended to be higher when patients were administered ATRA. At the time of this report, all 5 patients maintain their response.
Five patients with pretreatment thrombocytopenia exhibited increases in platelet count exceeding 30 × 109/L (range of platelet count increment, 33 to 92 × 109/L), and one patient (patient 16) had an MiR and an increase in platelet count of 21 × 109/L. When only patients with pretreatment platelet counts less than 100 × 109/L (n = 9) were considered, the response rate was 67% (95% CI, 0.28-1.05). In 12 patients who had normal counts before treatment, we observed platelet increases ranging from 18 to 169 × 109/L. Platelet response was maintained throughout treatment for 4 of the 6 patients, whereas patients 1 and 26 had relapses after 65 and 28 weeks of extended treatment, respectively. It is noteworthy that the platelet response was slower than the hemoglobin and neutrophil responses because it began to appear after 4 weeks from the start of treatment. Three patients (patients 5, 16, and 23) displayed trilineage hematologic improvement that was sustained during continuation therapy.
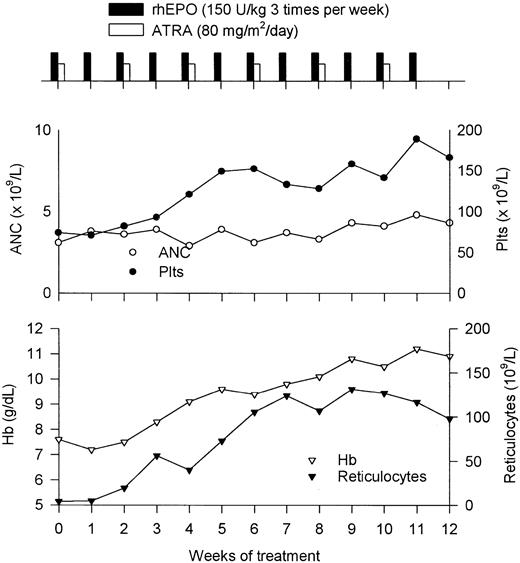
A representative responding patient's course (patient 18) is shown in Figure 2. The patient was a 70-year-old woman with a 4-year history of MDS and a transfusion need of 3 RBC units per month. Significant improvement of hemogram parameters was observed after 4 weeks of treatment, at which time her hemoglobin concentration rose to 8.3 g/dL without transfusions and her platelet count increased to 93 × 109/L from a baseline value of 74 × 109/L. A constant increase in hemoglobin and platelet values was noted thereafter, with hemoglobin (11.2 g/dL) and platelet (189 × 109/L) peaks recorded in week 11. This patient eventually maintained hemoglobin concentrations in the 10 to 11 g/dL range and platelet values above 100 × 109/L during the extension phase.
Response to combination therapy.
Clinical course of a patient with MDS responding to combination therapy with EPO and ATRA. See “Results” for details.
Response to combination therapy.
Clinical course of a patient with MDS responding to combination therapy with EPO and ATRA. See “Results” for details.
Examination of marrow aspirates on conclusion of the study showed a nonsignificant increase in the percentage of erythroid cells in 1 of 3 major responders and in 2 of 6 minor responders. As assessed by clinical parameters and bone marrow morphology, disease progression was not observed in any patient.
Laboratory studies
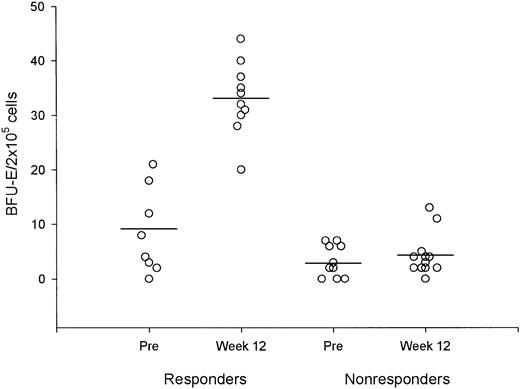
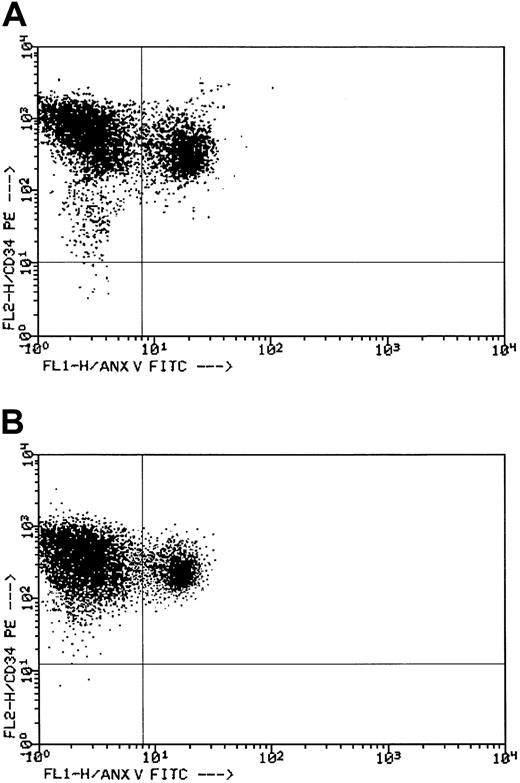
As shown in Figure 3, the number of circulating BFU-E in responders during week 12 of treatment consistently increased compared with baseline (P < .001). Analysis of karyotype at the end of the study (available in 12 patients) did not show remarkable changes. Pretreatment determination of the degree of apoptosis in hematopoietic progenitors by means of the annexin V method indicated no significant differences between the FAB subgroups. In refractory anemia (RA) and RA with ringed sideroblasts (RARS), the median CD34+ cell apoptosis was 52.3% (range, 24.3%-86.4%), whereas in RA with excess blasts (RAEB) it was 49.3% (range, 38.7%-61.2%; P not significant). Control patients had median CD34+ cell apoptosis of 14.8% (range, 5.5%-27.9%; P < .001). In week 12 of treatment, CD34+ cell apoptosis was significantly decreased in responders (median, 35.1%; range, 15.4%-56.4%) compared to nonresponders (median, 57.3%; range, 43.4%-81.2%;P < .001). Further determinations during the extension phase in 6 patients produced results almost superimposable with those obtained in week 12. Figure 4 reports the double-color analyses with antibodies against CD34 (phycoerythrin) and against Annexin V (fluorescein isothiocyanate) in patient 18. Double-positive cells (upper right quadrant) dropped from 38.6% before treatment (Figure 4A) to 15.4% during the extension phase (Figure 4B).
Responders versus nonresponders.
Comparison of circulating BFU-E before and after 12 weeks of treatment in responders and nonresponders. Horizontal lines represent mean values.
Responders versus nonresponders.
Comparison of circulating BFU-E before and after 12 weeks of treatment in responders and nonresponders. Horizontal lines represent mean values.
Flow cytometric evaluation of CD34+ cell apoptosis in a responder.
See “Results” for details. (A) Dot plot histogram before treatment. (B) Dot plot histogram during the extension phase.
Flow cytometric evaluation of CD34+ cell apoptosis in a responder.
See “Results” for details. (A) Dot plot histogram before treatment. (B) Dot plot histogram during the extension phase.
Safety
Treatment was well tolerated overall. Patient 15 had a mild increase in arterial blood pressure after 8 weeks of treatment that was easily controlled by medical therapy. Patient 3 had painful erythema at the site of recombinant human (rh)EPO injections, though it did not interrupt rhEPO administration. ATRA-related side effects were observed in all patients and are detailed in Table3. In particular, changes in the skin and mucous membranes developed in 22 (81%) patients and consisted of erythema (particularly in the areas of greater distribution of the sebaceous glands), hyperkeratosis, cheilitis, conjunctivitis, and nail dystrophy. All these side effects were grade 1 or 2 according to the National Cancer Institute toxicity scale. While on the protocol, approximately 50% of the patients experienced elevations of serum triglycerides; biochemical signs of liver cell damage or renal toxicity were not noted.
Side effects associated with therapy
| Side effect . | No. patients . |
|---|---|
| Dry skin | 22 |
| Cheilitis | 17 |
| Conjunctivitis | 7 |
| Nail dystrophy | 3 |
| Nausea | 2 |
| Headache | 1 |
| Myalgias | 1 |
| Hypertension | 1 |
| Side effect . | No. patients . |
|---|---|
| Dry skin | 22 |
| Cheilitis | 17 |
| Conjunctivitis | 7 |
| Nail dystrophy | 3 |
| Nausea | 2 |
| Headache | 1 |
| Myalgias | 1 |
| Hypertension | 1 |
Prognostic factors
Table 4 shows the clinical and laboratory characteristics of the 13 patients who had erythroid responses (MaR+MiR) to ATRA plus EPO treatment compared with those of the 14 patients who did not respond. No variable was significantly different between the 2 groups of patients in univariate analysis. Similarly, no combination of these characteristics demonstrated a significant association with response. We were unable to show the predictive value of these variables for neutrophil and platelet responses.
Comparison of clinical and laboratory characteristics of MDS patients responding and not responding to ATRA plus EPO treatment
| . | Responders (range) . | Nonresponders (range) . | P . |
|---|---|---|---|
| Median age, y | 66 (54-77) | 69 (52-78) | .319 330 |
| M/F | 6/7 | 7/7 | 1.000 000 |
| MDS duration, mo | 22 (12-56) | 25 (8-55) | .644 601 |
| Serum EPO | 322 (80-1115) | 467 (125-1482) | .468 262 |
| Prior RBC transfusion requirements, U/mo | 2 (0-4) | 2.5 (0-4) | .507 629 |
| Hb levels, g/dL | 7.9 (6.7-9.3) | 8.1 (6.1-9.5) | .884 009 |
| Reticulocytes, × 109/L | 22.33 (5.9-80.0) | 18.89 (4.86-86.3) | .752 408 |
| ANC, × 109/L | 2.4 (0.5-3.7) | 1.55 (0.9-4.1) | .144 909 |
| Platelets, × 109/L | 104 (32-220) | 133.5 (67-298) | .528 024 |
| Cytogenetics, normal/abnormal | 5/4 | 7/3 | .649 920 |
| . | Responders (range) . | Nonresponders (range) . | P . |
|---|---|---|---|
| Median age, y | 66 (54-77) | 69 (52-78) | .319 330 |
| M/F | 6/7 | 7/7 | 1.000 000 |
| MDS duration, mo | 22 (12-56) | 25 (8-55) | .644 601 |
| Serum EPO | 322 (80-1115) | 467 (125-1482) | .468 262 |
| Prior RBC transfusion requirements, U/mo | 2 (0-4) | 2.5 (0-4) | .507 629 |
| Hb levels, g/dL | 7.9 (6.7-9.3) | 8.1 (6.1-9.5) | .884 009 |
| Reticulocytes, × 109/L | 22.33 (5.9-80.0) | 18.89 (4.86-86.3) | .752 408 |
| ANC, × 109/L | 2.4 (0.5-3.7) | 1.55 (0.9-4.1) | .144 909 |
| Platelets, × 109/L | 104 (32-220) | 133.5 (67-298) | .528 024 |
| Cytogenetics, normal/abnormal | 5/4 | 7/3 | .649 920 |
Discussion
In this study, 27 patients with low- or intermediate-risk MDS were treated with a combination of intermittent oral ATRA and subcutaneous EPO for a 12-week study period. The EPO dose was doubled if after 6 weeks there was no erythroid response or a suboptimal erythroid response. Erythroid responses were seen in 13 (48%) patients (MaR in 5 patients, MiR in 8 patients), with 10 (37%) patients displaying a continued response during 6 months of extended treatment and 7 (26%) maintaining their response after a median follow-up period of 15 months. Neutrophil response was observed in 5 (42%) and platelet response in 6 (67%) patients, respectively. Three patients displayed trilineage response that was sustained during continuation therapy. Side effects were observed in all patients but were of mild entity and did not require the discontinuation of treatment.
Although in this study we used oral ATRA in alternate weeks at 80 mg/m2 per day, the optimal schedule in association with EPO is unknown because other schedules have not been systematically investigated. In addition, EPO has been used in MDS in a variety of doses and schedules. In most reports, we find starting doses of 450 U/kg per week, as in the current study.
When interpreting our results, it seems reasonable to ask what is the relative contribution of ATRA and EPO to the results and whether their effects are synergistic or simply additive. Although these issues were not investigated directly, some considerations can be inferred from our data and other reported studies. We know that improvement of hemoglobin levels in response to EPO therapy in patients with MDS stands, on average, at approximately 20%1 but that by patient selection (FAB types RA and RAEB, serum EPO levels less than 200 mIU/mL, no transfusion need) responses to EPO alone can be in excess of 50%.1 Because in our series only 2 patients displayed the pretreatment characteristics of good responders to EPO, it seems unlikely that the high response rate of this study could be obtained with EPO alone. Nevertheless, the limited sample size of this study and the wide confidence interval (28%-68%) did not allow us to fully support superiority of combination therapy over EPO alone or to conclude that the use of ATRA results in a synergistic effect on erythroid precursors. Regarding myeloid and megakaryocytic lineages, the response rate was in the magnitude of 25%, far above the expected additive effect of the single drugs, but again we have to acknowledge that the relatively small number of patients raises concerns about the reproducibility of these findings.
A remarkable result of our study is the sustained response to therapy in 26% of the patients because the clinical usefulness of any therapy in MDS depends on response duration and on response rate. Actually, we do not know whether these patients would stay in prolonged remission only with the combination therapy or with either agent as single therapy. For example, in the study by Negrin et al16 in which EPO was combined with granulocyte–colony-stimulating factor, approximately half the responders maintained an erythroid response with EPO alone. Incidentally, in that study the variables that turned out to be predictive of erythroid response were serum EPO, reticulocyte count, and cytogenetic pattern, whereas in our own study no pretreatment characteristic was significantly associated with response. As stated before, the good compliance with combination therapy and requests from the patients themselves induced us to prolong unchanged treatment until relapse occurred.
Results of laboratory investigations performed in this study indicate that response to treatment is associated with higher concentrations of BFU-E in the peripheral blood and with a remarkable decrease of the bone marrow fraction of apoptotic CD34+ cells. Whether these findings represent a stimulation of residual polyclonal hematopoiesis or an actual reduction in the degree of ineffective hematopoiesis remains undetermined. Studies suggest that one mechanism of the positive effects of combination therapy with growth factors is in fact the inhibition of apoptosis.17 The current study confirms previous findings that bone marrow of patients with MDS contains significantly more apoptotic cells than bone marrow of healthy persons; it also confirms that there is ample variation between patients.18-20 There was no significant relationship between pretreatment values for apoptosis and treatment outcome. This suggests that apoptosis in itself is not the only factor for a clinical response to the combination therapy we used. In drawing these conclusions, however, we must emphasize that there are many techniques now available to assess apoptosis, each with a different sensitivity. Although our results are perfectly in line with those of other researchers,17 20 this aspect should be carefully evaluated when comparing results from different studies.
In conclusion, the combination of intermittent oral ATRA and subcutaneous EPO is an effective and well-tolerated treatment for patients with low- and intermediate-risk MDS. Several issues such as optimal ATRA and EPO schedules, role of maintenance treatment, and mechanisms of response remain to be determined and deserve further investigation.
We thank Janssen-Cilag and Roche Companies (Milan, Italy) for supplying, in part, the recombinant human erythropoietin and the all-trans retinoic acid used in this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Roberto Stasi, Department of Medical Sciences, Regina Apostolorum Hospital, Via S. Francesco 50, 00041 Albano Laziale, Italy; e-mail: roberto.stasi@libero.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal