We introduce a novel in vivo model of human mucosal immunity, based on the implantation of human fetal bronchial mucosa and autologous peribronchial lymph node (PLN) in the severe combined immunodeficiency (SCID) mouse. In the SCID host, human fetal bronchi implanted alone retain macrophages and mast cells but lose T cells. In contrast, fetal bronchi co-implanted with PLN contain, in addition to macrophages and mast cells, numerous T cells and B cells, often clustered in intramucosal bronchus-associated lymphoid tissue (BALT). Functionally, bronchus–PLN cografts are able to mount robust αβ and γδ T-cell–mediated immune responses to Pseudomonas aeruginosa and 3,4-epoxy-3-methyl-1-butyl-diphosphate challenges. No other autologous lymphoid organ (bone marrow, thymus, liver) allows for BALT development in co-implanted bronchi, which suggests special ontogenetic and functional relations between extramucosal PLN and intramucosal BALT. Overall, the bronchus–PLN cograft appears as a promising model for human bronchial immune development and function. Our study is the first to document long-term ex vivo maintenance of functional human lymph nodes as native appendices to mucosal tissue. Our results, therefore, suggest a simple strategy for developing similar experimental models of human immune function in other mucosae.
Introduction
Mucosae are highly differentiated, epithelium-lined tissues that regulate most aspects of our relations with the environment, including the development of protective immunity, tolerance, and allergy.1 In that respect, the bronchial mucosa2 is as important as that of the gut or the skin. Resident immune cells in the bronchial mucosa are dispersed or are organized in densely packed cell clusters, known as lymphoid follicles, with discrete areas featuring mature B cells, immunoglobulin (Ig)–producing plasma cells, and T cells. Such lymphoid follicles can be found externally to the bronchial mucosa, within peribronchial lymph nodes (PLN), or internally, within so-called bronchus-associated lymphoid tissue (BALT).3 Adaptive immune responses depend on the production of T and B cells in primary lymphoid tissues (liver, bone marrow, thymus), followed by their migration to secondary lymphoid tissues (eg, PLN and BALT), where they are exposed to antigens and are activated. Besides allowing for normal immune function, secondary lymphoid tissues are involved in other significant events, such as organ transplant rejection.4
The development of secondary lymphoid tissues follows complex developmental cues investigators are just beginning to unravel.5 Using mouse models and focusing on gut intramucosal immune structures (Peyer patches), recent studies have emphasized at least 3 different homing and differentiation pathways that may distinctly affect extramucosal and intramucosal secondary lymphoid tissues.6 Regarding the development of human bronchial immune structures, data are less abundant. During normal gestation, PLNs appear during the second trimester,7whereas BALT is rarely, if at all, present.8 After birth, BALT develops rapidly and can be identified in the mucosa of normal human bronchi throughout childhood and adolescence but not in adulthood, except under particular conditions such as smoking9 or chronic lung illness.10Thus, BALT may play a normal inductive role in immunity and may signal a pathologic context. For now, the conditions leading to BALT emergence in the human bronchial mucosa are unknown.
In previous work, we showed that human fetal bronchi implanted in SCID mice reach complete epithelial differentiation within less than 8 weeks of engraftment, whatever their initial gestational age and genotype.11 This model was used to recapitulate the early events of cystic fibrosis airway disease12,13and the dynamics of stem cell–mediated bronchial development.14 In functional terms, we demonstrated that innate responses, manifested by human macrophage and murine macrophage and granulocyte transepithelial migration, can be elicited in simple bronchial grafts after Pseudomonas aeruginosa infection.13 Here, we asked whether this model could be further used to investigate the ontogenesis and function of human bronchial immune structures, including PLN and BALT, and to mimic αβ and γδ T-cell–mediated responses.
Materials and methods
Human tissues and xenografts
Six- to 38-week gestational age (GA) human airways were obtained after elective or medically indicated termination of pregnancy, under guidelines approved by the Ethics Committee for Life Sciences of the Centre National de la Recherche Scientifique. Simple bronchial grafts and bronchus–lymphoid tissue cografts were prepared as described,11 with some modifications. Portions of liver co-implanted with airways measured approximately 1 cm3. Portions of bone were 2- to 4 cm-long segments of femur. Portions of thymus included approximately 10 lobules. For bronchus–PLN cografts, the external PLN was dissected from the airway or left adherent to it. Compound cografts of bronchus–PLN–thymus were prepared by leaving external PLN attached to the airway and by placing approximately 10 thymic lobules adjacent to the PLN. In optimal conditions, a given fetal tissue yielded up to 4 simple bronchial grafts (that could be co-implanted with nonattached lymphoid tissue) and 2 bronchus-attached PLN–thymus cografts. Mice were implanted with a maximum of 2 grafts–cografts (one on each flank). At biopsy (duration of engraftment was greater than 8 weeks), all grafts and cografts displayed complete epithelial differentiation of surface epithelium and submucosal glands as described.13 14 Before any manipulation, SCID mice were anesthetized with intraperitoneal injections of 0.4 mL Hypnomidate (Janssen-Cilag, Issy-Les-Moulineaux, France).
Immunohistochemistry
Tissues were fixed in 10% formaldehyde in phosphate-buffered saline (PBS), frozen, and cut into serial 5 μm-thick sections as previously described.13 Serial sections were stained with either mouse isotype control antibodies (DAKO, Trappes Le Font du Claix, France) or mouse monoclonal primary antibodies against human CD3, CD38, and CD45 from BD PharMingen (France); human tryptase, CD68, IgA, and HLA-DR from DAKO; or CD20 and CD25 from Immunotech (Marseilles, France). Appropriate biotinylated secondary antibodies were added, followed by streptavidin-conjugated peroxidase (DAKO), revealed by chromogenous substrates diaminobenzidine or aminoethylcarbazole, both from Sigma (Lyons, France), or followed by avidin–fluorescein isothiocyanate (FITC) (DAKO) or streptavidin-Cy3 (Dupont-NEN, Paris, France), yielding green or red fluorescence signals, respectively. For double-immunofluorescence stainings, green fluorescence was obtained using secondary antibodies directly coupled to FITC, to avoid cross-reactivity. Sections were counterstained with Gill hematoxylin.
Response to in vivo challenge with P aeruginosa
Two series of 2 matched bronchial grafts (GA, 21 weeks; duration of engraftment [DE], 27 weeks) and 2 bronchus–PLN cografts (GA, 16 weeks; DE, 10 weeks) were exposed and challenged with 100 μL of either 107 cfu/mL P aeruginosa PAO1 strain suspension or RPMI medium as a negative control, as described.13 In independent studies, we had observed that the innate immune response to intraluminal P aeruginosachallenge in simple bronchial grafts led to massive infiltration with host neutrophils, eventually destroying the graft within 4 days (R.T., et al13 and R.T., unpublished observations, January 1999). Therefore, to assay the human immune response in simple bronchial grafts and bronchus–PLN cografts, those were left for 2 days only in the SCID host after injection and were harvested for immunohistochemical analysis as described above.
Response to in vivo challenge with EpoxPP
Challenge with 3,4-epoxy-3-methyl-1-butyl-diphosphate (EpoxPP) was performed on 2 independent series (GA, 20 weeks, DE, 36 weeks; GA, 14 weeks, DE, 15 weeks, respectively), each containing 2 bronchus–PLN cografts. For each series, 100 μL EpoxPP at 0.3 mM in saline was injected into the lumen of one cograft; the other was kept uninjected. After 3 weeks, resident mucosal T cells were isolated, expanded in vitro (see below), and analyzed by flow cytometry using antibodies against human T-cell receptor (TCR) δ chain, Vγ9, and Vδ2 subsets (Immunotech), as described previously.15 The responsiveness of graft-derived T-cell lines to EpoxPP was confirmed in vitro. Responding cells were plated at 104 cells/well in 96-well plates and were incubated with EpoxPP. After 6 hours, culture supernatants were recovered and tested for their tumor necrosis factor content.16 Cytotoxic activity of graft-derived T cells against the B-cell tumor Daudi was estimated by a regular chromium Cr 51-release assay, as previously described.17
In vitro analysis of mucosal T cells
Grafts were harvested and carefully dissected to isolate internal (ie, epithelium and mesenchyme of the airway) from external (connected LN or LN + thymus) regions. Then the internal region was cut longitudinally in half. One half was processed for immunohistochemistry as described above. The other half was dissociated enzymatically for 30 minutes in PBS–0.1% collagenase–dispase (Boehringer-Mannheim, Mannheim, Germany), followed by 2 washes with PBS–5% newborn calf serum on ice. Single-cell suspensions were obtained by repeated pipetting, with a viability exceeding 95% for leukocytes as judged by Trypan blue exclusion. Cells were then seeded in 96-well plates and were expanded for 12 days,17 after which they were stained with monoclonal antibodies against mouse Thy 1.1/1.2 (PharMingen), HLA class I (W6/32; Immunotech), and human TCRβ and TCRδ chains (Immunotech) and were analyzed by flow cytometry as described previously.15 Analysis of length distribution of TCRβ junctional sequences in resident mucosal T cells from a bronchus–PLN–thymus cograft (GA, 21 weeks; DE, 27 weeks) was performed using the Immunoscope technique.18 The presence of xenoreactive clones was further tested in an in vitro cytotoxicity assay using an H-2d cell line, syngeneic to the SCID mouse host, as a target for human T cells.19
Results
Macrophages, T lymphocytes, and mast cells reside in human fetal bronchi
Human tracheas and stem bronchi from 6 to 38 weeks of development were screened for the presence of leukocytes. Three main populations were found in the mucosa—macrophages, T lymphocytes, and mast cells, in their order of first appearance—whereas B lymphocytes, natural killer cells, and granulocytes were not found in significant amounts (Table 1). Leukocyte colonization starts at 7 weeks and proceeded steadily until term, with 2 major waves occurring at approximately 12 and 20 weeks and yielding numerous T lymphocytes and mast cells. In the mature stage (from 23 weeks on), the intraepithelial compartment excluded mast cells, the subepithelial compartment included all 3 populations with T cells dominating, and the mesenchymal compartment included all 3 populations with mast cells dominating. No evidence for intramucosal lymphoid tissue was found at any stage during gestation, thus confirming previous findings.8
Presence and localization of human leukocyte populations in human fetal bronchial tissue during gestation and after implantation in the SCID host
| Leukocyte populations . | Bronchial tissues during gestation . | Bronchial grafts . | ||
|---|---|---|---|---|
| Early stage (1st appearance) . | Medium stage . | Mature stage . | Mature stage . | |
| CD3+ | IE, ME (10 wk) | IE, SB, ME | IE, SB, ME | (IE), (SB) |
| CD15+ | — | — | — | — |
| CD20+ | — | — | — | — |
| CD38+ | — | — | — | — |
| CD56+ | — | — | — | — |
| CD68+ | ME (7 wk) | (IE), SB, ME | (IE), SB, ME | (IE), SB, ME |
| Tryptase+ | (ME) (13 wk) | SB, ME | SB, ME | SB, ME |
| Leukocyte populations . | Bronchial tissues during gestation . | Bronchial grafts . | ||
|---|---|---|---|---|
| Early stage (1st appearance) . | Medium stage . | Mature stage . | Mature stage . | |
| CD3+ | IE, ME (10 wk) | IE, SB, ME | IE, SB, ME | (IE), (SB) |
| CD15+ | — | — | — | — |
| CD20+ | — | — | — | — |
| CD38+ | — | — | — | — |
| CD56+ | — | — | — | — |
| CD68+ | ME (7 wk) | (IE), SB, ME | (IE), SB, ME | (IE), SB, ME |
| Tryptase+ | (ME) (13 wk) | SB, ME | SB, ME | SB, ME |
Leukocyte populations expressing CD3 (T lymphocytes), CD15 (granulocytes), CD20 (B cells), CD38 (plasma cells), CD56 (NK cells), CD68 (macrophages), and tryptase (mast cells) were localized in the intraepithelial (IE), subepithelial (SB), and mesenchymal (ME) compartments of human fetal tracheas and stem bronchi at early (6-15 wk), medium (15-23 wk), and mature (23-38 wk) stages of epithelial differentiation27 and in the bronchial grafts, having attained full maturation of surface epithelium and submucosal glands (GA, 16-25 wks; DE, 8-36 wks) as previously described.13 14 Parentheses indicate scarce presence in a compartment.
Peribronchial lymph nodes, but no other lymphoid organs, trigger the formation of BALT in co-implanted bronchi
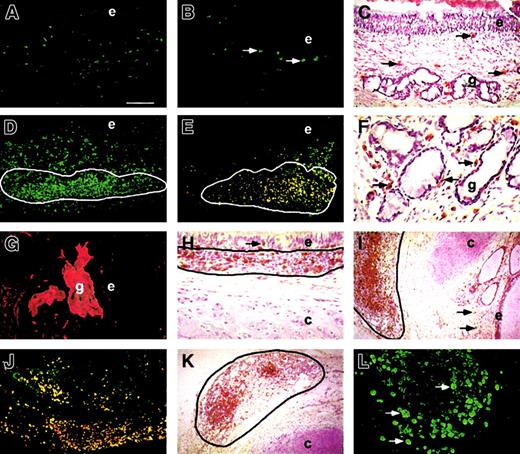
With the objective of providing a model for human bronchial immunity, we implanted fetal bronchi alone or with autologous lymphoid tissues into SCID hosts. When implanted alone, bronchi maintained limited mucosal human leukocyte populations (Table 1; Figure1A) featuring mast cells (Figure 1B) and macrophages (Figure 1C) but scarce, if any, T cells. By contrast, when co-implanted with attached PLNs, bronchial grafts underwent a spectacular enrichment of the mucosa with human leukocytes (Figure 1D). In addition to mast cells and macrophages, bronchus–PLN cografts displayed numerous B and T cells (16 of 16 cases; Table2), found dispersed in the mucosa or within intramucosal clusters homologous to BALT (12 of 16 cases). Mature B cells and plasma cells comprise most cells in these clusters (Figure 1E). Plasma cells are also found dispersed in the mesenchyme, especially at the basal aspect of submucosal glands (Figure 1F), allowing for IgA secretion in the gland ducts (16 of 16 cases, Figure1G). T cells are found dispersed in the mesenchyme, within BALT, and in the subepithelial and intraepithelial compartments (Figure 1H-I). No other cograft combinations tested led to any enrichment of the bronchial mucosa with leukocytes (Table 2). No impact on host survival was noted with any of the cograft combinations.
Human leukocyte populations in simple bronchial grafts and bronchus–PLN cografts.
Mature simple bronchial grafts (A-C) show dispersed CD45+ mucosal leukocytes (A), including tryptase+ mast cells (B, arrows) and CD68+macrophages (C, arrows). Bronchus–PLN cografts (D-L) show CD45+ leukocytes (D) dispersed in the mesenchyme and clustered in BALT (D, circled area). BALT cells (E, circled area) include mature CD20+ B cells (green fluorescence) and CD20+CD38+ (orange fluorescence) plasma cells. Some CD38+ plasma cells are found at the basal aspect of submucosal glands (F, arrows), allowing for transcytosis-mediated IgA secretion (G) into the gland lumen. CD3+ T lymphocytes are found within the epithelium (H, arrow) of bronchus–PLN cografts, among BALT cells (H, circled area) and are dispersed in the mesenchyme (I, arrows). Co-implanted PLNs stay well delimited (I-K, circled area) externally to the cartilage and show numerous CD3+ T lymphocytes (I), mature CD20+ B cells (green fluorescence), and CD20+CD38+ (orange fluorescence) plasma cells (J) and CD68+ macrophages (K). Human PLNs maintained long term in the SCID host also show evidence of proliferating Ki67+ leukocytes (L). Bar represents 100 μm in A, B, D, E, J; 250 μm in I, K; 50 μm in C, H; 25 μm in F, G, L. e indicates epithelium; g, gland; and c, cartilage. Original magnification ×50 (A, B, D, E, J); ×20 (I, K); ×100 (C, H); ×200 (F, G, L).
Human leukocyte populations in simple bronchial grafts and bronchus–PLN cografts.
Mature simple bronchial grafts (A-C) show dispersed CD45+ mucosal leukocytes (A), including tryptase+ mast cells (B, arrows) and CD68+macrophages (C, arrows). Bronchus–PLN cografts (D-L) show CD45+ leukocytes (D) dispersed in the mesenchyme and clustered in BALT (D, circled area). BALT cells (E, circled area) include mature CD20+ B cells (green fluorescence) and CD20+CD38+ (orange fluorescence) plasma cells. Some CD38+ plasma cells are found at the basal aspect of submucosal glands (F, arrows), allowing for transcytosis-mediated IgA secretion (G) into the gland lumen. CD3+ T lymphocytes are found within the epithelium (H, arrow) of bronchus–PLN cografts, among BALT cells (H, circled area) and are dispersed in the mesenchyme (I, arrows). Co-implanted PLNs stay well delimited (I-K, circled area) externally to the cartilage and show numerous CD3+ T lymphocytes (I), mature CD20+ B cells (green fluorescence), and CD20+CD38+ (orange fluorescence) plasma cells (J) and CD68+ macrophages (K). Human PLNs maintained long term in the SCID host also show evidence of proliferating Ki67+ leukocytes (L). Bar represents 100 μm in A, B, D, E, J; 250 μm in I, K; 50 μm in C, H; 25 μm in F, G, L. e indicates epithelium; g, gland; and c, cartilage. Original magnification ×50 (A, B, D, E, J); ×20 (I, K); ×100 (C, H); ×200 (F, G, L).
Cografts of human fetal bronchus and autologous lymphoid tissues
| Type of cograft . | N . | DE (wk) . | Lymphoid tissue fate . | Enrichment in leukocytes . | Presence of BALT/IgA . | Impact on host survival . |
|---|---|---|---|---|---|---|
| Bronchus + liver | 3 | 11-21 | Involuted (3 of 3) | No (3 of 3) | No (3 of 3)/no (3 of 3) | No (3 of 3) |
| Bronchus + bone | 3 | 12-18 | Conserved (3 of 3) | No (3 of 3) | No (3 of 3)/no (3 of 3) | No (3 of 3) |
| Bronchus + thymus | 4 | 12-34 | Conserved (4 of 4) | No (4 of 4) | No (4 of 4)/no (4 of 4) | No (4 of 4) |
| Bronchus + dissected PLN | 4 | 8-28 | Involuted (4 of 4) | No (4 of 4) | No (4 of 4)/no (4 of 4) | No (4 of 4) |
| Bronchus + attached PLN | 16 | 8-36 | Conserved (16 of 16) | Yes (16 of 16) | Yes (12 of 16)/yes (16 of 16) | No (16 of 16) |
| Bronchus + attached PLN + thymus | 3 | 12-27 | Conserved (3 of 3) | Yes (3 of 3) | Yes (3 of 3)/yes (3 of 3) | No (3 of 3) |
| Type of cograft . | N . | DE (wk) . | Lymphoid tissue fate . | Enrichment in leukocytes . | Presence of BALT/IgA . | Impact on host survival . |
|---|---|---|---|---|---|---|
| Bronchus + liver | 3 | 11-21 | Involuted (3 of 3) | No (3 of 3) | No (3 of 3)/no (3 of 3) | No (3 of 3) |
| Bronchus + bone | 3 | 12-18 | Conserved (3 of 3) | No (3 of 3) | No (3 of 3)/no (3 of 3) | No (3 of 3) |
| Bronchus + thymus | 4 | 12-34 | Conserved (4 of 4) | No (4 of 4) | No (4 of 4)/no (4 of 4) | No (4 of 4) |
| Bronchus + dissected PLN | 4 | 8-28 | Involuted (4 of 4) | No (4 of 4) | No (4 of 4)/no (4 of 4) | No (4 of 4) |
| Bronchus + attached PLN | 16 | 8-36 | Conserved (16 of 16) | Yes (16 of 16) | Yes (12 of 16)/yes (16 of 16) | No (16 of 16) |
| Bronchus + attached PLN + thymus | 3 | 12-27 | Conserved (3 of 3) | Yes (3 of 3) | Yes (3 of 3)/yes (3 of 3) | No (3 of 3) |
Portions of fetal bronchi (total of 10 independent tissues from 16-25 wks gestation) were implanted in SCID hosts either alone (total of 16 simple bronchial grafts; DE, 8-36 wks) or with autologous lymphoid tissues (number of each type, N, indicated in table) and studied as described (see “Materials and methods”) after variable duration of engraftment (DE). The fate of lymphoid tissues was assessed morphologically. Bronchus–lymphoid tissue cografts were compared to simple bronchial grafts from the same series (same fetal tissue of origin, grafted in littermates) to detect any enrichment in human leukocytes, the presence of BALT, IgA, and impact on host survival.
Lymph nodes are maintained in SCID hosts as appendices to bronchial grafts
Fetal PLNs are natively attached to the external side of bronchi by connective tissue. When PLNs were dissected and then implanted in the SCID host away from the bronchial mucosa, no BALT formation was induced (Table 2). In addition, dissected PLNs quickly involuted on implantation, leaving only fibrous remnants after more than 4 weeks in the host. Conversely, PLNs that remained attached not only induced the formation of BALT, they were also maintained as functional lymphoid organs in the host (Figure 1I). Co-implanted PLNs contain numerous macrophages (Figure 1K), T cells (Figure 1I), and mature B cells and plasma cells (Figure 1J) organized in lymphoid follicles, among which cycling cells could be detected (Figure 1L).
αβ T-cell–mediated immunity is active in bronchus–PLN cografts
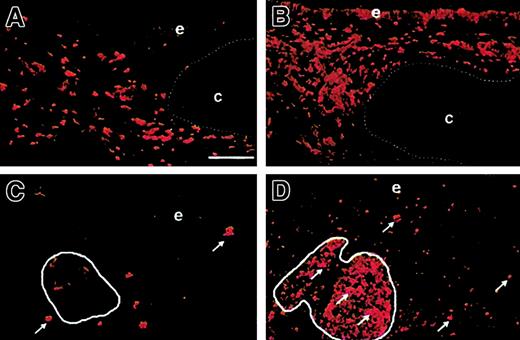
Antigen presentation function is found in simple bronchial grafts in which major histocompatibility complex class II (MHC II) is detected on macrophages and rare epithelial cells (Figure2A). MHC II expression is more widespread in bronchus–PLN cografts and is found among macrophages and fibroblasts, epithelial cells, and T and B cells (Figure 2B). Before stimulation, CD25 (IL-2 receptor α chain) expression is absent in simple bronchial grafts and only detected on scarce macrophages in bronchus–PLN cografts (Figure 2C), consistent with the lack of T-cell preactivation. On in vivo infection with P aeruginosa, CD25 expression is induced in a large subset of mesenchymal leukocytes in bronchus–PLN cografts, notably among cells in BALT (Figure 2D). No such induction of CD25 expression is found in mock-infected cografts or in simple bronchial grafts mock-infected or infected with P aeruginosa (not shown). These results are consistent with the induction of an αβ T-cell–mediated response in P aeruginosa–infected cografts.
Markers of αβ T-cell–mediated immunity in simple bronchial grafts and bronchus–PLN cografts before and after infection with P aeruginosa.
Before P aeruginosa infection, the up-regulation of HLA-DR is visible in the bronchus–PLN cograft (B) compared with the simple bronchial graft (A), with strong staining of the epithelium and the mesenchymal macrophages. Before infection, few mesenchymal macrophages express CD25 (C, arrows) in the bronchus–PLN cograft, whereas BALT cells (C, circled area) are mostly CD25−. After in vivo infection with P aeruginosa, CD25 expression is markedly up-regulated (D, arrows) in the mesenchyme of the bronchus–PLN cograft, notably among BALT cells (D, circled area), with CD25+ cells spreading in the whole mucosa and the epithelium. Bar, 100 μm. e indicates epithelium; and c, cartilage. Original magnification ×100 for all panels.
Markers of αβ T-cell–mediated immunity in simple bronchial grafts and bronchus–PLN cografts before and after infection with P aeruginosa.
Before P aeruginosa infection, the up-regulation of HLA-DR is visible in the bronchus–PLN cograft (B) compared with the simple bronchial graft (A), with strong staining of the epithelium and the mesenchymal macrophages. Before infection, few mesenchymal macrophages express CD25 (C, arrows) in the bronchus–PLN cograft, whereas BALT cells (C, circled area) are mostly CD25−. After in vivo infection with P aeruginosa, CD25 expression is markedly up-regulated (D, arrows) in the mesenchyme of the bronchus–PLN cograft, notably among BALT cells (D, circled area), with CD25+ cells spreading in the whole mucosa and the epithelium. Bar, 100 μm. e indicates epithelium; and c, cartilage. Original magnification ×100 for all panels.
γδ T-cell–mediated immunity is active in bronchus–PLN cografts
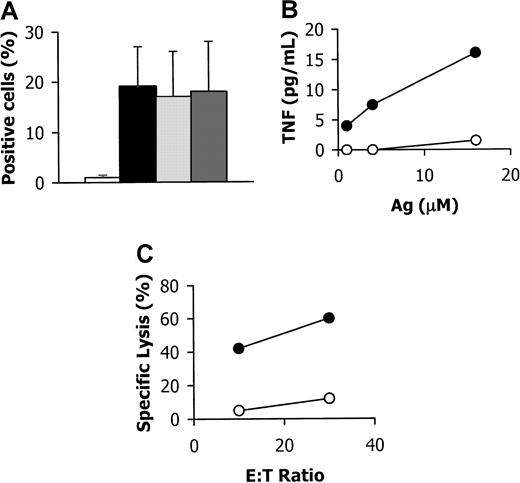
In addition to αβ T cells, γδ T cells have been shown to exert a crucial protective role in mucosae. To test γδ T-cell function in bronchus–PLN cografts, we studied their response to the in vivo injection of a synthetic phospho-antigen, 3,4-epoxy-3-methyl-1-butyl-diphosphate (or EpoxPP), known to selectively activate the Vγ9Vδ2 subset. The frequency of Vγ9Vδ2 T cells was dramatically increased within bronchus–PLN cografts injected with EpoxPP compared with noninjected cografts (Figure3A). Accordingly, T cells derived from EpoxPP-injected cografts, but not from noninjected cografts, secreted high levels of tumor-necrosis factor when exposed to phospho-antigens in vitro (Figure 3B). Moreover, the former but not the latter cells efficiently killed Daudi cells, a B-cell tumor specifically recognized by Vγ9Vδ2 T cells (Figure 3C). These data demonstrate that human γδ T cells in bronchus–PLN cografts are functional and are able to respond to an antigenic challenge in vivo.
Responsiveness of human γδ T cells from bronchus–PLN cografts to in vivo EpoxPP challenge.
(A) Flow cytometry analysis using TCR-Cδ, -Vγ9, and -Vδ2–specific mAbs. Frequency (mean ± SD) of TCR-Cδ–positive cells is shown for mock-injected (open bar) and EpoxPP-injected (closed bar) grafts from two series. Among TCR-Cδ–positive cells from EpoxPP-injected grafts, most cells co-expressed TCR-Vγ9 (light grey bar) and TCR-Vδ2 (dark grey bar). (B) T cells derived from EpoxPP-injected (open circles) or noninjected (closed circles) bronchus–PLN cografts from the same series were incubated in vitro with EpoxPP (Ag) at various concentrations, and culture supernatants were tested for their tumor necrosis factor content. (C) Cytolytic activity of T cells derived from EpoxPP-injected (open circles) or noninjected (closed circles) bronchus–PLN cografts from the same series against 51Cr-labeled Vγ9Vδ/2-susceptible Daudi cells was estimated at 2 effector-to-target (E:T) ratios in a 4-hour51Cr-release assay.
Responsiveness of human γδ T cells from bronchus–PLN cografts to in vivo EpoxPP challenge.
(A) Flow cytometry analysis using TCR-Cδ, -Vγ9, and -Vδ2–specific mAbs. Frequency (mean ± SD) of TCR-Cδ–positive cells is shown for mock-injected (open bar) and EpoxPP-injected (closed bar) grafts from two series. Among TCR-Cδ–positive cells from EpoxPP-injected grafts, most cells co-expressed TCR-Vγ9 (light grey bar) and TCR-Vδ2 (dark grey bar). (B) T cells derived from EpoxPP-injected (open circles) or noninjected (closed circles) bronchus–PLN cografts from the same series were incubated in vitro with EpoxPP (Ag) at various concentrations, and culture supernatants were tested for their tumor necrosis factor content. (C) Cytolytic activity of T cells derived from EpoxPP-injected (open circles) or noninjected (closed circles) bronchus–PLN cografts from the same series against 51Cr-labeled Vγ9Vδ/2-susceptible Daudi cells was estimated at 2 effector-to-target (E:T) ratios in a 4-hour51Cr-release assay.
Adjunction of autologous thymus to bronchus–PLN cografts increases the representation of αβ T cells
Having proven that αβ and γδ T cells within the mucosa of bronchus–PLN cografts are able to respond to immune challenges, we sought to further characterize their relative representation and to test whether the adjunction of autologous thymus could influence the αβ–γδ balance. To this end, grafts from 2 series—including simple bronchial grafts, bronchus–PLN cografts, and bronchus–PLN–thymus cografts—were partly dissociated, and resident T cells were analyzed by flow cytometry after short-term in vitro expansion. As shown in Table 2, bronchus–PLN–thymus cografts allowed for the development of BALT in the airway mucosa and for the maintenance of PLN, as in bronchus–PLN cografts without thymus. Moreover, as presented in Table 3, bronchus–PLN–thymus cografts exhibited a marked increase in the proportion of αβ T cells compared with bronchus–PLN cografts.
Flow cytometric analysis of graft-derived T cells
| Sample . | Recovered cells (× 106) . | Positive cells (%) . | |||
|---|---|---|---|---|---|
| Thy-1 . | W6/32 . | TCRαβ . | TCRγδ . | ||
| G1 | 1.8 | NS | 99.8 | 0.9 | 4.5 |
| G2 | 3.4 | NS | 99.5 | 0.8 | 28.8 |
| G3 | 1.3 | NS | 99.7 | 37.4 | 15.8 |
| G4 | 40.0 | NS | 99.9 | 95.9 | 1.0 |
| Sample . | Recovered cells (× 106) . | Positive cells (%) . | |||
|---|---|---|---|---|---|
| Thy-1 . | W6/32 . | TCRαβ . | TCRγδ . | ||
| G1 | 1.8 | NS | 99.8 | 0.9 | 4.5 |
| G2 | 3.4 | NS | 99.5 | 0.8 | 28.8 |
| G3 | 1.3 | NS | 99.7 | 37.4 | 15.8 |
| G4 | 40.0 | NS | 99.9 | 95.9 | 1.0 |
Cells were isolated from the mucosa of 1 simple bronchial graft (G1), 1 airway–LN cograft (G2), and 2 airway–LN–thymus cografts (G3 and G4) from 2 independent series (GA, 16 wk; DE, 10 wk for G1, G2, and G3; GA, 21 wk; DE, 27 wk for G4), expanded in vitro for 12 days and finally counted and analyzed by flow cytometry (see “Materials and methods”). Levels below 0.3% were considered not significant (NS).
Repertoire of αβ T cells in bronchus–PLN–thymus cografts is restricted
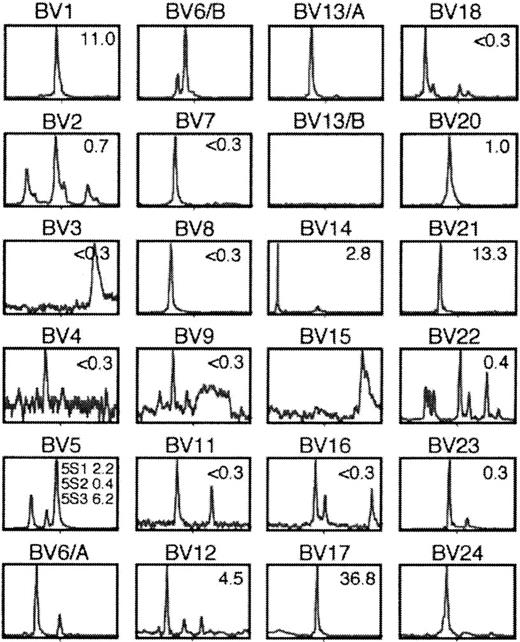
We further evaluated TCR diversity within a bronchus–PLN–thymus cograft, using a previously described reverse transcription–polymerase chain reaction approach, termed Immunoscope.18 According to the size distribution of junctional regions of rearranged TCRβ chains, graft-derived αβ T cells were polyclonal, comprising at least 40 clonotypes (Figure 4). Although polyclonal, this repertoire was significantly restricted compared to the pseudo-gaussian patterns obtained with peripheral blood T cells from healthy adults.18 One explanation for this restriction could be that graft T cells undergo host antigen–driven oligoclonal expansion, a form of graft-versus-host reaction previously observed in SCID mice engrafted with adult human peripheral blood leukocytes.20 However, mucosal T cells were consistently CD25− in the basal state, thus ruling out any chronic activation of bronchus–PLN (–thymus) cografts. Besides, no impairment of host survival was observed, which also argues against any ongoing graft-versus-host reaction. Furthermore, no xenoreactivity was found in a cytotoxicity assay in which graft T cells were co-incubated with a mouse cell line syngeneic to the SCID host (not shown).
Analysis of length distribution of TCRβ junctional sequences in cells from the mucosa of a bronchus–PLN–thymus cograft.
Shown are the profiles of cDNA amplified with pairs of Vβ–Cβ primers plus run-off with internal fluorescence Cβ primer. The percentage of cells expressing corresponding TCR Vβ, as estimated by flow cytometry, is indicated in the upper right corner of each histogram. Staining was considered significant when it was greater than 0.3%. According to this criterion, at least 12 of 24 distinct Vβ subsets were detected by flow cytometry. Immunoscope analysis suggested that the culture contained at least 40 distinct clonotypes.
Analysis of length distribution of TCRβ junctional sequences in cells from the mucosa of a bronchus–PLN–thymus cograft.
Shown are the profiles of cDNA amplified with pairs of Vβ–Cβ primers plus run-off with internal fluorescence Cβ primer. The percentage of cells expressing corresponding TCR Vβ, as estimated by flow cytometry, is indicated in the upper right corner of each histogram. Staining was considered significant when it was greater than 0.3%. According to this criterion, at least 12 of 24 distinct Vβ subsets were detected by flow cytometry. Immunoscope analysis suggested that the culture contained at least 40 distinct clonotypes.
Discussion
The colonization of human bronchi by leukocyte populations remains ill defined, as do the conditions in which intramucosal leukocyte clusters or BALT emerge. By studying human fetal bronchial mucosae before and after implantation as xenografts in SCID mice, we are able to offer several insights into the development of these human bronchial immune structures. First, we show that macrophages, mast cells, and T cells colonize human bronchi during gestation, with no evidence of intramucosal cluster formation. On implantation of the bronchial mucosa in the SCID host, macrophages and mast cells are maintained; both are known to play prominent roles in bronchial immunopathology, notably in asthma.2,21 Conversely, T cells are found to decline, possibly because of the lack of antigenic stimulation or growth factors. Similar T-cell decline was found in an adult bronchial xenograft model.22
In testing several graft combinations of lymphoid tissues and fetal bronchi to increase the representation of human leukocytes in the mucosa, we found that the bronchus–PLN cografts had outstanding properties. Indeed, this strategy allowed us, for the first time, to maintain a human lymph node long-term ex vivo. Although successful engraftment of human LNs in the SCID mouse host was reported by Kaneshima et al in 1991,23 they later acknowledged that they were incapable of maintaining isolated human LNs for the long term because of the absence of adequate vascular and lymphatic connections, with surrounding (host) tissues held responsible for LN involution after 8 to 12 weeks.24 Accordingly, we found that PLN maintenance in the SCID host (up to 36 weeks) was strictly dependent on the conservation of connective tissue attachments to the adjacent bronchial mucosa. The bronchial epithelium, glands, and mesenchyme may thus provide necessary growth factor activity for the PLN.
Conversely, co-implanted PLNs induce the enrichment of the bronchial mucosa with T and B cells, often leading to BALT formation. No other lymphoid tissue led to similar enrichment, which argues for a crucial role of PLNs in BALT formation. This is in contrast to findings in the mouse gut suggesting separate developmental pathways for extramucosal and intramucosal lymphoid structures.6 We also identified IgA-secreting plasma cells in gland ducts of all bronchus–PLN cografts in the basal state. In vivo, bronchial IgA secretion is thought to depend on prior activation of gut-associated lymphoid tissues and is not observed before 6 months after birth.25 Our results suggest that IgA secretion may arise in the bronchial mucosa independent of other sites.
It is also possible that, in our model, exposure to host antigens might have led to the development of xenoreactivity among human lymphocytes. In B cells, the secretion of IgA and other immunoglobulin isotypes may ensue. We cannot rule out this possibility, and we look forward to further detailed analysis of B-cell function and immunoglobulin specificity in the model to shed insight. In T cells, exposure to host antigens was shown to induce oligoclonal expansion of xenoreactive clones in a model of human adult T-cell development in SCID mice.20 Our first experiments using bronchus–PLN–thymus cografts, in which αβ T cells are largely represented (whereas γδ T cells predominate in bronchus–PLN cografts), showed a restricted repertoire for TCRβ chain rearrangements, which may indicate an ongoing xenoreaction. Yet several arguments dispute this hypothesis. First, our model uses fetal T cells, which, because of their immaturity, are more likely than adult T cells to undergo tolerization toward the host. Second, we determined that graft T cells did not exert cytotoxicity toward a mouse cell line syngeneic to the SCID host, though arguably this result may also be explained by the absence of relevant antigens (ie, those involved in a putative in vivo xenoreaction) in the cell line used in our assay. Third, we found that T cells were consistently CD25− in unchallenged bronchus–PLN (–thymus) cografts, which rules out any chronic immune activation, as would happen in xenoreactivity. Fourth, we did not find evidence that any of the graft combinations used altered host survival. Therefore, we believe that the restricted distribution of chain rearrangements within graft αβ T cells is more likely explained by the limited number of T cells initially present within implanted tissues than by xenoantigen-induced oligoclonal expansion.
Although more work is needed to confirm our current hypotheses regarding the αβ T-cell repertoire in our model, results presented here provide clear evidence that αβ T cells within bronchus–PLN cografts are responsive to infection with P aeruginosa, a pathogen causing life-threatening lung infections notably in patients with cystic fibrosis. We further show that although antigen-presenting function is present in simple bronchial grafts and bronchus–PLN cografts, only the latter can mount αβ T-cell–mediated responses. Because our experimental conditions did not allow for the maintenance of live bacteria in contact with human graft lymphocytes for more than 2 days (because of the parallel development of a massive host neutrophil–mediated response leading to graft tissue destruction within 3-4 days), we based our demonstration of the development of an αβ T-cell–mediated response to P aeruginosa infection on the early sign of activation provided by the up-regulation of CD25. Later signs of activation, as provided, for example, by a quantitation of αβ T-cell expansion, would be meaningful only with longer incubation times (typically in the 1- to 2-week range). Infectious challenge of bronchus–PLN cografts with mutant or heat-killed P aeruginosa strains may reduce host responsiveness and therefore allow the full tracking of human αβ T-cell responses as they unfold.
In addition to αβ T cells, we demonstrate that γδ T cells, more precisely the Vγ9Vδ2 subset, are also present and activatable in bronchus–PLN cografts. Human γδ T cells are believed to play a major role in antitumor and antibacterial immunity. In particular, most peripheral blood γδ T cells in human adults, which express T-cell receptors with a combination of variable regions mostly restricted to Vγ9 and Vδ2,26 are known to recognize in vitro bacterial and synthetic nonpeptidic phosphorylated compounds and a wide range of B-cell tumors.15 Until now, the lack of rodent homologues of this major human γδ subset had hampered its analysis. To our knowledge, our data represent the first indication that human γδ T cells developed in SCID mice can keep their antigen responsiveness. Therefore, our model could be of great value to help gain insight into the in vivo physiology of γδ T cells.
In summary, we demonstrated that bronchus–PLN (–thymus) cografts allow for the study of human bronchial epithelial cells, glands, and mesenchyme in relation to diverse human leukocytes subsets, namely macrophages, mast cells, mature B cells, plasma cells, αβ, and γδ T cells, which are all found in the postnatal bronchial mucosa. Additional work is now required to address important concerns, such as (1) antigen repertoire and specificity of αβ and γδ T cells and B cells found in PLN and BALT; (2) levels of active molecules—eg, epithelial antimicrobials, cytokines, IgA, and possibly other immunoglobulin isotypes; and (3) responsiveness to additional varied types of immune challenges. Potential applications of this model include studies of innate and adaptive immunity in normal airways and in diseased states, such as asthma and cystic fibrosis. Developmentally, our results point to an inductive role of extramucosal PLNs in the histogenesis of intramucosal BALT, which deserves further investigation. In essence, our study suggests an experimental strategy (ie, the co-implantation of LNs with adjacent mucosa) for the development of models of immune function in other human mucosae such as that of the skin, gut, or genital tract.
We thank P. Kourilsky, L. A. Herzenberg, and E. Puchelle for helpful discussions; M. Catala, A.-L. Delezoide, C. Ferec, F. Menez, F. Narcy, J. Martinovic, and J. Tantau for providing fetal tissues; the Centre Régional de Transfusion Sanguine (Nantes, France) for providing human serum and feeder cells; and C. Balmant (INSERM U395, Toulouse, France) for providing the γδ T-cell antigens.
M.B. and B.P. contributed equally to this work.
Supported by grants from Centre National de la Recherche Scientifique (CNRS), Institut National de la Santé et de la Recherche Médicale (INSERM), SyStemix Inc, Association pour la Recherche contre le Cancer, and Vaincre la Mucoviscidose.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Bruno Péault, INSERM U506, Batiment Lavoisier, Groupe Hospitalier Paul Brousse, 12 Ave Paul Vaillant-Couturier, 94807 Villejuif Cedex, France; e-mail:U506@infobiogen.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal