Herein, we show that CD8dull, CD8intermediate, and CD8bright natural killer (NK) cell clones can be identified. Triggering of CD8 with its natural ligand(s), represented by soluble HLA class I (sHLA-I), isolated either from serum of healthy donors or from HLA-I− 721.221 lymphoblastoid cell line transfected with HLA-A2, -Cw4, and -Bw46 alleles, or HLA-G1 leads to NK cell apoptosis. The magnitude of this effect directly correlated with the level of CD8 expression. sHLA-I–induced apoptosis depends on the interaction with CD8, as it was inhibited by masking this molecule with specific monoclonal antibodies (mAbs). Moreover, sHLA-I or CD8 cross-linking with specific mAbs elicited intracellular calcium increases, Fas ligand (FasL) messenger RNA transcription, and FasL secretion, which were needed for delivering the death signal. Indeed, this apoptosis was inhibited by preincubation of NK cell clones with Fas or FasL antagonist mAbs, indicating that the Fas/FasL pathway is involved. Furthermore, members of the inhibitory receptor superfamily, such as CD94/NKG2 complex or killer inhibitory receptors, were shown to exert an inhibitory effect on sHLA-I–mediated apoptosis and secretion of FasL. These findings suggest that interaction between sHLA-I and CD8 evokes an apoptotic signal that is down-regulated by inhibitory receptor superfamily that function as survival receptors in NK cells.
Introduction
CD8 antigen is expressed on a subpopulation of T-cell receptor (TCR) αβ+ T lymphocytes as a heterodimer composed of an α and a β chain, whereas natural killer (NK) cells bear a homodimer of α chain.1 CD8 binds to most of the known HLA class I (HLA-I) molecules by recognizing the α3 or membrane-proximal domain, and it functions as a costimulatory molecule in cytotoxic + lymphocytes (CTLs) by favoring the interaction of TCR and HLA-I expressed on target cells.2-9However, the functional role of CD8 in NK cells is not fully understood: in fact, only a fraction of peripheral blood NK cells express this molecule, and its role in the recognition of HLA-I on target cells has not been investigated so far. Several additional receptors for HLA-I–specific alleles are present on NK cells, represented by some members of the inhibitory receptor superfamily (IRS).10-12 IRS can be subdivided in 2 structural types of molecules: one consists of the immunoglobulin-superfamily inhibitory receptors (ISIRs) KIR2DL and KIR3DL and the other of the C-type lectin inhibitory receptors (CLIRs) CD94/NKG2 complex. On interaction with HLA-I expressed on target cells, these IRSs can deliver an inhibitory signal, leading to the reduction of cytolytic activity as well as to the inhibition of cytokine secretion.10-12 It is not clear why NK cells express several inhibitory receptors for different alleles of HLA-I and a unique receptor, CD8, for a monomorphic portion of HLA-I. It is well known that soluble HLA-I (sHLA-I) heavy chain is present in human serum either free or associated to β2-microglobulin.13-16 sHLA-I serum levels vary among different individuals and are significantly affected by inflammatory diseases and transplant rejection.16-19 sHLA-I has been shown to inhibit cytolytic activity of both alloreactive CTL and NK cells in vitro.13-17 More recently, it has been reported that sHLA-I, interacting with CD8 molecules, induces apoptosis of CD8+ T lymphocytes on binding of soluble Fas ligand (FasL) to Fas antigen expressed by these cells. This phenomenon may play a role in the regulation of antigen-specific immune responses by inducing programmed cell death of effector T lymphocytes.13-16 18-24 However, it is not clear whether sHLA-I delivers an apoptotic signal by interacting with CD8 only or through the simultaneous engagement of CD8 and TCR.
Herein, we show that the interaction of sHLA-I with CD8α molecules leads to NK cell apoptosis through the secretion of FasL that, in turn, binds to Fas at the NK cell surface. This effect is calcium dependent, and it is down-regulated by the engagement of IRSs that, thus, function as survival receptors in NK cells.
Materials and methods
Monoclonal antibodies and reagents
The anti-CD16 (NK54, immunoglobulin [Ig]G1) monoclonal antibody (mAb); the anti-CD56 (TA181H12, IgG2a) mAb; the anti-CD54 (14D12D2, IgG1) mAb; the anti-CD8α (astra 102, IgG1) mAb; the NKVFS1 mAb, recognizing a common epitope of CD158a and CD158b; and the anti-CD69 mAb (31C4, IgG2a) were produced as described.25 26 The anti-CD3 (Leu4, IgG1), the anti-CD4 (Leu3a, IgG1), and the anti-CD8 (Leu2a, IgG1) mAbs were from Becton Dickinson (Palo Alto, CA). The anti-FasL (NOK-1, IgG1) was from PharMingen (San Diego, CA). The anti-CD94 (HP-3B1, IgG2a), the anti-CD158a (EB6, IgG1), and the anti-CD158b (GL183, IgG1) mAbs were from Serotec (Oxford, United Kingdom). The blocking anti-Fas mAb ZB4 (IgG1) and the apoptosis-inducing anti-Fas mAb CH11 (IgM) were from MBL (Naka-ku Nagoya, Japan); the anti-FasL mAb Alf-2.1a was from Ancell (Bayport, MN). The anti-CD8α-chain OKT8 mAb was purchased from Ortho (Milan, Italy). The mAbs W6/32 and TP25.99 to HLA-I heavy chains α3 domain were a kind gift of S. Ferrone (Roswell Park Memorial, Buffalo, NY). Annexin-V–fluorescein isothiocyanate (FITC) and –propidium iodide (PI) were from Sigma (Milan, Italy). The affinity-purified goat antimouse (GAM) anti-isotype–specific antiserum was from Southern Biotechnology (Birmingham, AL). Purified GAM anti-immunoglobulin (H + L) was purchased from Sigma, the immunomagnetic beads coated with GAM were from Oxoid (Dynal A.S., Oslo, Norway), and the recombinant interleukin 2 (rIL-2) was from Chiron (Proleukin; Chiron Italia, Siena, Italy). Cells were cultured in RPMI 1640 medium with glutamine and penicillin-streptomycin (Biochrom, Berlin, Germany) supplemented with 10% fetal calf serum (Sigma). The L-type calcium channel blocker verapamil and the calcium chelator EGTA were from Sigma.
Indirect immunofluorescence
Single fluorescence staining was performed as described.25 Briefly, aliquots of 105 cells were stained with the corresponding mAb followed by PE-conjugated anti-isotype–specific GAM serum or with an unrelated mAb followed by the fluorescent second reagent. Samples were analyzed on a flow cytometer (FACSort; Becton Dickinson), and results are expressed as Log red mean fluorescence intensity (MFI) in arbitrary units (au) (X-axis) versus number of cells (Y-axis).
Isolation and culture of polyclonal and clonal NK cell populations
Peripheral blood mononuclear cells from healthy volunteers were isolated by Ficoll-Hypaque gradient. CD3−CD4−cells were isolated after negative immunodepletion as described.25 The resulting cell population was 50% to 70% CD16+ (range of 8 different experiments) but 99% CD3−CD4−. Highly purified CD3−CD4− cells were stimulated with 10 μg/mL phytohemagglutinin (PHA) and cultured in 96-well U-bottomed microplates (Becton Dickinson) with complete medium in the presence of 100 U/mL rIL-2 in a final volume of 200 μL/well in the presence of 105/well irradiated allogeneic peripheral blood mononuclear cells and 104/well 721.221 lymphoblastoid cell line.25 Under these culture conditions, by 15 days all cells expressed CD16 and CD56 antigens. CD3−CD16+ clones were obtained by culturing highly purified CD3−CD4− NK cells under limiting dilution conditions as previously reported.25Cloning efficiency was of 5% to 10% calculated as described.28
sHLA-I antigen preparations
sHLA-I molecules were obtained from serum of healthy subjects by precipitation with ammonium sulfate, low-medium pressure chromatography, strong anionic and strong cationic ion exchange, and gel filtration as described19 and were purified by affinity chromatography on anti–HLA-I mAb W6/32 (10 μg/mL) coupled to cyanogen-bromide–activated Sepharose 4B (Pharmacia). The purity of sHLA-I molecule preparations was analyzed by one-dimensional polyacrylamide gel electrophoresis under nonreducing/nondenaturing or reducing/denaturing conditions followed by silver staining or immunoblotting with anti–HLA-I mAb TP25.99.19 Soluble HLA-A2, -Bw46, -Cw4, and -G1 were prepared from culture supernatants (SNs) of 721.221 cells transfected with the corresponding HLA-I alleles.10 20
Determination of soluble FasL in culture SNs
Soluble FasL (sFasL) present in culture SNs derived from NK cell clones after different incubation times (6, 12, 24, 36, and 48 hours), with medium alone, or under the various culture conditions indicated in the “Results” section and figure legends was evaluated by enzyme-linked immunosorbent assay.19 Standard curve was obtained by using progressive dilution of recombinant FasL from Alexis (Leufelfingen, Switzerland). Results were expressed as mean ± SD of triplicate wells.
Induction and detection of apoptosis
Bulk NK cell populations or NK cell clones (105/mL) were cultured in 24-well flat-bottomed plates with culture medium either alone or with different amounts of sHLA-I molecules (from 0.5 to 8 μg/mL) for different time periods (6, 12, 24, 36, 48, 60, and 72 hours) at 37°C in a 5% CO2 atmosphere. In some experiments cells were incubated with anti-CD8 mAb (OKT8, or astra102 orLeu2a) alone or in combination with anti-CD94 (HP-3B1) or anti-CD158b (GL183) or with anti-CD54 mAb (14D12D2) for 30 minutes at 4°C, washed, and then incubated for different times with 4-per-cell GAM-coated magnetic beads.19,29 Cells were then washed, and early apoptotic events were evaluated by annexin-V labeling method to show the exposure of phosphatidyl-serine at the external side of the plasma membrane. Viable apoptotic cells were differentiated from necrotic cells by flow cytometry after PI staining of nonpermeabilized cells. Apoptotic cells were identified as annexin V+PI− cells.19,29 Some experiments were performed in the presence of 0.1, 1, or 10 μM verapamil diluted in dimethyl sulfoxide or with the dimethyl sulfoxide solvent as control, or with 2 mM EGTA (calcium chelator). Analysis of 104cells/sample was done, and results were plotted as the percentage of annexin V+ cells and PI− cells. Apoptosis was also detected by PI staining after permeabilization (DNA content < 2n) and by DNA extraction and agarose gel electrophoresis.19 29
Isolation of RNA, reverse transcription, and polymerase chain reaction amplification
Total RNA was isolated from cell pellets by using the RNAzol B (Biotech Lab, Houston, TX) method.19 Complementary DNA (corresponding to 2 μg RNA) was synthesized from oligo(dT)-primed RNA as described.19 The polymerase chain reaction (PCR) mixture was amplified by using the following primer sequences: β-actin 5′-GTGGGGCGCCCCAGGCACCA, β-actin 3′-CTCCTTAATGTCACGCACGATTTC (548-base pair [bp] fragment); Fas-L 5′-CAAGTCCAACTCAAGGTCCATGCC, Fas-L 3′CAGAGAGAGCTCAGATACGTTGAC (350-bp fragment).19 PCR products were size-fractionated by agarose electrophoresis and normalized according to the amount of β-actin detected in the same messenger RNA (mRNA) sample.
Calcium mobilization assay
NK cells were loaded with the acetoxymethyl-ester of Fura-2 (Fura-2-am, 1 μM; Sigma), placed in a quartz 2-mL cuvette, and maintained at 37°C by a thermostatically controlled water bath.27,30 Fura-2-am was excited at 334 nm and 380 nm, emitted light was filtered at 510 nm, and fluorescence was monitored with the LS-50B spectrofluorimeter (Perkin-Elmer, Beaconsfield, England). The intracellular free calcium concentration ([Ca++]i) was calculated as described.30 [Ca++]i increases were measured on addition of sHLA-I (the optimal concentration, 4 μg/mL, was determined after titration experiments by using concentrations from 0.5 to 8 μg/mL) or on cross-linking of the CD8 molecule obtained by the addition of 20 μg/mL GAM after the preincubation for 20 minutes at 4°C of NK cells with anti-CD8–specific mAb (astra 102 or OKT8). Some experiments were performed in the presence of EGTA (2 mM) followed by 4 mM CaCl2. Inhibition of [Ca++]iincreases induced by CD8 was performed by incubating Fura-2–labeled NK cell clones with anti-CD8 mAb (OKT8 or astra 102) together with anti-CD94 mAb (HP-3B1) or with anti-CD158b mAb (GL183). In control experiments anti-CD8 mAb was added in combination with anti-CD54 mAb (14D12D2) or anti-CD56 mAb (TA181H12). Cells were then analyzed as above and co-engagement of the indicated surface molecules was achieved by the addition of 20 μg/mL GAM.27
Results
sHLA-I induces NK cell apoptosis on engagement of CD8
We first analyzed the expression of CD8 on a panel of NK cell clones obtained from peripheral blood CD3−CD4− cells stimulated with PHA and cultured with 100 U/mL rIL-2 under limiting dilution condition at 25-, 12-, and 6-cells/well. Clonal efficiency was relatively low (<10%) in different experiments (n = 8), and, on this basis, these microcultures could be operationally considered as clones.10,11,25,28 NK cell clones were selected for the homogeneous expression of a panel of NK cell surface molecules, including CD56, CD16, CD158a, and/or CD158b CD94,10-12further supporting that they can be considered as clones.
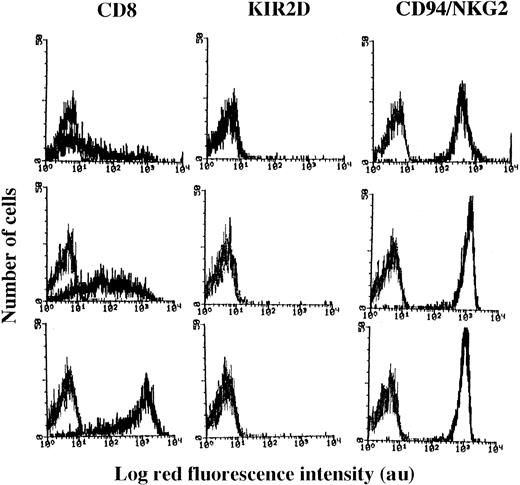
We observed that these selected NK cell clones (n = 90) could be divided into 3 groups with different CD8 expressions (Figure1). Indeed, in some NK clones (35 of 90) most of the cells were surface CD8−, and a small percentage (10% to 15%) displayed a broad expression of this antigen (ranging from 10 to 1000 au MFI; Figure 1, upper panels), whereas in 20 (22%) of 90 NK cell clones all cells expressed high levels of CD8 (MFI > 500 au; Figure 1, lower panels). These 2 groups of clones were arbitrarily termed CD8dull and CD8bright, respectively. It is of note that all cells of CD8dullclones expressed CD8 in the cytoplasm as assessed by indirect immunofluorescence after cell permeabilization by using 3 different anti-CD8 mAbs (OKT8 or Leu2a or astra102; not shown). Furthermore, in 35 of 90 NK cell clones, most of the cells were CD8+ but displayed an intermediate intensity of expression (range of MFI, 10-1000), and these clones were termed CD8intermediate(Figure 1, middle panels). Although not shown, the expression of CD8 did not vary on CD8bright NK cell clones, whereas CD8dull and CD8intermediate clones up-regulated CD8 during the culture.
Surface expression of CD8 antigen on NK cell clones.
NK cell clones were incubated with mAb recognizing CD8 (Leu2a) or KIR2D (NKVFS1) or CD94/NKG2 complex (HP-3B1), followed by PE-conjugated GAM, and analyzed on a FACSort. Results are expressed as Log red fluorescence intensity (au) versus number of cells. Left histograms in each panel represent fluorescence of samples incubated with an unrelated mAb followed by GAM-PE. Results are representative of 90 clones isolated from 3 different healthy donors.
Surface expression of CD8 antigen on NK cell clones.
NK cell clones were incubated with mAb recognizing CD8 (Leu2a) or KIR2D (NKVFS1) or CD94/NKG2 complex (HP-3B1), followed by PE-conjugated GAM, and analyzed on a FACSort. Results are expressed as Log red fluorescence intensity (au) versus number of cells. Left histograms in each panel represent fluorescence of samples incubated with an unrelated mAb followed by GAM-PE. Results are representative of 90 clones isolated from 3 different healthy donors.
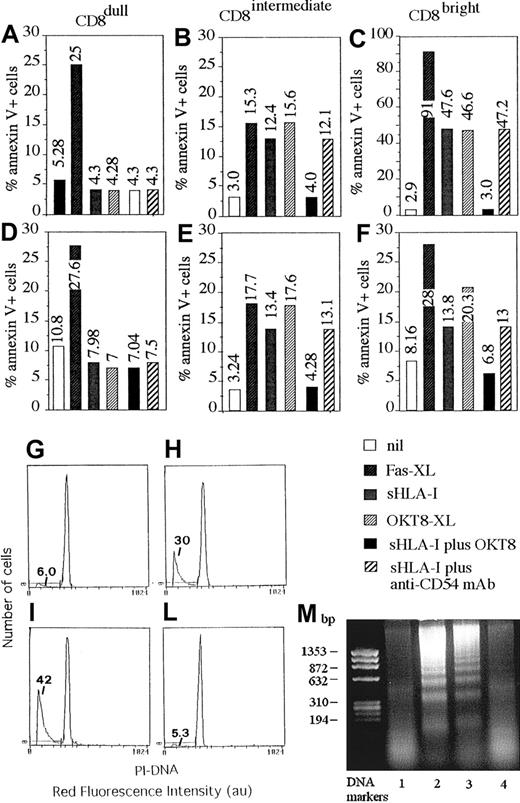
Although not shown, all NK cell clones displayed a similar cytolytic activity against a panel of tumor target cells independently of the different expression of CD8 antigen and the percentage of CD8+ cells present. Furthermore, the addition of anti-CD8 mAb to the cytolytic assay did not have any effect. Finally, cytotoxicity of these clones was not triggered, in a redirected killing assay, using a panel of anti-CD8 mAb, whereas mAbs specific for activating surface molecules, such as CD16, and CD69 were effective (not shown). This finding would indicate that NK cells that bear CD8 antigen do not use this molecule to trigger cytolysis but rather to regulate some other functions of NK cells. In this context, it has been reported that CD8 can deliver an apoptotic signal in T lymphocytes by interacting with its soluble natural ligand represented by sHLA-I molecule.13-16,19,20 Thus, we analyzed whether sHLA-I could induce apoptosis of NK cell clones by interacting with CD8 and whether this effect was correlated with the CD8 antigen expression of each clone tested. As shown in Figure 2, sHLA-I induced apoptosis in CD8bright (C,F) and in CD8intermediate (B,E) but not in CD8dull (A,D) NK cell clones. Indeed, the detectable percentage of cells among CD8bright (13% to 46%; C,F) and CD8intermediate clones (12% to 14%; B,E) was annexin V+ within 48 hours from the addition of sHLA-I to culture medium; that is, they expressed phosphatidylserine at the cell surface, an early marker of programmed cell death (PCD).17,19 31-33Apoptosis was also confirmed by DNA staining with PI after incubation with sHLA-I (Figure 2H) and by DNA laddering (Figure 2M, lane 2). This apoptosis was mediated by the specific interaction of sHLA-I with CD8 antigen as the preincubation of NK cells with an anti-CD8 mAb (but not with anti-CD54 mAb used as negative control; Figure 2A-F), which masked CD8, preventing sHLA-I/CD8 interaction, almost completely inhibited the sHLA-I–induced apoptosis (Figure 2B,C,E,F; compare panel H with L and lane 2 with 4). Furthermore, optimal cross-linking of CD8 achieved by anti-CD8 mAb followed by GAM-coated beads led to NK cell apoptosis (Figure 2B,C,E,F,I,M lane 3), suggesting that CD8 can actually deliver an apoptotic signal. In addition, cross-linking of Fas antigen, achieved by the anti-Fas–specific CH-11 mAb, induced apoptosis in any kind of NK cell clone (Figure 2A-F), indicating that CD8dull NK cell clones were also susceptible to Fas-mediated PCD. Kinetics experiments showed that NK cell apoptosis reached a maximum after 2 to 3 days of incubation with sHLA-I (Figure3A-C) and that the optimal sHLA-I concentration was 4 μg/mL, although detectable levels of apoptotic cells were found also at 0.5 μg/mL (Figure 3D). Finally, sHLA-I did not induce apoptosis of CD8+ resting NK cells, supporting the idea that NK cells should be activated to become sensitive to CD8-mediated PCD (not shown).
Soluble HLA-I induces apoptosis in NK cells through its interaction with CD8.
NK cell clones expressing different levels of CD8 (CD8dull, clones Cl.8.25 and Cl.S4, panels A,D; CD8intermediate, clones Cl.8.6 and Cl.4.25, panels B,E; and CD8bright, clones Cl. 77.12 and Cl.S2, panels C,F), but homogeneously expressing CD94/NKG2 complex and KIR2D− were incubated with medium alone (nil), or anti-Fas mAb (CH-11, 1 μg/mL) (Fas-XL) or 4 μg/mL sHLA-I or anti-CD8 mAb (OKT8, 1 μg/mL) followed by 4-per-cell GAM-coated magnetic beads (OKT8-XL) to achieve CD8 cross-linking or 4 μg/mL sHLA-I either after preincubation with anti-CD8 mAb (OKT8, 1 μg/mL) to avoid sHLA-I interaction with CD8 or anti-CD54 mAb as control mAb. Cells were then analyzed for the reactivity with FITC–annexin V after 48 hours. Results are expressed as the percentage of annexin V+ PI− (numbers in each panel indicate the percentage of apoptotic cells). (G-L) DNA staining with PI of fixed and permeabilized NK cells (Cl.77.12) incubated with medium alone (G), or 4 μg/mL sHLA-I (H) or anti-CD8 mAb (OKT8, 1 μg/mL) followed by 4-per-cell GAM-coated magnetic beads (I) to achieve CD8 cross-linking or 4 μg/mL sHLA-I after preincubation with anti-CD8 mAb (L) to avoid sHLA-I interaction with CD8. Numbers in each panel indicate the percentage of NK cells with a less than 2n DNA content (apoptotic cells, markers). (M) DNA laddering of NK cells (Cl.77.12) incubated with medium alone (lane 1), or 4 μg/mL sHLA-I (lane 2) or anti-CD8 mAb (OKT8, 1 μg/mL) followed by 4-per-cell GAM-coated magnetic beads (lane 3) or 4 μg/mL sHLA-I, after preincubation with anti-CD8 mAb (OKT8, 1 μg/mL, lane 4). DNA markers are shown on the left.
Soluble HLA-I induces apoptosis in NK cells through its interaction with CD8.
NK cell clones expressing different levels of CD8 (CD8dull, clones Cl.8.25 and Cl.S4, panels A,D; CD8intermediate, clones Cl.8.6 and Cl.4.25, panels B,E; and CD8bright, clones Cl. 77.12 and Cl.S2, panels C,F), but homogeneously expressing CD94/NKG2 complex and KIR2D− were incubated with medium alone (nil), or anti-Fas mAb (CH-11, 1 μg/mL) (Fas-XL) or 4 μg/mL sHLA-I or anti-CD8 mAb (OKT8, 1 μg/mL) followed by 4-per-cell GAM-coated magnetic beads (OKT8-XL) to achieve CD8 cross-linking or 4 μg/mL sHLA-I either after preincubation with anti-CD8 mAb (OKT8, 1 μg/mL) to avoid sHLA-I interaction with CD8 or anti-CD54 mAb as control mAb. Cells were then analyzed for the reactivity with FITC–annexin V after 48 hours. Results are expressed as the percentage of annexin V+ PI− (numbers in each panel indicate the percentage of apoptotic cells). (G-L) DNA staining with PI of fixed and permeabilized NK cells (Cl.77.12) incubated with medium alone (G), or 4 μg/mL sHLA-I (H) or anti-CD8 mAb (OKT8, 1 μg/mL) followed by 4-per-cell GAM-coated magnetic beads (I) to achieve CD8 cross-linking or 4 μg/mL sHLA-I after preincubation with anti-CD8 mAb (L) to avoid sHLA-I interaction with CD8. Numbers in each panel indicate the percentage of NK cells with a less than 2n DNA content (apoptotic cells, markers). (M) DNA laddering of NK cells (Cl.77.12) incubated with medium alone (lane 1), or 4 μg/mL sHLA-I (lane 2) or anti-CD8 mAb (OKT8, 1 μg/mL) followed by 4-per-cell GAM-coated magnetic beads (lane 3) or 4 μg/mL sHLA-I, after preincubation with anti-CD8 mAb (OKT8, 1 μg/mL, lane 4). DNA markers are shown on the left.
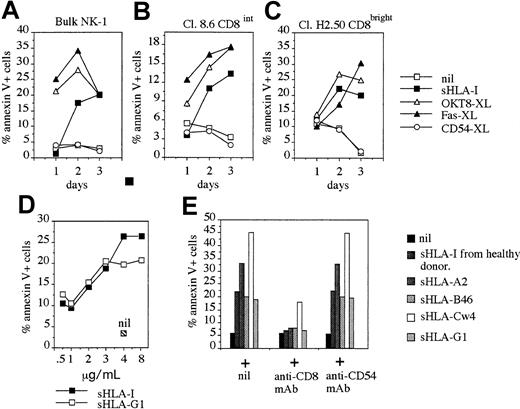
Kinetics and dose-response of NK cell apoptosis induced by different sHLA-I alleles.
The NK cell population NK-1 (90% CD8+, A), the NK cell clones Cl. C8.6 (CD8intermediate, B), and Cl. H2-50 (CD8bright, C) were incubated with medium alone (nil), or 4 μg/mL sHLA, or anti-CD8 mAb (OKT8, 1 μg/mL; OKT8-XL), or anti-CD54 mAb (14D12D2, 1 μg/mL; CD54-XL) followed by 4-per-cell GAM-coated magnetic beads to achieve cross-linking of the corresponding molecule or anti-Fas mAb (CH-11, 1 μg/mL; Fas-XL) and analyzed for their reactivity with FITC–annexin V at different time points (1, 2, and 3 days). Results are expressed as the percentage of annexin V+ PI− cells. The NK-1 cell population and the NK cell clones analyzed were CD94+ and KIR2D−. (D) The NK cell clone Cl.S2 (CD8bright) was incubated with increasing amounts of either sHLA-I or with sHLA-G1 (0.5, 1, 2, 3, 4, and 8 μg/mL). After 48 hours, apoptosis was assessed by staining with FITC–annexin V. Nil: apoptosis in the absence of any addition. (E) The NK cell clone Cl.S2 (CD8bright) was incubated with 4 μg/mL sHLA-I isolated from serum of healthy donors or with sHLA-A2, sHLA-Cw4, sHLA-B46, or sHLA-G1 (from 721.221 cells transfected with the corresponding HLA-I allele) alone or after pretreatment of NK cells with anti-CD8 mAb (OKT8, 1 μg/mL), to avoid interaction of sHLA-I with CD8 or with anti-CD54 mAb as control mAb, and apoptosis was evaluated after 48 hours. Results are expressed as the percentage of annexin V+ PI− cells and are representative of 4 independent experiments by using 4 different NK cell clones from 4 healthy donors.
Kinetics and dose-response of NK cell apoptosis induced by different sHLA-I alleles.
The NK cell population NK-1 (90% CD8+, A), the NK cell clones Cl. C8.6 (CD8intermediate, B), and Cl. H2-50 (CD8bright, C) were incubated with medium alone (nil), or 4 μg/mL sHLA, or anti-CD8 mAb (OKT8, 1 μg/mL; OKT8-XL), or anti-CD54 mAb (14D12D2, 1 μg/mL; CD54-XL) followed by 4-per-cell GAM-coated magnetic beads to achieve cross-linking of the corresponding molecule or anti-Fas mAb (CH-11, 1 μg/mL; Fas-XL) and analyzed for their reactivity with FITC–annexin V at different time points (1, 2, and 3 days). Results are expressed as the percentage of annexin V+ PI− cells. The NK-1 cell population and the NK cell clones analyzed were CD94+ and KIR2D−. (D) The NK cell clone Cl.S2 (CD8bright) was incubated with increasing amounts of either sHLA-I or with sHLA-G1 (0.5, 1, 2, 3, 4, and 8 μg/mL). After 48 hours, apoptosis was assessed by staining with FITC–annexin V. Nil: apoptosis in the absence of any addition. (E) The NK cell clone Cl.S2 (CD8bright) was incubated with 4 μg/mL sHLA-I isolated from serum of healthy donors or with sHLA-A2, sHLA-Cw4, sHLA-B46, or sHLA-G1 (from 721.221 cells transfected with the corresponding HLA-I allele) alone or after pretreatment of NK cells with anti-CD8 mAb (OKT8, 1 μg/mL), to avoid interaction of sHLA-I with CD8 or with anti-CD54 mAb as control mAb, and apoptosis was evaluated after 48 hours. Results are expressed as the percentage of annexin V+ PI− cells and are representative of 4 independent experiments by using 4 different NK cell clones from 4 healthy donors.
Different alleles of sHLA-I induce apoptosis of NK cells on CD8 engagement
It is well known that CD8 is a surface receptor for the α3 domain of HLA-I.2-8 Indeed, it has been reported that in T lymphocytes CD8 can interact with sHLA-I isolated from serum of healthy donors13-16,19 or with sHLA-G1.20 Thus, we assessed the question of whether different sHLA-I alleles can induce apoptosis in CD8-bearing NK cells. sHLA-I isolated from serum of healthy donors, as well as sHLA-A2, -Cw4, -Bw46, and -G1 isolated from culture SN of the HLA-I− cell line 721.221 transfected with these HLA-I alleles, induced apoptosis of NK cells (Figure 3E). Again, this effect was strongly reduced by masking the CD8 surface molecule with specific mAb (Figure 3E). The pretreatment of NK cells with anti-CD54 mAb, used as negative control, did not affect NK cell apoptosis induced by different sHLA-I alleles (Figure 3E). Furthermore, titration experiments have shown that the amount of sHLA that induces NK cell apoptosis was similar for sHLA-I and sHLA-G1 (Figure 3D), as for sHLA-A2, -Cw4, and -Bw46 (not shown). This finding suggests that CD8 recognizes a constant portion of sHLA-I and that any sHLA-I allele could induce NK cell apoptosis through the engagement of CD8.
FasL/Fas interaction is responsible for sHLA-I–induced apoptosis via CD8 in NK cells
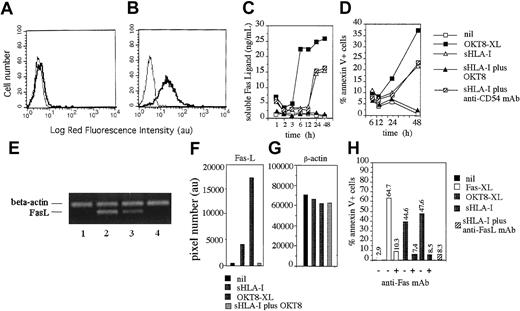
Apoptosis of lymphocytes is mainly mediated by the interaction of Fas expressed at the cell surface and FasL present on neighboring cells or released in the extracellular milieu.31-33 Thus, to determine whether FasL/Fas interaction was responsible for sHLA-I–mediated PCD of NK cells on CD8 engagement, we analyzed NK cells for the surface or cytoplasmic expression of FasL and cultured SN derived from NK cell on incubation with sHLA-I for the presence of FasL. As shown in Figure 4, FasL was present in the cytoplasm (Figure 4B) but not at the cell surface of NK cells (Figure 4A). More importantly, sFasL was detectable in the SN of NK cells incubated with sHLA-I for 1 hour (Figure 4C) at 10-fold higher amounts than in SN from untreated NK cells (5.3 ng/mL versus 0.5 ng/mL), suggesting that FasL is released from NK cells during this incubation time. Importantly, the sHLA-I–induced apoptosis of NK cells (Figure 4D) was evident with a delay from the time when FasL was detectable in the SN (Figure 4C). This finding suggests that FasL is secreted into the extracellular milieu before inducing apoptosis. This phenomenon was more evident when sFasL was analyzed in SN derived from NK cell cultures in which CD8 was cross-linked (Figure 4C,D) by GAM-coated beads. The amount of sFasL detectable in the SN 3 hours after optimal cross-linking of CD8 was similar to that observed by using sHLA-I; however, sFasL recovered starting from 6 to 48 hours was higher in CD8–cross-linked than sHLA-I–incubated NK cell cultures (Figure 4C). Although not shown, sFasL present in these SNs was functional, as it induced apoptosis of the Fas+ T-cell line Jurkat. In addition, FasL was almost undetectable at different time points (1, 2, 3, 6, 12, 24, and 48 hours) in the SN of NK cells incubated with sHLA-I when CD8 antigen was masked with specific anti-CD8 mAbs (Figure 4C). Finally, although anti-Fas mAb CH-11 induced apoptosis at similar level of sHLA-I, a low amount of FasL was found in these culture SNs compared with that present in SNs of untreated NK cells (0.75 ng/mL versus 0.5 ng/mL) (not shown). This suggests that sFasL detected in SNs on incubation with sHLA-I or CD8 cross-linking was not simply because of the unspecific release from dying NK cells.
sHLA-I induces transcription of Fas-L mRNA, Fas-L secretion, and apoptosis by Fas-FasL interaction.
(A,B) Indirect immunofluorescence analysis of the NK cells clone Cl.77.12 (CD8bright) for surface (A) or cytoplasmic (B) expression of FasL. Left histograms in each panel represent cells stained with an unrelated isotype matched mAb followed by GAM-PE. Data are representative of results obtained analyzing 10 different NK cell clones. (C,D) FasL concentration (nanogram per milliliter) in culture SN (C) or NK cell apoptosis (annexin-V+ PI−, D) were evaluated at different time points after incubation of NK cells with medium alone (nil) or with 4 μg/mL sHLA-I or the anti-CD8 mAb (OKT8, 1 μg/mL) followed by 4-per-cell GAM-coated magnetic beads (OKT8-XL) to achieve CD8 cross-linking, or sHLA-I (4 μg/mL) after covering CD8 with anti-CD8 mAb (sHLA-I plus OKT8) or with anti-CD54 mAb as control mAb. (E) Normalized aliquots of total RNA (10 μg), isolated from the NK-1 population, were incubated for 3 hours with medium alone (lane 1) or anti-CD8 mAb (OKT8, 1 μg/mL) followed by 4-per-cell GAM-coated magnetic beads (lane 2) or with 4 μg/mL sHLA-I (lane 3), or sHLA-I (4 μg/mL) after covering of CD8 with anti-CD8 mAb (lane 4) and amplified by PCR with the specific primers for β-actin or FasL, as indicated, and PCR products were size-fractionated by agarose electrophoresis. Bands corresponding to FasL (F) and β-actin (G) were subjected to densitometric analysis, and results are expressed in pixel number (au). (H) NK cells (clone 35.6, CD8bright) were either untreated or pretreated with the apoptosis-blocking anti-Fas mAb (ZB4) and incubated with medium alone (nil), or with the apoptosis-inducing anti-Fas mAb (CH-11, Fas-XL) or OKT8 followed by 4-per-cell GAM-coated magnetic beads (OKT8-XL) or 4 μg/mL sHLA-I. Similar results were obtained with 4 additional CD8bright NK cell clones.
sHLA-I induces transcription of Fas-L mRNA, Fas-L secretion, and apoptosis by Fas-FasL interaction.
(A,B) Indirect immunofluorescence analysis of the NK cells clone Cl.77.12 (CD8bright) for surface (A) or cytoplasmic (B) expression of FasL. Left histograms in each panel represent cells stained with an unrelated isotype matched mAb followed by GAM-PE. Data are representative of results obtained analyzing 10 different NK cell clones. (C,D) FasL concentration (nanogram per milliliter) in culture SN (C) or NK cell apoptosis (annexin-V+ PI−, D) were evaluated at different time points after incubation of NK cells with medium alone (nil) or with 4 μg/mL sHLA-I or the anti-CD8 mAb (OKT8, 1 μg/mL) followed by 4-per-cell GAM-coated magnetic beads (OKT8-XL) to achieve CD8 cross-linking, or sHLA-I (4 μg/mL) after covering CD8 with anti-CD8 mAb (sHLA-I plus OKT8) or with anti-CD54 mAb as control mAb. (E) Normalized aliquots of total RNA (10 μg), isolated from the NK-1 population, were incubated for 3 hours with medium alone (lane 1) or anti-CD8 mAb (OKT8, 1 μg/mL) followed by 4-per-cell GAM-coated magnetic beads (lane 2) or with 4 μg/mL sHLA-I (lane 3), or sHLA-I (4 μg/mL) after covering of CD8 with anti-CD8 mAb (lane 4) and amplified by PCR with the specific primers for β-actin or FasL, as indicated, and PCR products were size-fractionated by agarose electrophoresis. Bands corresponding to FasL (F) and β-actin (G) were subjected to densitometric analysis, and results are expressed in pixel number (au). (H) NK cells (clone 35.6, CD8bright) were either untreated or pretreated with the apoptosis-blocking anti-Fas mAb (ZB4) and incubated with medium alone (nil), or with the apoptosis-inducing anti-Fas mAb (CH-11, Fas-XL) or OKT8 followed by 4-per-cell GAM-coated magnetic beads (OKT8-XL) or 4 μg/mL sHLA-I. Similar results were obtained with 4 additional CD8bright NK cell clones.
To determine whether sFasL present in NK cell culture SN was newly synthesized, we analyzed NK cells for the expression of mRNA coding for FasL on stimulation with either sHLA-I or on CD8 cross-linking. To this aim, NK cells were incubated for different periods of time with sHLA or on CD8 cross-linking, total mRNA was isolated, and the normalized amount of mRNA was amplified by RT-PCR simultaneously with primers for FasL and β-actin as a control. A detectable increase in FasL mRNA was found on NK cell incubation (3 hours) with sHLA-I (Figure 4E, lane 3), and a similar, although stronger, effect was detectable by the engagement of CD8 antigen with specific mAb, followed by cross-linking with GAM-coated beads (Figure 4E, lane 2). Importantly, sHLA-I did not induce FasL transcription when CD8 antigen was masked with specific anti-CD8 mAb (Figure 4E, lane 4), indicating that sHLA-I should interact with CD8 to deliver an appropriate signal leading to FasL transcription in NK cells. Densitometric analysis of mRNA bands corresponding to FasL (Figure 4F) revealed that FasL mRNA was strongly induced after incubation of NK cells with sHLA-I or on CD8 cross-linking (4500 au and 17 500 au, respectively, versus 320 au NK cells in medium alone; Figure 4F), whereas bands corresponding to β-actin mRNA from the different culture conditions were similar (Figure 4G). That FasL/Fas interaction was needed to induce NK cell apoptosis was indicated by blocking experiments that used anti-Fas mAbs. Indeed, pretreatment of NK cells with the blocking anti-Fas mAb (ZB4) strongly reduced (by 80% to 95%) sHLA-I–mediated apoptosis (Figure 4H). Similarly, the anti-FasL mAb added at the onset of the apoptotic assay inhibits up to 95% NK cell apoptosis induced by sHLA-I (Figure 4H).
Taken together these findings indicate that sHLA-I, interacting with CD8 at the NK cell surface, leads to transcription of FasL mRNA and production of functional FasL protein. Thus, FasL is secreted in the extracellular milieu and, on binding with Fas, induces NK cell apoptosis.
Intracellular calcium increases are involved in sHLA-I–mediated apoptosis of NK cells
Secretion of synthesized proteins is a cellular function dependent on the rise of [Ca++]i.34 35Thus, we analyzed whether (1) sHLA-I or CD8 engagement could induce calcium rises in NK cells and (2) calcium was needed for sHLA-I–induced apoptosis. To this aim, NK cell clones were labeled with Fura-2-am, and variations in [Ca++]i were monitored in real time. As shown in Figure 5B, the addition of sHLA-I to CD8bright NK cell clones induces a strong and prompt [Ca++]i increase, with a maximum of 450 nM after about 200 seconds, lasting for at least 550 seconds at lower levels (400 nM). Masking of CD8 with anti-CD8 mAb (but not with anti CD54 mAb, used as control, not shown) abolished sHLA-I–mediated [Ca++]i rise (Figure 5C), suggesting that [Ca++]i increase was elicited by the binding of sHLA-I to CD8. Thus, we addressed the question of whether CD8 could also trigger [Ca++]i increases. As shown in Figure 5H, the engagement of CD8 in CD8bright NK cell clones led to a strong [Ca++]i rise, reaching a peak (750 nM) after about 100 seconds and lasting for more than 400 seconds at lower levels (400 nM). CD8 engagement in CD8dullNK cell clones did not induce any [Ca++]irise (Figure 5E). However, CD16 evoked a strong [Ca++]i increase both in CD8bright and CD8dull NK cell clones (Figure5I,F), indicating that both clones were able to mobilize calcium on engagement of appropriate molecules. Importantly, sHLA-I and the CD8-mediated [Ca++]i increase were mainly due to the entry of calcium from the extracellular medium, as the addition of the extracellular calcium chelator EGTA, prior to the addition of sHLA-I (Figure 5N) or CD8 cross-linking (Figure 5M), almost abrogated the [Ca++]i increase. The addition of an excess (4 mM) of CaCl2 to the extracellular milieu, in the presence of EGTA, after the addition of sHLA-I (Figure 5N) or CD8 cross-linking (Figure 5M) led to a strong [Ca++]i increase. This finding suggests that sHLA-I induces the opening of calcium channels by engaging CD8 at the cell surface of NK cells.
Soluble HLA-I and CD8 engagement induce intracellular calcium increases in NK cells.
(A-C) The NK cell clone S2 (CD8bright) was labeled with Fura-2 and fluorescence monitored in real time on a spectrofluorometer LS50B at 37°C. (A) Baseline, [Ca++]ioscillations in the absence of any addition. (B,C) Calcium mobilization induced by sHLA-I (4 μg/mL, B) before or after pretreatment with anti-CD8 mAb (OKT8, C) to avoid the interaction of sHLA-I with CD8 antigen (CD8 masking). (D-I) The NK cell clones S4 (CD8dull, D-F) and S2 (CD8bright, G-I) were treated with the anti-CD8 mAb astra102 (E,H) or the anti-CD16 mAb NK54 (F,I). Cross-linking of CD8 or CD16 was achieved by adding 20 μg/mL GAM. (D,G) Cells treated with anti-CD54 mAb (14D12D2) matched for isotype followed by GAM, as negative control. (M,N) CD8 cross-linking (M) or addition of 4 μg/mL sHLA-I (N) were performed in the presence of 2 mM calcium chelator EGTA. An excess of calcium (4 mM of CaCl2) was added (on 250 seconds) (right arrows) after the addition of GAM or sHLA-I (left arrows). (L) Calcium oscillations in the presence of EGTA without any stimulus (baseline). Arrows in each histogram indicate the addition of either GAM (D-I,M) or sHLA-I (B,C,N); the addition of CaCl2 is indicated in panels M and N by the second arrow on the right. Results are expressed as [Ca++]i nM and are representative of 3 independent experiments performed by using NK cell clones from 3 different healthy donors.
Soluble HLA-I and CD8 engagement induce intracellular calcium increases in NK cells.
(A-C) The NK cell clone S2 (CD8bright) was labeled with Fura-2 and fluorescence monitored in real time on a spectrofluorometer LS50B at 37°C. (A) Baseline, [Ca++]ioscillations in the absence of any addition. (B,C) Calcium mobilization induced by sHLA-I (4 μg/mL, B) before or after pretreatment with anti-CD8 mAb (OKT8, C) to avoid the interaction of sHLA-I with CD8 antigen (CD8 masking). (D-I) The NK cell clones S4 (CD8dull, D-F) and S2 (CD8bright, G-I) were treated with the anti-CD8 mAb astra102 (E,H) or the anti-CD16 mAb NK54 (F,I). Cross-linking of CD8 or CD16 was achieved by adding 20 μg/mL GAM. (D,G) Cells treated with anti-CD54 mAb (14D12D2) matched for isotype followed by GAM, as negative control. (M,N) CD8 cross-linking (M) or addition of 4 μg/mL sHLA-I (N) were performed in the presence of 2 mM calcium chelator EGTA. An excess of calcium (4 mM of CaCl2) was added (on 250 seconds) (right arrows) after the addition of GAM or sHLA-I (left arrows). (L) Calcium oscillations in the presence of EGTA without any stimulus (baseline). Arrows in each histogram indicate the addition of either GAM (D-I,M) or sHLA-I (B,C,N); the addition of CaCl2 is indicated in panels M and N by the second arrow on the right. Results are expressed as [Ca++]i nM and are representative of 3 independent experiments performed by using NK cell clones from 3 different healthy donors.
To determine whether extracellular calcium was needed for sHLA-mediated apoptosis, we performed a series of experiments in the presence of the calcium chelator EGTA or by blocking calcium entry with verapamil as described.27 36 Apoptosis induced by sHLA-I (Table 1) or on cross-linking of CD8 (not shown) was strongly reduced (by 60%) in the absence of extracellular calcium or by blocking calcium channels with verapamil. Altogether, these findings indicate that the engagement of CD8 by sHLA-I activates NK cells that open their calcium channels, allowing extracellular calcium entry that is essential to induce apoptosis of NK cells.
Extracellular calcium is necessary for the soluble HLA class I–mediated apoptosis
| . | Nil . | sHLA-I . | SHLA-I + EGTA . | sHLA-I + DMSO . | sHLA-I + verapamil . |
|---|---|---|---|---|---|
| Cl.1.25 CD8bright | 1.0 | 38.0 | 15.0 | 37.0 | 12.0 |
| Cl.10.50 CD8dull | 3.0 | 5.0 | 3.0 | 3.0 | 2.1 |
| NK-2 | 2.0 | 45.3 | 17.4 | 44.2 | 10.5 |
| NK-3 | 5.6 | 35.5 | 15.3 | 36.0 | 12.6 |
| . | Nil . | sHLA-I . | SHLA-I + EGTA . | sHLA-I + DMSO . | sHLA-I + verapamil . |
|---|---|---|---|---|---|
| Cl.1.25 CD8bright | 1.0 | 38.0 | 15.0 | 37.0 | 12.0 |
| Cl.10.50 CD8dull | 3.0 | 5.0 | 3.0 | 3.0 | 2.1 |
| NK-2 | 2.0 | 45.3 | 17.4 | 44.2 | 10.5 |
| NK-3 | 5.6 | 35.5 | 15.3 | 36.0 | 12.6 |
A total of 5 × 104 cells (from natural killer [NK] cell clones Cl.1.25, Cl.10.50, NK-2 bulk population, 90% CD8+; NK-3 bulk population, 80% CD8+) were cultured for 48 hours in medium alone (nil) or with 4 μg/mL soluble HLA class-I (sHLA-I), either in the absence or in the presence of the extracellular calcium chelator EGTA (2 mM) or of the L-type calcium channel blocker verapamil (10 μM) diluted in dimethyl sulfoxide (DMSO) or with the solvent DMSO alone. Apoptosis was evaluated by staining with fluorescein isothiocyanate–annexin V. Samples were run on a FACSort (Becton Dickinson), and results are expressed as the percentage of annexin V+ PI− cells.
Down-regulation of sHLA-I–induced apoptosis by the engagement of IRS in NK cells
It is well established after interaction with HLA-I that ISIR or CLIR may deliver an inhibitory signal that leads to the down-regulation of different NK cell–mediated functions.10-12 Thus, the observed sHLA-I–induced apoptosis in CD8+ NK cell clones would represent the result of opposing effects mediated by the interaction of sHLA-I with CD8 (proapoptotic) or with IRS (antiapoptotic). To test this hypothesis, NK cell clones were incubated with sHLA-I from healthy donors after covering of inhibitory receptors with specific mAbs to block the interaction of sHLA-I and IRS, thus up-regulating sHLA-I–induced apoptosis. A representative experiment is shown in Figure6A using the NK cell clone S2, which was CD8bright and expressed the inhibitory receptor CD94 (a CLIR). The clone S2 did not express other NK cell receptors for HLA-I, as it was KIR2D− (CD158a− and CD158b−). The covering of CD94 with HP-3B1 mAb strongly augmented the sHLA-I–mediated apoptosis (from 47% to 77%). A similar effect was detected by using sHLA-G1 isoform, as HLA-I was recognized specifically by CD94 (Table 2). Importantly, the amount of FasL detected in the SN of NK cells pretreated with HP-3B1 mAb and then incubated with sHLA-I was increased by about 70-fold (from 12.37 to 827.79 ng/mL; Figure 6B). Moreover, simultaneous cross-linking of CD8 and CD94 reduced by 50% the apoptosis mediated by CD8 alone (from 47% to 24%; Figure 6A) and by 90% the CD8-mediated [Ca++]i rise in NK cells (Figure 6C). Similar results were obtained in all the NK cell clones expressing, as IRS, only the CD94 complex (n = 5, not shown). We further analyzed also whether the engagement of a KIR2D molecule plays a role in the negative regulation of sHLA-I–mediated apoptosis. As shown in Figure 6D, sHLA-I–induced apoptosis was strongly increased by the covering, with a specific mAb, the KIR2D inhibitory receptor GL183 in the KIR2D+ (CD158b+) NK cell clone 209 (CD8bright, CD158b+). A similar effect was found by using sHLA-Cw3, as HLA-I was specifically recognized by KIR2D GL183 (Table 2). Again, FasL detected in the culture SNs of NK cells precoated with anti-KIR2D mAb and incubated with sHLA-I was strongly increased compared with NK cells incubated with sHLA-I alone (from 27 to 119 ng/mL; 4.4-fold increase; Figure 6E). Finally, the co-engagement of CD8 and KIR2D (GL183) reduced by 30% apoptosis and by 90% [Ca++]i rise elicited via CD8 (Figure 6F). Altogether these findings suggest that IRSs for HLA-I inhibit the sHLA-I–mediated FasL secretion and the consequent apoptotic signal.
IRSs are involved in the negative regulation of sHLA-mediated apoptosis.
The NK cell clone Cl.S2 (CD8bright CD94+KIR2D−, A-C) or the NK cell clone Cl.209 (CD8bright CD158b+ [GL183+], D-F) was incubated with the following reagents: medium (nil), sHLA-I (4 μg/mL) alone or after masking of either CD8 (sHLA-I + OKT8, A, or sHLA-I + astra 102, D) or CD94 (sHLA-I + HP-3B1, A) or CD158b (sHLA-I + GL183, D) with specific mAbs, or with anti-CD94 mAb (HP-3B1, A), or anti-CD158b mAb (GL183, D). Some samples were treated with anti-CD8 mAb (OKT8, A, or astra 102, D) alone or in combination with either anti-CD94/NKG2 or anti-CD158b mAb followed by 4-per-cell GAM-coated magnetic beads to induce cross-linking of the indicated molecules (OKT8-XL, OKT8-XL + HP-3B1-XL, A; astra 102-XL, astra 102-XL + GL183-XL, D). Results are expressed as the percentage of annexin V+ PI− cells. (B,E) Aliquots of SN derived from cells incubated for 48 hours with medium (nil), or sHLA-I (B,E), or HP-3B1 mAb (B) or GL183 mAb (E) were analyzed for the presence of FasL by enzyme-linked immunosorbent assay. In some samples, cells were exposed to sHLA-I after incubation with anti-CD94/NKG2 (sHLA-I + HP-3B1, B) or with anti-CD158b (sHLA-I + GL183, E) mAbs. Results are expressed as nanogram per milliliter of FasL. (C,F) NK cell clone Cl.S2 (C) or Cl.209 (F) was labeled with Fura-2 and treated with anti-CD8 mAb (OKT8, C, astra102, F), or with anti-CD8 plus either anti-CD94/NKG2 (OKT8-XL + HP-3B1-XL, C) or anti-CD158b (astra 102-XL + GL183-XL, F) mAb. The cross-linking (XL) of the corresponding surface molecules was achieved by adding 20 μg/mL GAM as indicated. Results are expressed as [Ca++]i nM and are representative of 4 independent experiments by using 4 different NK cell clones with a comparable surface phenotype.
IRSs are involved in the negative regulation of sHLA-mediated apoptosis.
The NK cell clone Cl.S2 (CD8bright CD94+KIR2D−, A-C) or the NK cell clone Cl.209 (CD8bright CD158b+ [GL183+], D-F) was incubated with the following reagents: medium (nil), sHLA-I (4 μg/mL) alone or after masking of either CD8 (sHLA-I + OKT8, A, or sHLA-I + astra 102, D) or CD94 (sHLA-I + HP-3B1, A) or CD158b (sHLA-I + GL183, D) with specific mAbs, or with anti-CD94 mAb (HP-3B1, A), or anti-CD158b mAb (GL183, D). Some samples were treated with anti-CD8 mAb (OKT8, A, or astra 102, D) alone or in combination with either anti-CD94/NKG2 or anti-CD158b mAb followed by 4-per-cell GAM-coated magnetic beads to induce cross-linking of the indicated molecules (OKT8-XL, OKT8-XL + HP-3B1-XL, A; astra 102-XL, astra 102-XL + GL183-XL, D). Results are expressed as the percentage of annexin V+ PI− cells. (B,E) Aliquots of SN derived from cells incubated for 48 hours with medium (nil), or sHLA-I (B,E), or HP-3B1 mAb (B) or GL183 mAb (E) were analyzed for the presence of FasL by enzyme-linked immunosorbent assay. In some samples, cells were exposed to sHLA-I after incubation with anti-CD94/NKG2 (sHLA-I + HP-3B1, B) or with anti-CD158b (sHLA-I + GL183, E) mAbs. Results are expressed as nanogram per milliliter of FasL. (C,F) NK cell clone Cl.S2 (C) or Cl.209 (F) was labeled with Fura-2 and treated with anti-CD8 mAb (OKT8, C, astra102, F), or with anti-CD8 plus either anti-CD94/NKG2 (OKT8-XL + HP-3B1-XL, C) or anti-CD158b (astra 102-XL + GL183-XL, F) mAb. The cross-linking (XL) of the corresponding surface molecules was achieved by adding 20 μg/mL GAM as indicated. Results are expressed as [Ca++]i nM and are representative of 4 independent experiments by using 4 different NK cell clones with a comparable surface phenotype.
Inhibitory receptor superfamily down-regulate natural killer cell apoptosis induced by specific soluble HLA class I alleles
| . | Nil . | Specific sHLA-I . | Specific sHLA-I + specific anti-IRS mAb . | Irrelevant sHLA-I + specific anti-IRS mAb . |
|---|---|---|---|---|
| Bulk NK-3 KIR2D+(CD158b+/GL183+) | 2 | 35 | 70 | 37 |
| Clone MZ25.4 KIR2D+(CD158b+/GL183+) | 3 | 25 | 40 | 28 |
| Bulk NK-4 (CD94+KIR2D−) | 5 | 37 | 60 | 40 |
| Clone RB50.3 (CD94+ KIR2D−) | 2 | 40 | 65 | 37 |
| . | Nil . | Specific sHLA-I . | Specific sHLA-I + specific anti-IRS mAb . | Irrelevant sHLA-I + specific anti-IRS mAb . |
|---|---|---|---|---|
| Bulk NK-3 KIR2D+(CD158b+/GL183+) | 2 | 35 | 70 | 37 |
| Clone MZ25.4 KIR2D+(CD158b+/GL183+) | 3 | 25 | 40 | 28 |
| Bulk NK-4 (CD94+KIR2D−) | 5 | 37 | 60 | 40 |
| Clone RB50.3 (CD94+ KIR2D−) | 2 | 40 | 65 | 37 |
A total of 5 × 104 cells (from natural killer [NK]-3 KIR2D+ bulk population 95% CD8+, or clone MZ25.4 KIR2D+ CD8bright, or NK-4 bulk population 90% CD8+CD94+ KIR2D−, or clone RB50.3 CD94+KIR2D−CD8bright) were cultured for 48 hours in medium alone (nil) or with 4 μg/mL specific soluble HLA class I (sHLA-I; HLA-Cw3 for KIR2D+[CD158b+/GL183+] or HLA-G1 for CD94+KIR2D− NK cells) or irrelevant sHLA-I (sHLA-A2 allele), either in the absence or after pretreatment with anti-inhibitory receptor superfamily (IRS) monoclonal antibody (mAb; GL183 for KIR2D+ or anti-CD94 for CD94+KIR2D− NK cells). Apoptosis was evaluated by staining with fluorescein isothiocyanate–annexin V. Samples were run on a FACSort (Becton Dickinson), and results are expressed as the percentage of annexin V+ PI− cells.
Discussion
Herein, we provide evidence that sHLA-I induces apoptosis of NK cell clones by interacting with CD8 antigen and triggering the Fas/FasL pathway. Indeed, the engagement of CD8 by sHLA-I activates NK cells to synthesize and secrete FasL that, in turn, binds to Fas expressed by NK cells and, finally, induces their programmed suicide. This phenomenon is calcium dependent and is down-regulated by the binding of sHLA-I to IRS, represented by either CLIR or KIR.
We have analyzed in detail the expression of CD8 on NK cell clones, and we found that this antigen is not present on all cells of a given NK cell clone. Thus, NK cell clones were grouped on the basis of the percentage of CD8+ cells and the level of expression of CD8 into CD8dull, CD8intermediate, and CD8bright. In addition, we observed that CD8 antigen was present in the cytoplasm of all cells of CD8dull NK cell clones and that it was up-regulated on CD8dull and CD8intermediate cells along the culture period. In addition, the NK cell–mediated lysis of both HLA-I+ and HLA-I− tumor target cells is independent of the level of CD8 expression and that the engagement of CD8 does not trigger NK cell killing. Altogether, these findings suggest that CD8 antigen is not stable at the NK cell surface, and it is not essential for the activation of NK cell cytolysis. This finding supports the idea that CD8 could regulate a function other than cytolysis in NK cells. It has been shown that sHLA-I induces cell death of PHA blasts by interacting with CD8.13,17,19,20 We found that sHLA-I induces apoptosis only in CD8intermediate and CD8bright, but not CD8dull, NK cell clones. More importantly, masking of CD8 with specific mAbs, thus not allowing its interaction with sHLA-I, blocked sHLA-I–mediated apoptosis. This would imply that sHLA-I can affect NK cell survival by binding to CD8. Because NK cells express CD8αα homodimer and do not bear TCR,37 our findings indicate that sHLA-I is able to deliver an apoptotic signal by CD8α and without interaction with TCR. Moreover, we observed that different sHLA-I alleles induced NK cell apoptosis, further supporting the notion that CD8 recognizes the α3 constant region of HLA-I. After the interaction of sHLA-I with CD8, a strong increase in intracellular calcium concentration was detected, and this calcium rise was mainly due to calcium influx from the extracellular milieu. Calcium influx was needed for induction of NK cell apoptosis, as either calcium chelator EGTA or the L-type calcium channel blockers verapamil strongly inhibited sHLA-I–induced apoptosis.
We also provide evidence that Fas/FasL interaction plays a key role in sHLA-I–mediated NK cell death. Indeed, FasL was detectable in the culture SN of NK cells incubated with sHLA-I; this finding suggests that interaction of sHLA-I with CD8 delivers an activating signal, leading to synthesis and secretion of FasL, which, in turn, binds to Fas and induces NK cell death. This hypothesis is further supported by blocking experiments with anti-Fas antibody and by neutralization of SNs with anti-FasL mAb.
The molecular mechanism underlying sHLA-I–induced apoptosis of NK cells is reminiscent of that operating in CD8+ T lymphocytes13,19; however, at variance with most T lymphocytes, CD8+ NK cells express at the cell surface other receptors for HLA-I represented by IRS, either ISIR or CLIR.10-12 Some of these receptors recognize specific subgroups of HLA-I alleles, whereas others, such as CD94/NKG2, can interact with different HLA-I alleles, HLA-G, and/or HLA-E.10-12 It is conceivable that apoptosis of CD8+ NK cell clones, which bear receptors for HLA-I in addition to CD8, is the sum of opposing effects because of the engagement of proapoptotic (CD8) and antiapoptotic (IRS) HLA-I receptors. This finding is of particular interest, as, until now, IRSs have been considered as receptors that inhibit several NK cell–mediated functional activities, including cytolysis and cytokine production,10-12 whereas they might also represent a useful tool for NK cells to survive from apoptosis mediated by HLA-I/CD8 interaction. The finding that IRSs can protect NK cells from sHLA-I–mediated apoptosis is noteworthy. It has been recently shown that interaction of IRS with purified major histocompatibility complex class I molecules is sufficient to inhibit the release of interferon γ (IFN-γ) by activated murine NK cells.38 However, it is well known that HLA-I expression and shedding are strongly increased by IFN-γ. If this is the case in humans also, the interaction between sHLA-I and IRS could reduce the release of HLA-I from bystander cells in the SN. Thus, IRS could inhibit sHLA-I–mediated apoptosis with a double mechanism: directly by affecting CD8-mediated signal and indirectly by reducing IFN-γ–mediated effects.
Why does IRS not abolish sHLA-I–mediated apoptosis? In fact, it has been claimed that inhibitory signals delivered through IRS usually prevail on activating signals.10-12 Conversely, in our experimental system, sHLA-I could induce NK cell apoptosis despite the expression of functional IRS, including CD94 that recognizes most sHLA-I alleles. That CD94 actually interacts with sHLA-I, leading to a powerful inhibitory effect, is demonstrated by the finding that covering this receptor with specific mAbs, in those NK cell clones that bear only CD94 as known inhibitory receptor, led to a sharp increase of sHLA-I–mediated apoptosis and FasL secretion. A possible explanation of our results is that the proapoptotic signal from CD8 and the antiapoptotic signal from IRS do not take place at the cell membrane in close proximity, thus allowing the proapoptotic signal to overcome the inhibition mediated by IRS engagement. Indeed, in our experiments the natural ligand of CD8 was represented by the monomeric form of sHLA-I (>99%), which might interact selectively with either CD8 or IRS. Alternatively, as the binding site of certain IRS for HLA-I is different from that of CD8,10-12 binding of sHLA-I to either IRS or CD8 would determine a conformational change in HLA-I that does not allow a simultaneous binding to both IRS and CD8. In addition, experimental evidence for the inhibitory effect of IRS has often involved the use of antibodies, and not the natural ligand, to cross-link at the same time activating and inhibiting receptors.10-12 Along this line, we have found that engagement of IRS could almost abolish the CD8-mediated calcium mobilization and consequent cell death when these 2 molecules are co-engaged by specific mAbs.
NK cells play a key role in antiviral immune response.37,10-12 Further, it has been found that CD8 is present on the surface of some decidual NK cells.39 Thus, we speculate that NK cell–mediated lysis of virus-infected cells or trophoblastic cells would be down-regulated by the interaction of sHLA-I with CD8, which, in turn, induces NK cell apoptosis. In the first instance, this speculation would be relevant to self-limiting antiviral response. However, this response would allow the elimination of potentially harmful maternal CD8+ NK cells and could play a role in the immune tolerance of fetal allograft.
What is the pathophysiologic significance of the NK cell apoptosis induced by sHLA-I? sHLA-I derived from tumor targets can interact with CD8, thus inducing NK cell death without the need of NK-target cell interaction. However, NK cells during interaction with target cells might receive an apoptotic signal through the binding of CD8 with HLA-I expressed by the target cell. These phenomena may play a key role in regulating the response of innate immunity against tumors. In this context, we found NK cell apoptosis at sHLA-I concentrations as low as 1 pg/cell, and this amount is high compared with that of HLA-I present on a single lymphocyte (0.1 fg/cell8); thus, it is possible that tumor targets down-regulate NK cell activity when high amounts of cancer cells are dying as in a necrotic portion of the tumor. In addition, tumor cells might evade the control of innate immunity simply by releasing sHLA-I, which, in turn, leads to NK cell apoptosis. This situation could explain, at least in part, how tumors with low amounts of HLA-I at the cell surface escape both from NK cell–mediated killing (by releasing HLA-I) and T-cell recognition (by reducing presentation of tumor-specific antigens).
In conclusion, apoptosis of NK cells through sHLA-I/CD8 interaction could play a role in switching off NK cell–mediated responses (cytolysis and/or lymphokine production). The finding that CD8α is not essential for NK cytolysis suggests that blocking of CD8-mediated apoptosis would be a useful tool to enhance antitumor activity of NK cells.
Partially supported by grants from Ministero della Sanità(2000-2002), Associazione Italiana per la Ricerca sul Cancro (AIRC), and Istituto Superiore di Sanità (ISS) AIDS project and by grants from MURST National Program 2000: “Mechanisms and modulation of apoptosis: role in autoimmune and haematological diseases” (No. MM06118858-001). G.M.S. is a fellow of Federazione Italiana per la Ricerca sul Cancro (FIRC).
G.M.S. and P.C. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Alessandro Poggi, Laboratory of Immunology, National Institute for Cancer Research, c/o CBA, Torre A1, Largo R. Benzi, 10, 16132 Genoa Italy; e-mail: poggi@vega.cba.unige.it.

![Fig. 5. Soluble HLA-I and CD8 engagement induce intracellular calcium increases in NK cells. / (A-C) The NK cell clone S2 (CD8bright) was labeled with Fura-2 and fluorescence monitored in real time on a spectrofluorometer LS50B at 37°C. (A) Baseline, [Ca++]ioscillations in the absence of any addition. (B,C) Calcium mobilization induced by sHLA-I (4 μg/mL, B) before or after pretreatment with anti-CD8 mAb (OKT8, C) to avoid the interaction of sHLA-I with CD8 antigen (CD8 masking). (D-I) The NK cell clones S4 (CD8dull, D-F) and S2 (CD8bright, G-I) were treated with the anti-CD8 mAb astra102 (E,H) or the anti-CD16 mAb NK54 (F,I). Cross-linking of CD8 or CD16 was achieved by adding 20 μg/mL GAM. (D,G) Cells treated with anti-CD54 mAb (14D12D2) matched for isotype followed by GAM, as negative control. (M,N) CD8 cross-linking (M) or addition of 4 μg/mL sHLA-I (N) were performed in the presence of 2 mM calcium chelator EGTA. An excess of calcium (4 mM of CaCl2) was added (on 250 seconds) (right arrows) after the addition of GAM or sHLA-I (left arrows). (L) Calcium oscillations in the presence of EGTA without any stimulus (baseline). Arrows in each histogram indicate the addition of either GAM (D-I,M) or sHLA-I (B,C,N); the addition of CaCl2 is indicated in panels M and N by the second arrow on the right. Results are expressed as [Ca++]i nM and are representative of 3 independent experiments performed by using NK cell clones from 3 different healthy donors.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/5/10.1182_blood.v99.5.1706/5/m_h80522223005.jpeg?Expires=1769244170&Signature=BaPU9L4ZmGNNuaSt~qbwR7X7bKtCM-EJP5Br8VAGzVoD9P6L1tF7C-POSyCK3HMnwCuP52N40uR8r~ZpAi72-urGdlA1bwDV1-db7iSP5-6ChOeRuzhP3SZ15Sxgf~Fl73wpXkjrbc2tciwpqfxxhS1WkPtQAXZjM6gxnyaZNrY~6ApawD6gv8~4bvTGoIP6eC3PvFKcYuWDCgEOiOCg~okM44hPYXw2nkmwauS6ZJNTH0c2hGVRKS5nnUNSi9FvTlDIn251faZGTTXl7EJ-GTZbFE7X8S0bhqqGO2rgK4lrU0X2i0UfrSNAy1okJ8LQ3QIbRKJ6FGi7aE5JWIe0xw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. IRSs are involved in the negative regulation of sHLA-mediated apoptosis. / The NK cell clone Cl.S2 (CD8bright CD94+KIR2D−, A-C) or the NK cell clone Cl.209 (CD8bright CD158b+ [GL183+], D-F) was incubated with the following reagents: medium (nil), sHLA-I (4 μg/mL) alone or after masking of either CD8 (sHLA-I + OKT8, A, or sHLA-I + astra 102, D) or CD94 (sHLA-I + HP-3B1, A) or CD158b (sHLA-I + GL183, D) with specific mAbs, or with anti-CD94 mAb (HP-3B1, A), or anti-CD158b mAb (GL183, D). Some samples were treated with anti-CD8 mAb (OKT8, A, or astra 102, D) alone or in combination with either anti-CD94/NKG2 or anti-CD158b mAb followed by 4-per-cell GAM-coated magnetic beads to induce cross-linking of the indicated molecules (OKT8-XL, OKT8-XL + HP-3B1-XL, A; astra 102-XL, astra 102-XL + GL183-XL, D). Results are expressed as the percentage of annexin V+ PI− cells. (B,E) Aliquots of SN derived from cells incubated for 48 hours with medium (nil), or sHLA-I (B,E), or HP-3B1 mAb (B) or GL183 mAb (E) were analyzed for the presence of FasL by enzyme-linked immunosorbent assay. In some samples, cells were exposed to sHLA-I after incubation with anti-CD94/NKG2 (sHLA-I + HP-3B1, B) or with anti-CD158b (sHLA-I + GL183, E) mAbs. Results are expressed as nanogram per milliliter of FasL. (C,F) NK cell clone Cl.S2 (C) or Cl.209 (F) was labeled with Fura-2 and treated with anti-CD8 mAb (OKT8, C, astra102, F), or with anti-CD8 plus either anti-CD94/NKG2 (OKT8-XL + HP-3B1-XL, C) or anti-CD158b (astra 102-XL + GL183-XL, F) mAb. The cross-linking (XL) of the corresponding surface molecules was achieved by adding 20 μg/mL GAM as indicated. Results are expressed as [Ca++]i nM and are representative of 4 independent experiments by using 4 different NK cell clones with a comparable surface phenotype.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/5/10.1182_blood.v99.5.1706/5/m_h80522223006.jpeg?Expires=1769244170&Signature=dbNHjYrT~rwXBwCp4aqT3EAUZLJ0jvNVKdLw7UftHTtIwJ3vYURQz~q5EuNSM3WvP~cdZV1DEgSY~eTSCQOMk6bv38EodD2y3rHSs3T-eiNSQKfjNYl6Dw0MeM6-LrFqLUTeRUorN6B8qNLpIFnUPUlorFmYI3vkCP6MWZoRY8oV3ug6E7~c34fVW3MvLAp2bckRqJzD3Qo9S4RLRHRLLlxA-1mrUsiY7Gas37Mecl9q4XZ0ApDLg3KvZZh-vZ1OZKiUf9lDu4uDwyqos5xw2Ke6tBeIqN~kN8c7~tcrM6AgV3ItyUf-ElnGmeCAt95BDin2I1mDAiUisRTgZIt9fg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal