Abstract
Chronic myelogenous leukemia (CML) is caused by expression of the BCR-ABL tyrosine kinase oncogene, the product of the t(9;22) Philadelphia translocation. Patients with CML in accelerated phase have rapidly progressive disease and are characteristically unresponsive to existing therapies. Imatinib (formerly STI571) is a rationally developed, orally administered inhibitor of the Bcr-Abl kinase. A total of 235 CML patients were enrolled in this study, of whom 181 had a confirmed diagnosis of accelerated phase. Patients were treated with imatinib at 400 or 600 mg/d and were evaluated for hematologic and cytogenetic response, time to progression, survival, and toxicity. Imatinib induced hematologic response in 82% of patients and sustained hematologic responses lasting at least 4 weeks in 69% (complete in 34%). The rate of major cytogenetic response was 24% (complete in 17%). Estimated 12-month progression-free and overall survival rates were 59% and 74%, respectively. Nonhematologic toxicity was usually mild or moderate, and hematologic toxicity was manageable. In comparison to 400 mg, imatinib doses of 600 mg/d led to more cytogenetic responses (28% compared to 16%), longer duration of response (79% compared to 57% at 12 months), time to disease progression (67% compared to 44% at 12 months), and overall survival (78% compared to 65% at 12 months), with no clinically relevant increase in toxicity. Orally administered imatinib is an effective and well-tolerated treatment for patients with CML in accelerated phase. A daily dose of 600 mg is more effective than 400 mg, with similar toxicity.
Introduction
Accelerated phase marks the onset of advanced, rapidly progressive chronic myelogenous leukemia (CML), a clonal neoplastic disorder of hematopoietic stem cells.1,2 In more than 85% of all CML patients, accelerated phase is preceded by a prolonged chronic phase characterized by mild symptoms in most patients.1-7 In accelerated phase, cells develop genetic and karyotypic abnormalities leading to an increased number of poorly differentiated cells in peripheral blood and marrow, splenomegaly, and often to the onset of constitutional symptoms.2-8Accelerated phase generally leads to a rapidly fatal blast crisis within 6 months.3-8
Because accelerated phase CML is associated with numerous hematologic, cytogenetic, and clinical signs and symptoms, no single set of criteria for its onset is universally accepted. Some groups advocate practical criteria embracing any of several events or developments that are generally associated with advanced, progressive CML. These include progressive splenomegaly; treatment-refractory thrombocytosis; rapid leukocyte doubling time; myelofibrosis; atypical leukocytes or erythrocytes; extramedullary disease (chloromas); disease symptoms; the presence of elevated numbers of blasts, basophils, or eosinophils in peripheral blood or marrow; thrombocytopenia; or karyotypic evolution.5-9 Figure1 presents a set of criteria including only those factors retrospectively statistically correlated with reduced survival, comprising the presence of 15% to less than 30% blasts, or 30% blasts and promyelocytes, or 20% basophils in peripheral blood or marrow, or platelet counts less than 100 × 109/L unrelated to anticancer therapy.3 4
The causative event in CML is the genetic transposition of ABL and BCR sequences to form a BCR-ABL fusion gene, leading to the expression of a constitutively active, fusion Bcr-Abl protein-tyrosine kinase.1,10 In at least 90% of cases, this event appears as a t(9;22) (q34;q11) reciprocal translocation (the Philadelphia translocation), which is the most characteristic feature of CML.10,11 Expression of the BCR-ABL gene is sufficient to cause chronic phase CML; the progression of disease to accelerated phase or terminal blast crisis is thought to depend on the development of additional genetic changes leading to loss of differentiation and an increasingly aggressive clinical presentation.1,3,4 12-15
Accelerated phase CML responds poorly to therapy. The majority of patients will have received prolonged treatment for chronic phase disease, usually with hydroxyurea (HU) or recombinant interferon alfa (rIFNα), and disease control can be maintained in some patients by increasing the dosages of previously effective drugs.7,16In most patients, however, treatment with high-dose combination chemotherapy regimens commonly used for acute leukemia is the only effective therapeutic option, inducing responses in 30% to 60% of patients, but with response duration usually less than 6 months.16-18 Allogeneic matched donor bone marrow transplantation (BMT) generally produces poor results in this patient population.16 19-24
Imatinib (Glivec [Gleevec in the United States; imatinib mesylate; Novartis Pharma AG]) is a rationally designed, potent selective competitive inhibitor of the Bcr-Abl protein-tyrosine kinase.25-28 At therapeutic doses it also inhibits tyrosine kinase activity of the platelet-derived growth factor (PDGF) receptor β and c-Kit, but does not affect other members of the type III receptor kinase family, such as Flt-3 and Fms.28 In an ascending dose phase 1 study, imatinib induced substantial and durable responses with minimal toxicity, at daily doses of 300 mg and higher, in nearly all patients with chronic CML, including patients with evidence of accelerated disease.29 In expanded phase 1 trials, imatinib also showed clinically relevant activity in patients with blast crisis.30 The substantial activity observed in the subset of patients with accelerated or blast phase CML indicates that the selective inhibition of Bcr-Abl tyrosine kinase activity can have therapeutic benefit in these patients.
Because of the observed activity and favorable tolerability of imatinib in patients with accelerated phase CML, and because of the limited treatment options available to these patients, we have conducted a phase 2 trial of imatinib in patients meeting rigorous criteria for accelerated phase CML.3 4 The study objectives were to confirm its observed activity and favorable safety profile in a larger patient population and to characterize prognostic factors.
Patients and methods
Patients
Male or female patients were eligible for inclusion in this study if they were at least 18 years old and had a diagnosis of Philadelphia chromosome-positive (Ph+) CML, confirmed by cytology, histology, and cytogenetic or molecular analyses, in accelerated phase as defined below.
Accelerated phase CML was defined by either at least 15% to less than 30% blasts in peripheral blood or marrow, or 30% or more blasts plus promyelocytes in peripheral blood or marrow (provided that < 30% blasts were present), or at least 20% peripheral basophils, or thrombocytopenia defined as platelet counts of less than 100 × 109/L, unrelated to therapy.3Patients with karyotypic evolution suggesting advanced CML but without other evidence of accelerated phase were not eligible for enrollment.
Patients were required to have alanine aminotransferase (ALT) and aspartate amino transferase (AST) levels not higher than 3 times the upper normal limit in cases without suspected leukemic involvement of the liver, or not higher than 5 times the upper normal limit in cases of suspected liver involvement, to have serum total bilirubin levels not higher than 3 times the upper normal limit, and serum creatinine levels not higher than 2 times the upper normal limit. Women of childbearing potential were required to have a negative pregnancy test before starting treatment, and all patients were required to use barrier contraceptive measures throughout therapy with imatinib. Patients were excluded from the trial if they had an Eastern Cooperative Oncology Group (ECOG) performance status of grade 3 or higher, grade 3/4 cardiac disease, leukemic central nervous system involvement, or any serious concomitant medical condition. Exclusion criteria included treatment with either HU within 24 hours before starting therapy with imatinib, or with INFα within 48 hours before starting therapy; with 6-mercaptopurine, vinca alkaloids, steroids, or low-dose cytosine arabinoside (< 30 mg/m2 every 12-24 hours) within 7 days before starting therapy; with homoharringtonine or moderate-dose cytosine arabinoside (100-200 mg/m2 for 5-7 days) within 14 days before therapy; with anthracyclines, mitoxantrone, cyclophosphamide, etoposide, or methotrexate within 21 days before starting therapy; with any other investigational agent or with high-dose cytosine arabinoside (1-3 g/m2 every 12-24 hours for 6-12 doses) within 28 days before starting therapy; or with busulfan or any hematopoietic stem cell transplantation within 6 weeks before starting therapy; or if recovery from stem cell transplantation was not complete. Patients were also excluded from enrollment if they had a history of noncompliance to therapy or if they were considered by the investigator to be potentially unreliable.
All patients gave written informed consent to participate in the study, and the study was reviewed and approved by a recognized ethics review committee at each trial center. The study was performed in accordance with the Declaration of Helsinki (as amended in Tokyo, Venice, and Hong Kong).
Study design and treatment
This was an open-label, nonrandomized, multicenter, phase 2 trial designed to evaluate the clinical efficacy of imatinib, as determined by the rate of sustained hematologic response (lasting ≥ 4 weeks), and the safety of treatment.
Initially, enrolled patients received treatment with orally administered imatinib at daily doses of 400 mg. Following the availability of phase 1 dose escalation data demonstrating the safety of prolonged treatment with higher doses, this initial daily dose was increased by protocol amendment to 600 mg. For patients who had a relapse, dose escalation to a maximum of 400 mg twice daily was permitted at the discretion of the investigator. Dose escalation was also permitted for patients who did not achieve hematologic response after at least 1 month of therapy, on a case-by-case basis following discussion between the investigator and sponsor. Patients received treatment for 24 weeks, and then treatment was continued indefinitely in cases where the investigator judged that further treatment was of clinical benefit.
Treatment was interrupted or reduced in response to nonhematologic, hepatic, or hematologic toxicity, which was graded according to National Cancer Institute/National Institutes of Health (NCI/NIH) Common Toxicity Criteria (CTC). For patients requiring dose reduction, daily doses were reduced from 600 to 400 mg or from 400 to 300 mg. Dose reduction below 300 mg/d was discouraged in light of recent pharmacokinetic analyses indicating that treatment with imatinib at 200 mg/d results in subtherapeutic drug levels in a fraction of patients.41 If grade 2 nonhematologic toxicity occurred, therapy was interrupted until recovery to grade 1 or less, and resumed at the original dosage. If grade 2 toxicity recurred following resumption, treatment was again interrupted until recovery, and resumed at a reduced dose. If grade 3 or 4 nonhematologic toxicity occurred, therapy was interrupted until recovery to grade 2 or less, and resumed at a reduced dose. Specific dose reduction rules for hepatic toxicity were applied to patients who enrolled with elevated baseline transaminase levels (3-fold to 5-fold above upper normal limits). If such patients developed increases of more than 3-fold in one or more transaminase levels, therapy was interrupted until levels returned to baseline, and resumed at a reduced dose. For patients who experienced clinically relevant, but less than 3-fold increases in transaminase levels, treatment was interrupted until recovery, and resumed at the same dose. If patients experienced a subsequent clinically relevant increase in transaminase levels, treatment was interrupted until recovery, and resumed at a reduced dose. Dose reductions for hematologic toxicity were only to be considered for patients with grade 4 neutropenia (neutrophil count < 0.5 × 109/L) lasting at least 2 weeks, after a minimum of 28 days of therapy. In practice, however, imatinib doses were also reduced in cases of grade 4 thrombocytopenia considered to be unrelated to leukemia. Bone marrow examinations were performed routinely, and biopsies obtained in so far as possible, until recovery from grade 4 neutropenia or thrombocytopenia. For patients with persistent marrow cellularity less than 10% and blasts less than 10%, the daily dosage was successively reduced at 2-week intervals or was interrupted until recovery of neutropenia or thrombocytopenia to grade 2 or better. On recovery, treatment was resumed at the full initial dose. Treatment was not interrupted or reduced for patients with marrow cellularity or blasts more than 10%.
No concomitant anticancer drugs were administered, with the exception of HU, anagrelide, or leukopheresis, all 3 being permitted during the first 28 days of study treatment. Within the first 28 days of treatment, HU could be given at a maximum dose of 5 g/d for up to a total of 7 days if required to control elevated blast or platelet counts. Leukopheresis was allowed up to a maximum of 2 procedures per week or 4 procedures during the first 28 days. Treatment with allopurinol at 300 mg/d was recommended until stabilization of white blood cell (WBC) counts, and patients with febrile neutropenia or infection could receive treatment with colony-stimulating factors at the investigator's discretion.
Evaluation of patients
Patients were evaluated for hematologic and cytogenetic response and relapse at frequent intervals during the initial 24 weeks of imatinib treatment. Peripheral blood samples were obtained and analyzed at baseline, 3 times weekly for the first 4 weeks, weekly between weeks 5 and 13, every 2 weeks after week 13, and on the last day of treatment. Bone marrow aspirates were performed at screening, at weeks 5, 9, 13, and 25, and on the last day of treatment; bone marrow biopsies were optional and obtained as indicated. Physical examination to evaluate liver and spleen size and extramedullary involvement was carried out at screening, every 4 weeks during therapy, and on the last day of treatment. After the initial 24 weeks of treatment, bone marrow aspirates or biopsies for analysis of disease status were obtained every 3 months for the first 6 months, and every 4 months thereafter. Following treatment, patients were followed for survival at least every 3 months, until death. Treatment toxicity was evaluated by patient interview at each office visit. Toxicity was graded according to the NCI/NIH CTC.31
The primary efficacy end point in this study was sustained hematologic response lasting at least 4 weeks, assessed by the investigator as (1) complete hematologic response (CHR); (2) marrow response; or (3) return of chronic phase. CHR was defined according to conventional criteria as myeloblast count less than 5% in bone marrow, with no myeloblasts in peripheral blood, neutrophil count at least 1.5 × 109/L and platelet count at least 100 × 109/L, and no evidence of extramedullary involvement. In patients not achieving a CHR, marrow response was defined as myeloblast count less than 5% in bone marrow, with no myeloblasts in peripheral blood, neutrophil count at least 1.0 × 109/L, and platelet count at least 20 × 109/L (without platelet transfusion and without evidence of bleeding), and no evidence of extramedullary involvement. A return of chronic phase was defined as less than 15% myeloblasts in peripheral blood and bone marrow, with less than 30% myeloblasts plus promyelocytes in the peripheral blood and bone marrow, less than 20% peripheral basophils, and no extramedullary involvement other than in liver or spleen. Sustained responses were required to be observed at 2 consecutive evaluations at least 4 weeks apart.
Secondary efficacy end points were the induction of cytogenetic response, duration of hematologic response, time to disease progression, and overall survival. Cytogenetic response was based on the prevalence of Ph+ metaphases among at least 20 metaphase cells in each bone marrow sample and was defined as complete (0% Ph+ cells), partial (1%-35%), minor (36%-65%), minimal (66%-95%), none (> 95%). Major cytogenetic response was defined as either a complete or partial response. Duration of hematologic or major cytogenetic response was calculated for all responders as the time from the first reported date of response to the earliest date of reported relapse or death. Duration of response was censored at last examination date for patients with ongoing response or patients who discontinued treatment for reasons other than adverse events, progression, or death. A single determination not fulfilling the criteria for “return to chronic phase” was considered a relapse. Time to disease progression was calculated for all patients as the time from treatment start to the onset date of blast crisis, relapse (for responding patients), discontinuation of therapy, or death from any cause. This time was censored at last examination date for patients without progression or patients who discontinued treatment for reasons other than adverse events, progression, or death. Overall survival was calculated as the time from treatment start to the date of death from any cause. Survival was censored at the time treatment was discontinued to allow BMT, or at the last recorded contact or evaluation when patients were alive at time of analysis.
Statistical analysis
This study was designed to demonstrate whether the overall hematologic response rate (CHR, marrow response, or return to chronic phase) was at least 30% in patients with CML in accelerated phase. A required sample size of 68 evaluable patients was based on the Fleming single-stage procedure, testing H0: P ≤ 30% and H1: P ≥ 50%, with alpha = 2.5% (one-sided), and power of 90%. To allow for premature withdrawals, the planned sample size consisted of 100 patients with CML in accelerated phase.
Efficacy results are presented for all patients with a confirmed diagnosis of CML in accelerated phase and for all enrolled patients. Response rates are reported as intent-to-treat analyses. Patients who withdrew from treatment before a sustained response was reported were counted as nonresponders. Response duration, time to progression, and survival were computed using standard Kaplan-Meier methods. Safety results are reported for all enrolled patients who received at least one dose of imatinib.
Univariate and multivariate analyses were performed to test for effects of potential prognostic factors on the hematologic response rate, time to disease progression, and overall survival. The χ2 or log-rank tests were used to identify prognostic factors at a significance level of P < .2. Factors meeting this criterion were included as terms in logistic regression or multivariate Cox models. Factors with no significant effect at a level ofP < .10 in multivariate analysis were removed, whereas factors remaining in the multivariate model were interpreted as independently predictive of the corresponding efficacy outcome.
Results
Patients and treatment
A total of 235 patients were enrolled at 18 centers in France (2 centers), Germany (4 center), Italy (3 centers), Switzerland (1 center), the United Kingdom (2 center), and the United States (6 centers) from August 1999 to March 2000; efficacy and safety data for analysis were collected through January 2001. Patient enrollment was allowed to exceed the original planned accrual as follow-up data from an earlier phase 1 study became available and provided increasing evidence of the activity and safety of imatinib in patients with CML in advanced phases.30 Patients were diagnosed with Ph+ or BCR-ABL+ CML in accelerated phase during the screening period for patient selection. Based on a central review of data from screening and baseline tests, 181 (77%) patients had a confirmed diagnosis of CML with protocol-specified features of accelerated phase3 at the start of imatinib therapy, whereas this disease stage could not be confirmed at treatment start for 54 (23%) enrolled patients. For these 54 patients, disease features at screening, or developing between screening and treatment start, were consistent with chronic phase CML (16 patients), blast crisis (29 patients), or the disease phase could not be determined from available information (9 patients).
Table 1 presents a summary of patient disease history and baseline characteristics. Among the 235 enrolled patients and the 181 patients with confirmed diagnoses of accelerated phase CML, respectively, 77 (33%) and 62 (34%) started their initial therapy with imatinib at daily doses of 400 mg, whereas the initial daily dose was 600 mg for the remaining 158 (67%) and 119 (66%) patients. As shown in Table 1, patients in both dose groups had similar hematologic and cytogenetic characteristics. Among patients with confirmed diagnoses, accelerated phase was newly diagnosed in 62 patients (34%), whereas 119 patients (66%) had received prior therapy for CML in accelerated phase, most often with HU (101 patients), rIFNα (33 patients), or cytarabine (23 patients). In 132 of these 181 patients, accelerated phase criteria included elevated blast counts or blast plus promyelocyte counts. Approximately 12% of enrolled patients were older than 70.
Demographics, disease history, and characteristics of patients at baseline
| Characteristics . | Patients with confirmed diagnosis . | All enrolled patients . | ||
|---|---|---|---|---|
| All doses n = 181 . | 400-mg dose group n = 62 . | 600-mg dose group n = 119 . | All doses n = 235 . | |
| Age (y) | ||||
| Median (range) | 57 (22-86) | 56 (25-86) | 58 (22-80) | 56 (22-86) |
| Sex, n (%) | ||||
| Male | 92 (51) | 34 (55) | 58 (49) | 118 (50) |
| Female | 89 (49) | 28 (45) | 61 (51) | 117 (50) |
| ECOG score, n (%) | ||||
| Grade 0-1 | 143 (79) | 46 (74) | 97 (82) | 180 (77) |
| Grade 2 | 30 (17) | 15 (24) | 15 (13) | 41 (17) |
| Grade 3 | 0 | 0 | 0 | 2 (1) |
| Splenomegaly, n (%) | ||||
| Any splenomegaly | 104 (57) | 39 (63) | 65 (55) | 127 (54) |
| At least 10 cm | 48 (27) | 21 (34) | 27 (23) | 62 (26) |
| Prior therapy for accelerated phase, n (%) | 119 (66) | 41 (66) | 78 (66) | 157 (67) |
| WBC (× 109/L) | ||||
| Median | 25 | 21 | 25 | 21 |
| Range | 1-330 | 1-184 | 1-330 | 1-330 |
| At least 20 × 109/L, n (%) | 102 (56) | 31 (50) | 71 (60) | 124 (53) |
| Blasts in peripheral blood (%) | ||||
| Median | 4 | 4 | 5 | 4 |
| Range | 0-29 | 0-29 | 0-27 | 0-71 |
| At least 15% blasts, n (%) | 36 (20) | 10 (16) | 26 (22) | 54 (23) |
| Blasts in bone marrow (%) | ||||
| Median | 15 | 16 | 15 | 15 |
| Range | 0-29 | 0-27 | 0-29 | 0-77 |
| At least 15% blasts, n (%) | 94 (52) | 33 (53) | 61 (51) | 118 (50) |
| Basophils in peripheral blood | ||||
| At least 20%, n (%) | 24 (13) | 8 (13) | 16 (13) | 24 (10) |
| Hemoglobin (g/L) | ||||
| Median | 101 | 104 | 99 | 103 |
| Range | 62-163 | 70-148 | 62-163 | 62-163 |
| Platelets (× 109/L) | ||||
| Median | 241 | 211 | 267 | 263 |
| Range | 6-3067 | 12-2130 | 6-3067 | 6-3067 |
| Less than 100 × 1009/L, n (%) | 63 (35) | 23 (37) | 40 (34) | 73 (31) |
| Characteristics . | Patients with confirmed diagnosis . | All enrolled patients . | ||
|---|---|---|---|---|
| All doses n = 181 . | 400-mg dose group n = 62 . | 600-mg dose group n = 119 . | All doses n = 235 . | |
| Age (y) | ||||
| Median (range) | 57 (22-86) | 56 (25-86) | 58 (22-80) | 56 (22-86) |
| Sex, n (%) | ||||
| Male | 92 (51) | 34 (55) | 58 (49) | 118 (50) |
| Female | 89 (49) | 28 (45) | 61 (51) | 117 (50) |
| ECOG score, n (%) | ||||
| Grade 0-1 | 143 (79) | 46 (74) | 97 (82) | 180 (77) |
| Grade 2 | 30 (17) | 15 (24) | 15 (13) | 41 (17) |
| Grade 3 | 0 | 0 | 0 | 2 (1) |
| Splenomegaly, n (%) | ||||
| Any splenomegaly | 104 (57) | 39 (63) | 65 (55) | 127 (54) |
| At least 10 cm | 48 (27) | 21 (34) | 27 (23) | 62 (26) |
| Prior therapy for accelerated phase, n (%) | 119 (66) | 41 (66) | 78 (66) | 157 (67) |
| WBC (× 109/L) | ||||
| Median | 25 | 21 | 25 | 21 |
| Range | 1-330 | 1-184 | 1-330 | 1-330 |
| At least 20 × 109/L, n (%) | 102 (56) | 31 (50) | 71 (60) | 124 (53) |
| Blasts in peripheral blood (%) | ||||
| Median | 4 | 4 | 5 | 4 |
| Range | 0-29 | 0-29 | 0-27 | 0-71 |
| At least 15% blasts, n (%) | 36 (20) | 10 (16) | 26 (22) | 54 (23) |
| Blasts in bone marrow (%) | ||||
| Median | 15 | 16 | 15 | 15 |
| Range | 0-29 | 0-27 | 0-29 | 0-77 |
| At least 15% blasts, n (%) | 94 (52) | 33 (53) | 61 (51) | 118 (50) |
| Basophils in peripheral blood | ||||
| At least 20%, n (%) | 24 (13) | 8 (13) | 16 (13) | 24 (10) |
| Hemoglobin (g/L) | ||||
| Median | 101 | 104 | 99 | 103 |
| Range | 62-163 | 70-148 | 62-163 | 62-163 |
| Platelets (× 109/L) | ||||
| Median | 241 | 211 | 267 | 263 |
| Range | 6-3067 | 12-2130 | 6-3067 | 6-3067 |
| Less than 100 × 1009/L, n (%) | 63 (35) | 23 (37) | 40 (34) | 73 (31) |
At the time of data analysis, median treatment durations were 10 months (range, 0.2-17 months) for the 77 patients in the 400-mg dose group and 11 months (range, 0.2-15 months) for the 158 patients in 600-mg dose groups, and median actual dose intensities were 400 mg/d and 578 mg/d, respectively. Of the 235 patients enrolled, 100 (43%) patients were withdrawn from treatment. Reasons for withdrawal in the 400-mg and 600-mg dose groups, respectively, were disease progression or unsatisfactory therapeutic effect for 36 (47%) and 37 (23%) patients, adverse events for 2 (3%) and 12 (8%) patients, death during therapy for 6 (8%) and 1 (0.6%) patients, initiation of BMT therapy for 3 (4%) and 1 (0.6%) patients, or withdrawal of consent for 0 and 2 (1%) patients.
Efficacy
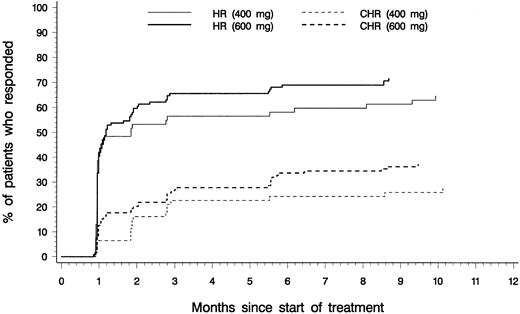
Efficacy analyses were based on the 181 patients with confirmed diagnoses of CML in accelerated phase. Table2 presents a summary of hematologic response rates for patients with confirmed diagnoses and for those in each initial dose group. Values represent the best response observed at any time during therapy. Of the 181 patients, 82% had reductions in blast counts in peripheral blood and in bone marrow corresponding to hematologic response on at least one occasion. Sustained hematologic responses lasting at least 4 weeks were reported for 69% of patients, including 34% CHR, with similar response rates for patients in the 400-mg and 600-mg dose groups. As shown in Figure2, the median time to hematologic response was 1 month in both groups, corresponding to the first scheduled evaluation of hematologic response, and response was usually achieved within 3 months after starting therapy.
Hematologic response in patients with confirmed diagnosis of accelerated phase
| Hematologic response . | All responses total n = 181 n (%) . | Sustained response . | ||
|---|---|---|---|---|
| Total n = 181 n (%) . | 400-mg dose group n = 62 n (%) . | 600-mg dose group n = 119 n (%) . | ||
| Overall* | 149 (82) | 125 (69) | 40 (65) | 85 (71) |
| 95% CI | 76.0-87.6 | 61.8-75.7 | 51.3-76.3 | 62.4-79.3 |
| Complete | 96 (53) | 61 (34) | 17 (27) | 44 (37) |
| Marrow response | 19 (10) | 22 (12) | 6 (10) | 16 (13) |
| Return to chronic phase | 34 (19) | 42 (23) | 17 (27) | 25 (21) |
| No response | 25 (14) | 46 (25) | 20 (32) | 26 (22) |
| Not evaluable | 7 (4) | 10 (6) | 2 (3) | 8 (7) |
| Hematologic response . | All responses total n = 181 n (%) . | Sustained response . | ||
|---|---|---|---|---|
| Total n = 181 n (%) . | 400-mg dose group n = 62 n (%) . | 600-mg dose group n = 119 n (%) . | ||
| Overall* | 149 (82) | 125 (69) | 40 (65) | 85 (71) |
| 95% CI | 76.0-87.6 | 61.8-75.7 | 51.3-76.3 | 62.4-79.3 |
| Complete | 96 (53) | 61 (34) | 17 (27) | 44 (37) |
| Marrow response | 19 (10) | 22 (12) | 6 (10) | 16 (13) |
| Return to chronic phase | 34 (19) | 42 (23) | 17 (27) | 25 (21) |
| No response | 25 (14) | 46 (25) | 20 (32) | 26 (22) |
| Not evaluable | 7 (4) | 10 (6) | 2 (3) | 8 (7) |
Overall hematologic response is complete response, marrow response, and return to chronic phase.
Time to overall and complete hematologic response.
Cumulative time to onset of hematologic response (HR; comprising complete hematologic response, marrow response, and return to chronic phase), and time to onset of complete hematologic response (CHR) for patients who started therapy with daily imatinib doses of 400 mg (n = 62) or 600 mg (n = 119).
Time to overall and complete hematologic response.
Cumulative time to onset of hematologic response (HR; comprising complete hematologic response, marrow response, and return to chronic phase), and time to onset of complete hematologic response (CHR) for patients who started therapy with daily imatinib doses of 400 mg (n = 62) or 600 mg (n = 119).
Most hematologic responses were induced at the initially assigned dose level, and the effects of imatinib dose escalation were not systematically studied. However, dose escalation (to a maximum daily dose of 800 mg) led to hematologic responses in 4 patients (of which one was a sustained response lasting at least 4 weeks) who had not responded to initial therapy.
Figure 3 depicts the duration of hematologic response for patients in each dose group with confirmed diagnoses of accelerated phase. Only patients with a response lasting at least 4 weeks were included in this analysis. The estimated median response duration was 13.4 months for patients in the 400-mg dose group; it had not been reached at the time of analysis for patients in the 600-mg dose group. Estimated response duration exceeded 12 months for 57% of patients in the 400-mg dose group (95% CI, 40%-73%), for 79% of patients in the 600-mg dose group (95% CI, 69%-88%), and for 70% of the 181 patients in either dose group (95% CI, 61%-80%).
Duration of hematologic response.
Of the 85 patients in the 600-mg dose group who had sustained hematologic responses, 68 are still in response (censored) between 2.3 and 13.8 months after response was first recorded. One of these patients discontinued to undergo BMT after 5 months in CHR. Of the 40 patients in the 400-mg dose group who had sustained hematologic responses, 21 are still in response (censored) between 4.2 and 16.0 months after the first report of response. The difference between dose groups was statistically significant (P = .014, log-rank test).
Duration of hematologic response.
Of the 85 patients in the 600-mg dose group who had sustained hematologic responses, 68 are still in response (censored) between 2.3 and 13.8 months after response was first recorded. One of these patients discontinued to undergo BMT after 5 months in CHR. Of the 40 patients in the 400-mg dose group who had sustained hematologic responses, 21 are still in response (censored) between 4.2 and 16.0 months after the first report of response. The difference between dose groups was statistically significant (P = .014, log-rank test).
As shown in Table 3, major cytogenetic responses were reported for 43 (24%) patients, and major, minor, or minimal cytogenetic response was reported for 86 (48%) patients. The median time to major cytogenetic response was similar for patients in the 400-mg dose group (2.4 months) and the 600-mg group (2.9 months), and mostly corresponded to the first evaluation of cytogenetic response. However, major cytogenetic responses were achieved up to 12 months after starting therapy. Cytogenetic response was only evaluated every 3 months, and therefore the duration of response cannot be calculated with precision. Among patients with major cytogenetic responses, the estimated duration of response was at least 9 months in 42% of patients in the 400-mg dose group (95% CI, 8%-76%), in 71% of patients in the 600-mg dose group (95% CI, 54%-88%), and in 64% of patients overall (95% CI, 48%-80%).
Cytogenetic response in patients with confirmed diagnosis of accelerated phase
| Cytogenetic response3-150 . | Total n = 181 n (%) . | 400-mg dose group n = 62 n (%) . | 600-mg dose group n = 119 n (%) . |
|---|---|---|---|
| Major3-151 | 43 (24) | 10 (16) | 33 (28) |
| 95% CI | 17.8-30.6 | 8.0-27.7 | 19.9-36.7 |
| Complete | 30 (17) | 7 (11) | 23 (19) |
| Partial | 13 (7) | 3 (5) | 10 (8) |
| Minor | 12 (7) | 5 (8) | 7 (6) |
| Minimal | 31 (17) | 9 (15) | 22 (18) |
| No response | 82 (45) | 36 (58) | 46 (39) |
| Not evaluated | 13 (7) | 2 (3) | 11 (9) |
| Cytogenetic response3-150 . | Total n = 181 n (%) . | 400-mg dose group n = 62 n (%) . | 600-mg dose group n = 119 n (%) . |
|---|---|---|---|
| Major3-151 | 43 (24) | 10 (16) | 33 (28) |
| 95% CI | 17.8-30.6 | 8.0-27.7 | 19.9-36.7 |
| Complete | 30 (17) | 7 (11) | 23 (19) |
| Partial | 13 (7) | 3 (5) | 10 (8) |
| Minor | 12 (7) | 5 (8) | 7 (6) |
| Minimal | 31 (17) | 9 (15) | 22 (18) |
| No response | 82 (45) | 36 (58) | 46 (39) |
| Not evaluated | 13 (7) | 2 (3) | 11 (9) |
Cytogenetic response defined by prevalence of Ph+ metaphases: 0%, complete; 1% to 35%, partial; 36% to 65%, minor; 66% to 95%, minimal; greater than 95%, none.
Major cytogenetic response indicates complete or partial response.
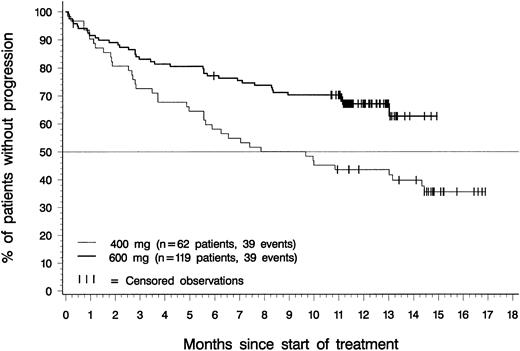
Figure 4 depicts time to disease progression (onset of blast crisis or loss of hematologic response) for patients in each dose group with confirmed diagnoses of accelerated phase. The median time to progression was 8.8 months for patients in the 400-mg dose group, and had not been reached at the time of analysis for patients in the 600-mg dose group. Estimated time to progression exceeded 12 months in 44% of patients in the 400-mg dosing group (95% CI, 31%-56%), in 67% of patients in the 600-mg dosing group (95% CI, 59%-76%), and in 59% of the 181 patients in either dosing group (85% CI, 52%-66%).
Time to disease progression.
Of the 119 patients in the 600-mg dose group, 39 have progressed, and time to disease progression was censored for the remaining 80. Of the 62 patients in the 400-mg dose group, 39 patients have progressed, and time to disease progression was censored for the remaining 23. The difference between dose groups was statistically significant (P = .002, log-rank test).
Time to disease progression.
Of the 119 patients in the 600-mg dose group, 39 have progressed, and time to disease progression was censored for the remaining 80. Of the 62 patients in the 400-mg dose group, 39 patients have progressed, and time to disease progression was censored for the remaining 23. The difference between dose groups was statistically significant (P = .002, log-rank test).
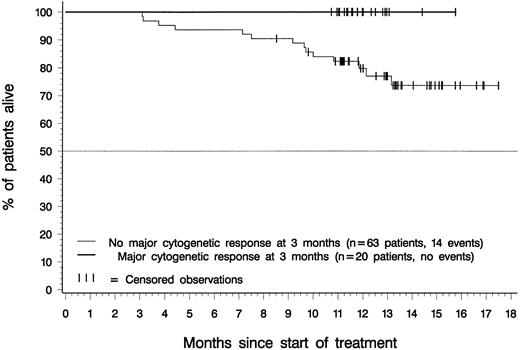
Figure 5 depicts overall survival for patients in the 400-mg and 600-mg dose groups with a confirmed diagnosis of CML in accelerated phase. At the time of analysis, median survival had not been reached for patients in either dosing group. Estimated overall survival rates at 12 months were 65% for patients in the 400-mg dose group (95% CI, 53%-77%), 78% for patients in the 600-mg dose group (95% CI, 70%-87%), and 74% for all 181 patients (95% CI, 68%-81%). Notably, all patients who achieved a major cytogenetic response within 3 months of therapy were alive at the time of analysis, regardless of their initial imatinib dose (Figure 6).
Overall survival.
Of the 119 patients in the 600-mg dose group, 24 have died, and overall survival was censored for the remaining 95; 1 of these 95 patients discontinued therapy with imatinib to undergo BMT. Of the 62 patients in the 400-mg dose group, 27 have died, and overall survival was censored for the remaining 35; 1 of these 35 patients discontinued treatment with imatinib to undergo BMT. The difference between dose groups was statistically significant (P = .014, log-rank test).
Overall survival.
Of the 119 patients in the 600-mg dose group, 24 have died, and overall survival was censored for the remaining 95; 1 of these 95 patients discontinued therapy with imatinib to undergo BMT. Of the 62 patients in the 400-mg dose group, 27 have died, and overall survival was censored for the remaining 35; 1 of these 35 patients discontinued treatment with imatinib to undergo BMT. The difference between dose groups was statistically significant (P = .014, log-rank test).
Landmark analysis: overall survival by major cytogenetic response status at 3 months.
Of 83 patients with an evaluation of cytogenetic response at 3 months after start of therapy, 20 (24%) had a major cytogenetic response at that time, and none of these 20 patients had died at the time of analysis. Of the 63 evaluated patients without major cytogenetic response after 3 months, 14 (22%) had died.
Landmark analysis: overall survival by major cytogenetic response status at 3 months.
Of 83 patients with an evaluation of cytogenetic response at 3 months after start of therapy, 20 (24%) had a major cytogenetic response at that time, and none of these 20 patients had died at the time of analysis. Of the 63 evaluated patients without major cytogenetic response after 3 months, 14 (22%) had died.
Univariate analyses and regression models were applied to test for the effects of several baseline variables on time to disease progression. Table 4 lists the prognostic variables included in these analyses, the cutoff values used to define patient subgroups, and the results. Data for 2 variables (blasts in bone marrow and additional chromosomal abnormalities) were unavailable in a substantial number of patients, and for this reason 2 separate analyses were performed, one excluding these factors but including all patients with confirmed diagnosis, and secondary analyses to evaluate the additional effects of these 2 factors separately, limited to the subset of patients with data for all variables.
Prognostic factors associated with time to disease progression
| Factor . | Category . | N . | Patients progressing, n (%) . | P4-150 log rank . | P4-150 Wald χ . | Relative risk . |
|---|---|---|---|---|---|---|
| Initial dose | 400 mg | 59 | 37 (63) | .0020 | .0005 | 2.34 |
| 600 mg | 112 | 36 (32) | ||||
| Age | Less than 60 y | 101 | 46 (46) | .31 | ||
| At least 60 y | 70 | 27 (39) | ||||
| Sex | Male | 87 | 42 (48) | .099 | .076 | 1.53 |
| Female | 84 | 31 (37) | ||||
| Weight | Less than 70 kg | 83 | 37 (46) | .55 | ||
| At least 70 kg | 88 | 36 (41) | ||||
| Hepatomegaly | No | 133 | 54 (41) | .23 | ||
| Yes | 38 | 19 (50) | ||||
| Splenomegaly | No | 72 | 25 (35) | .14 | ||
| Yes | 99 | 48 (48) | ||||
| Hemoglobin | Less than 100 g/L | 82 | 45 (55) | .0017 | .0002 | 2.50 |
| At least 100 g/L | 89 | 28 (31) | ||||
| WBC count | Less than 30 × 109/L | 99 | 43 (43) | .94 | ||
| At least 30 × 109/L | 72 | 30 (42) | ||||
| Platelets | Less than 100 × 109/L | 59 | 29 (49) | .099 | ||
| At least 100 × 109/L | 112 | 44 (39) | ||||
| Basophils in peripheral blood | Less than 20% | 149 | 67 (45) | .12 | ||
| At least 20% | 22 | 6 (27) | ||||
| Blasts in peripheral blood | Less than 15% | 138 | 56 (41) | .23 | ||
| At least 15% | 33 | 17 (52) | ||||
| Blasts in bone marrow4-151 | Less than 15% | 65 | 20 (31) | .092 | ||
| At least 15% | 88 | 41 (47) | ||||
| Other chromosome abnormalities4-151 | No | 100 | 38 (38) | .086 | ||
| Yes | 60 | 30 (50) |
| Factor . | Category . | N . | Patients progressing, n (%) . | P4-150 log rank . | P4-150 Wald χ . | Relative risk . |
|---|---|---|---|---|---|---|
| Initial dose | 400 mg | 59 | 37 (63) | .0020 | .0005 | 2.34 |
| 600 mg | 112 | 36 (32) | ||||
| Age | Less than 60 y | 101 | 46 (46) | .31 | ||
| At least 60 y | 70 | 27 (39) | ||||
| Sex | Male | 87 | 42 (48) | .099 | .076 | 1.53 |
| Female | 84 | 31 (37) | ||||
| Weight | Less than 70 kg | 83 | 37 (46) | .55 | ||
| At least 70 kg | 88 | 36 (41) | ||||
| Hepatomegaly | No | 133 | 54 (41) | .23 | ||
| Yes | 38 | 19 (50) | ||||
| Splenomegaly | No | 72 | 25 (35) | .14 | ||
| Yes | 99 | 48 (48) | ||||
| Hemoglobin | Less than 100 g/L | 82 | 45 (55) | .0017 | .0002 | 2.50 |
| At least 100 g/L | 89 | 28 (31) | ||||
| WBC count | Less than 30 × 109/L | 99 | 43 (43) | .94 | ||
| At least 30 × 109/L | 72 | 30 (42) | ||||
| Platelets | Less than 100 × 109/L | 59 | 29 (49) | .099 | ||
| At least 100 × 109/L | 112 | 44 (39) | ||||
| Basophils in peripheral blood | Less than 20% | 149 | 67 (45) | .12 | ||
| At least 20% | 22 | 6 (27) | ||||
| Blasts in peripheral blood | Less than 15% | 138 | 56 (41) | .23 | ||
| At least 15% | 33 | 17 (52) | ||||
| Blasts in bone marrow4-151 | Less than 15% | 65 | 20 (31) | .092 | ||
| At least 15% | 88 | 41 (47) | ||||
| Other chromosome abnormalities4-151 | No | 100 | 38 (38) | .086 | ||
| Yes | 60 | 30 (50) |
Results are shown for univariate (log-rank) and multivariate analysis (Wald χ). Factors significant atP < .2 in univariate analysis were included in the multivariate model. Factors in the multivariate model were sequentially added in order of greatest significance until the final model included only factors showing an effect with P < .1.
Multivariate analysis limited to patients with available data for all prognostic factors.
In multivariate analysis, the factors most strongly predictive of longer time to disease progression were hemoglobin at least 100 g/L, and a starting dose of 600 mg imatinib. Female sex was marginally predictive in this analysis. In subgroup analyses excluding patients without data for blasts in bone marrow or additional chromosomal abnormalities, neither variable added further to the prediction of hematologic response in multivariate analyses (P > .10). Further analyses indicated that varying the cutoff values used to define patient subgroups had little effect on most prognostic factors. Platelet counts of less than 40 × 109/L were independently predictive of shorter time to disease progression in exploratory analyses, but the reliability of this result was limited by the small number of patients in this subgroup. The use of different cutoff values for hemoglobin (< 110 g/L) and blasts in peripheral blood (< 5%) led to the elimination of male/female sex as a marginal prognostic factor in the final multivariate model, and to the inclusion of high platelet counts and low blast counts in peripheral blood as predictive of longer time to disease progression. In further exploratory multivariate analyses, a hemoglobin value of 100 g/L or higher was the sole factor significantly predictive for higher rates of sustained hematologic response. Exploratory multivariate analyses of prognostic factors for overall survival were limited by the small number of patients who had died, but results identified 4 factors as predictive of longer survival: a starting dose of 600 mg imatinib, WBC count less than 30 × 109/L, platelets at least 100 × 109/L, and absence of splenomegaly.
Efficacy results for all 235 enrolled patients were similar in all respects to those for the 181 patients with confirmed diagnoses of accelerated phase. For the 235 enrolled patients, the overall rate of hematologic response lasting at least 4 weeks was 68%, including 35% CHR. The rate of major cytogenetic response was 23%, with 17% complete cytogenetic responses. The estimated duration of hematologic response exceeded 12 months in 71% of patients. Estimated time to disease progression exceeded 12 months in 60% of patients, and estimated overall survival exceeded 12 months in 75% of patients. Prognostic factors identified for the 181 patients with confirmed diagnosis (Table 4) were similar for all 235 enrolled patients.
Toxicity
The toxicity of imatinib in this trial was generally similar to that observed in a previous phase 1 study at comparable doses. Table5 presents treatment-related adverse events observed in 5% of patients or more. The most frequently reported events were nausea, edema, vomiting, diarrhea, and muscle cramps. Grade 1 or 2 edema, dermatitis, vomiting, muscle cramps, myalgia, arthralgia, and weight increase/fluid retention were generally more frequent in the 600-mg dose group than for patients treated with imatinib at 400 mg/d, but the incidences of other grade 1 or 2 reactions, and of all grade 3 and 4 reactions, were similar in both dose groups. Routine laboratory tests revealed infrequent development of grade 3 abnormalities in AST (2% of patients), in ALT (3%), and bilirubin (2%) during treatment.
Adverse events related to treatment with imatinib
| Adverse event5-150 . | All patients (n = 235) . | |
|---|---|---|
| All grades n (%) . | Grade 3 or 4 n (%) . | |
| Nausea | 153 (65) | 8 (3) |
| Edema | 150 (64) | 6 (3) |
| Vomiting | 114 (49) | 3 (1) |
| Diarrhea | 88 (37) | 1 (0.4) |
| Muscle cramps | 75 (32) | 1 (0.4) |
| Dermatitis | 52 (22) | 3 (1) |
| Dyspepsia | 38 (16) | 0 |
| Fatigue | 25 (11) | 6 (3) |
| Myalgia | 30 (13) | 3 (1) |
| Headache | 30 (13) | 3 (1) |
| Arthralgia | 29 (12) | 7 (3) |
| Hemorrhage | 29 (12) | 4 (2) |
| Weight increase/fluid retention | 27 (11) | 3 (1) |
| Pain in limb | 27 (11) | 2 (0.9) |
| Abdominal pain, upper | 24 (10) | 1 (0.4) |
| Bone pain | 20 (9) | 1 (0.4) |
| Pruritus | 20 (9) | 1 (0.4) |
| Anorexia | 18 (8) | 3 (1) |
| Abdominal pain | 16 (7) | 1 (0.4) |
| Adverse event5-150 . | All patients (n = 235) . | |
|---|---|---|
| All grades n (%) . | Grade 3 or 4 n (%) . | |
| Nausea | 153 (65) | 8 (3) |
| Edema | 150 (64) | 6 (3) |
| Vomiting | 114 (49) | 3 (1) |
| Diarrhea | 88 (37) | 1 (0.4) |
| Muscle cramps | 75 (32) | 1 (0.4) |
| Dermatitis | 52 (22) | 3 (1) |
| Dyspepsia | 38 (16) | 0 |
| Fatigue | 25 (11) | 6 (3) |
| Myalgia | 30 (13) | 3 (1) |
| Headache | 30 (13) | 3 (1) |
| Arthralgia | 29 (12) | 7 (3) |
| Hemorrhage | 29 (12) | 4 (2) |
| Weight increase/fluid retention | 27 (11) | 3 (1) |
| Pain in limb | 27 (11) | 2 (0.9) |
| Abdominal pain, upper | 24 (10) | 1 (0.4) |
| Bone pain | 20 (9) | 1 (0.4) |
| Pruritus | 20 (9) | 1 (0.4) |
| Anorexia | 18 (8) | 3 (1) |
| Abdominal pain | 16 (7) | 1 (0.4) |
Events reported in at least 5% of patients.
Table 6 presents the numbers of patients who developed grade 3 or 4 hematologic abnormalities during treatment with imatinib. Frequencies were comparable in the 400-mg and 600-mg groups for all grade 3 or 4 abnormalities, and in both groups the most commonly reported abnormality was neutropenia. Median times from treatment start to nadir were 74 days for neutropenia and 57 days for thrombocytopenia. Among patients who developed grade 3 or 4 abnormalities, median times to recovery to grade 2 values or better were 21 days for neutropenia and 30 days for thrombocytopenia.
Hematologic abnormalities during treatment with imatinib
| Parameter6-150 and grade6-151 . | All patients (n = 235) n (%) . | 400-mg dose group (n = 77) n (%) . | 600-mg dose group (n = 158) n (%) . |
|---|---|---|---|
| Anemia | |||
| Grade 3 | 77 (33) | 27 (35) | 50 (32) |
| Grade 4 | 15 (6) | 7 (9) | 8 (15) |
| Thrombocytopenia | |||
| Grade 3 | 73 (31) | 23 (30) | 50 (32) |
| Grade 4 | 29 (12) | 11 (14) | 18 (11) |
| Leukopenia | |||
| Grade 3 | 77 (33) | 21 (27) | 56 (35) |
| Grade 4 | 34 (14) | 14 (18) | 20 (13) |
| Neutropenia | |||
| Grade 3 | 55 (23) | 16 (21) | 39 (25) |
| Grade 4 | 82 (35) | 27 (35) | 55 (35) |
| Parameter6-150 and grade6-151 . | All patients (n = 235) n (%) . | 400-mg dose group (n = 77) n (%) . | 600-mg dose group (n = 158) n (%) . |
|---|---|---|---|
| Anemia | |||
| Grade 3 | 77 (33) | 27 (35) | 50 (32) |
| Grade 4 | 15 (6) | 7 (9) | 8 (15) |
| Thrombocytopenia | |||
| Grade 3 | 73 (31) | 23 (30) | 50 (32) |
| Grade 4 | 29 (12) | 11 (14) | 18 (11) |
| Leukopenia | |||
| Grade 3 | 77 (33) | 21 (27) | 56 (35) |
| Grade 4 | 34 (14) | 14 (18) | 20 (13) |
| Neutropenia | |||
| Grade 3 | 55 (23) | 16 (21) | 39 (25) |
| Grade 4 | 82 (35) | 27 (35) | 55 (35) |
Abnormalities occurring during treatment or worsening from baseline to the indicated grade.
CTC: NCI/NIH Common Toxicity Criteria include the following:
CTC grade 3: neutrophils 0.5 to less than 1.0 × 109/L; platelets 10.0 to less than 50.0 × 109/L; hemoglobin 65 to less than 80 g/L; leukocytes 1.0 to less than 2.0 × 109/L.
CTC grade 4: neutrophils less than 0.5 × 1009/L; platelets less than 10.0 × 109/L; hemoglobin less than 65 g/L; leukocytes less than 1.0 × 109/L.
Adverse events, whether related or unrelated to treatment, led to dose reductions on one or more occasions in 38 (49%) patients in the 400-mg dose group and in 82 (52%) patients in the 600-mg dose group. Adverse events related to treatment led to the discontinuation of imatinib therapy in 6 patients, all in the 600-mg dose group, and comprised skin rash (2 patients), thrombocytopenia (1 patient), hepatobiliary disorders (Budd-Chiari syndrome and hepatotoxicity in 1 patient each), and gastric ulcer hemorrhage (1 patient). Treatment-related adverse events regarded as serious were reported for 8 (10.4%) patients in the 400-mg dose group and 24 (15.2%) patients in the 600-mg dose group, and most frequently comprised cytopenia or gastrointestinal events. One death due to liver failure was suspected to be related to therapy. This patient had undergone prior BMT and had received daily doses of 3000 to 3500 mg acetaminophen for approximately 1 month before starting treatment with imatinib at 600 mg/d. After 6 days of treatment the patient developed upper quadrant discomfort and jaundice, laboratory tests revealed markedly elevated bilirubin and transaminases, and imatinib therapy was stopped on day 7. Tests for infection were negative. The patient's condition deteriorated from day 10 and death occurred on day 12.
Discussion
We conducted this phase 2 study to determine whether imatinib, a potent inhibitor of the oncogenic Bcr-Abl tyrosine kinase, could induce hematologic responses in at least 30% of patients with CML in accelerated phase, when administered at well-tolerated doses defined in earlier trials.28-30 Results indicate that imatinib induced hematologic response lasting at least 4 weeks in 69% of patients, including CHR in 34% of patients, with manageable hematologic toxicity and few nonhematologic grade 3 or 4 adverse reactions. Treatment with imatinib also induced major cytogenetic responses in 24% of patients, 12-month progression-free survival in 59% of patients, and 12-month overall survival in 74% of patients. The efficacy of imatinib in this study far exceeded the planned success criteria defined in the protocol.
The demographic features, disease history and baseline characteristics, and major prognostic factors of the patients enrolled in this trial appear to be consistent with those described in other studies with similar patients.3,5-9 Notably, the criteria3,4 used to define accelerated phase in this trial are more stringent than those of the International Bone Marrow Transplant Registry (IBMTR)7 or Sokal8 classification systems, and patients with cytogenetic clonal evolution as a sole criterion for accelerated phase, representing a heterogeneous group with generally favorable prognosis,32 33 were excluded from this trial. Accordingly, the positive results observed with imatinib in this study are unlikely to be attributable to the selection of patients with favorable prognosis.
The analysis of prognostic factors for response, time to progression, and survival must be interpreted with caution because of the high correlation between many of the factors included in the model and the limited follow-up at the time of analysis. Although the effects of prognostic factors were not completely consistent across all 3 end points, an initial dose of 600 mg imatinib and absence of baseline anemia were found to be strongly predictive of favorable outcome. Although this trial was not designed as a randomized dose-finding study, the identification of a high starting dose of imatinib as a positive prognostic factor, and inspection of efficacy results in general, suggest that disease control is more durable when imatinib therapy is initiated and maintained at 600 mg/d rather than 400 mg/d. Hematologic response duration, cytogenetic response rates, progression-free survival, and overall survival were consistently better for patients who started imatinib therapy at 600 mg/d, although hematologic response rates did not differ greatly between dosage groups. These groups of patients had similar disease characteristics and histories, indicating that outcomes were unlikely to be influenced by differences in prognosis (Table 1). Because the toxicity of imatinib at 600 mg/d is manageable, overall results of this trial support the conclusion that patients with accelerated phase CML derive greater benefit from imatinib therapy at 600 mg/d than with 400 mg/d. Dose escalation above 600 mg/d was not systematically studied, but an increase in the initial dose to a maximum of 800 mg/d led to the induction of sustained or transient hematologic responses in a small number of cases.
A comparison of the results of this study with historical reports suggests that imatinib may bring substantially increased efficacy to the treatment of patients in CML accelerated phase, compared to current alternative biologic agents or chemotherapies. Among patients in the 600-mg dose group, overall and complete hematologic response rates of 71% and 37%, respectively, compare favorably with the range of approximately 20% to 60% overall response in similar patients treated with single agent therapies,16-18 rIFNα and low-dose cytarabine,33,34 or HU and 6-mercaptopurine.35 The induction of major cytogenetic responses in 28% of patients treated with imatinib is encouraging, because fewer than 5% of patients with CML in accelerated phase achieve cytogenetic response with alternative therapies.18,33 The 12-month progression-free and overall survival rates of 67% and 78%, respectively, also compare favorably with the more transient responses observed with other therapies.16-18 33-35 Nonhematologic adverse reactions associated with imatinib therapy were generally mild, and hematologic toxicity was manageable. In summary, the results of this phase 2 trial suggest that imatinib is substantially more active than current alternative chemotherapies or biologic agents for CML in accelerated phase.
Despite treatment-related mortality rates of approximately 50%, allogeneic stem cell transplantation is potentially curative, even in patients with advanced CML. Disease-free survival rates ranging from 20% to 40% at 4 years have been reported for patients meeting various definitions of CML in accelerated phase.19-24 Because allogeneic transplantation is more often successful in patients with early rather than advanced CML,25 clinical trials may be warranted to test whether preparative induction of response with imatinib improves the outcome of subsequent transplantation.
Although imatinib showed remarkable clinical activity in this group of patients, a substantial fraction of them ultimately had a relapse with resistant disease. Mechanisms of in vivo resistance to imatinib do not appear to involve drug absorption or metabolism.30 Resistance in many patients has been due to point mutations in the BCR-ABL oncogene.36 Other plausible resistance mechanisms obtained from in vitro models are postulated to involve amplification of the BCR-ABL fusion gene, drug efflux, increased expression of the Bcr-Abl protein, or decreased cellular bioavailability of imatinib.37-40
Because imatinib is well tolerated, it may be feasible to combine imatinib with existing agents used to treat CML in accelerated phase. Accordingly, further clinical trials are warranted to test the optimal use of imatinib in combination with other active agents.
We wish to thank the numerous coinvestigators, nursing staff, and clinical trial monitors who participated in this study. The contributions of data managers and programmers at Novartis Pharmaceuticals are gratefully acknowledged. Dr David Parkinson and Dr Greg Burke provided invaluable support, and we thank Dr John Ford, Dr Elisabeth Wehrle, and Dr Marianne Rosamilia for their collaboration in implementing the protocol and reporting the study results, and Dr Thomas Brown for assistance in preparing the manuscript.
In addition to the authors, the following investigators participated in this trial: M. Schuster, New York, NY; M. O'Dwyer, Portland, OR; J. F. Apperly, London, United Kingdom; G. Corneo, Milan, Italy; C. Karanes, Detroit, MI; J. Beck, Mainz, Germany; H.-K. Al-Ali, Leipzig, Germany; S. G. O'Brien, Newcastle upon Tyne, United Kingdom; R. Hehlmann, Mannheim, Germany; B. Wassmann, Frankfurt, Germany; D. Heim, Basel, Switzerland; D. Russo, Udine, Italy; D. Deangelo, Boston, MA; G. Rosti, Bologna, Italy; and R. Paquette, Los Angeles, CA.
Supported by a grant from Novartis Pharmaceuticals AG, Basel, Switzerland. This study was presented in part at the 42nd Annual Meeting of the American Society of Hematology, December 1-5, 2000, San Francisco, CA.
Three authors (S.F.-R., I.G., and R.C.) are employees of Novartis Pharma AG.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
M. Talpaz, M.D. Anderson Cancer Center, Houston, TX; e-mail: mtalpaz@mail.mdanderson.org.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal