E21R is a modified granulocyte macrophage–colony-stimulating factor (GM-CSF) protein which results in antagonism of GM-CSF function via selective binding to the GM-CSF receptor complex. Juvenile chronic myelomonocytic leukemia (JMML) is a rare leukemia where spontaneous proliferation of myeloid and monocytic precursors in patients' bone marrow cultures is dependent on GM-CSF. For patients who progress after systemic chemotherapy, there are no effective therapies. In vitro and in vivo studies in an animal model demonstrating that E21R exerts an antileukemic action prompted us to consider its potential utility in a child with end-stage JMML. E21R was well-tolerated during the 3 courses of subcutaneous treatment. A clear in vivo efficacy was observed after 2 courses of E21R but the disease appeared completely refractory during the third course. This novel therapeutic approach clearly deserves further evaluation in JMML.
Introduction
Juvenile chronic myelomonocytic leukemia (JMML) is a rare leukemia characterized by monocytosis and a moderate increase in blasts.1 Spontaneous proliferation of myeloid and monocytic precursors, dependent on granulocyte macrophage–colony-stimulating factor (GM-CSF), is a characteristic of these patients' bone marrow cultures. Myeloid and monocytic JMML progenitors are hypersensitive to GM-CSF. For patients exhibiting poor prognostic features, mainly thrombocytopenia, bone marrow transplantation is the only curative therapeutic option.2A transient response may result from chemotherapy such as Ara-C, VP-16, 6-mercaptopurine, and hydroxyurea, but these responses are never complete. For patients who progress after systemic chemotherapy there are no effective therapies.
Study design
E21R is a GM-CSF analogue generated by substitution of the glutamate (E) at position 21 with an arginine (R) residue, the overall result being to abolish the interaction of GM-CSF with the beta chain of its receptor, and thus eliminate signaling.3,4 E21R has a dual mechanism of action: antagonism of GM-CSF function, via selective binding to the receptor alpha chain, and induction of apoptosis in cells carrying the GM-CSF receptor. In vitro, E21R inhibits GM-CSF activity on myeloid leukemic cells5 and in vivo, E21R exerts an antileukemic action in a transplanted animal model,6 prompting us to consider its potential utility in JMML.
The protein is produced by BresaGen (Adelaide, Australia). A phase I study in adult solid tumor patients indicated that at the maximum dose tested subcutaneously (1 mg/kg per day for 10 days), E21R has a good safety profile (Olver et al, unpublished data, December 2001) and thus, these conditions were utilized in the current study. The level of E21R and anti-E21R antibodies were monitored during each course of treatment. Serum concentrations of E21R were determined using a quantitative human GM-CSF enzyme-linked immunosorbent assay (ELISA) kit from R&D Systems (Minneapolis, MN). Measurement of antibodies toward E21R was performed using a sandwich ELISA (Olver et al, unpublished data, December 2001).
Our patient is a boy who was born in 1992. The diagnosis of JMML was made in 1996, with associated liver and spleen enlargement and skin infiltration. Initial complete blood count (CBC) showed 28 400 white blood cells (WBC)/μL, 10 508 monocytes/μL, 10 224 neutrophils/μL, 6248 lymphocytes/μL, Hb 9.7 g/dL, and 26 000 platelets/μL. Initial bone marrow cytogenetics were normal. Oral chemotherapy (hydroxyurea) was ineffective. Since his brother was HLA antigen identical, an allogeneic bone marrow transplantation was performed in 1997 with busulfan/cyclophosphamide as the conditioning regimen. Complete remission occurred but the disease relapsed 18 months later and a second transplantation was performed in 1999 with the same donor and with TBI/Ara-C/melphalan as the conditioning regimen. The disease relapsed again 6 months later. No graft-versus-host-disease was observed after either transplantation. Three donor lymphocyte infusions were performed without any response. Different chemotherapy was attempted (hydroxyurea, 6-mercaptopurine) with no antileukemic effect. The general status rapidly worsened with pain, fever, and splenomegaly at 12 cm below the left costal margin as well as hematologic deterioration with leukocytosis, monocytosis, and peripheral blasts. At the beginning of August 2000, CBC showed 51 700 WBC/μL, 20 163 monocytes/μL, 3619 blasts/μL, and 31 000 platelets/μL. A bone marrow examination showed infiltration with 17% blast cells. Bone marrow cultures were unsuccessful. After informed consent was signed by the parents and an individual authorization for compassionate use was provided by the French drug agency, treatment with E21R was commenced. Three courses were administered. The first 2 courses lasted 10 days and the third lasted 14 days. The second course began on day 21 and the third on day 70. The delay for the third course was due to shipping considerations. No other treatment was given except prednisolone at 0.5 mg/kg per day.
Results and discussion
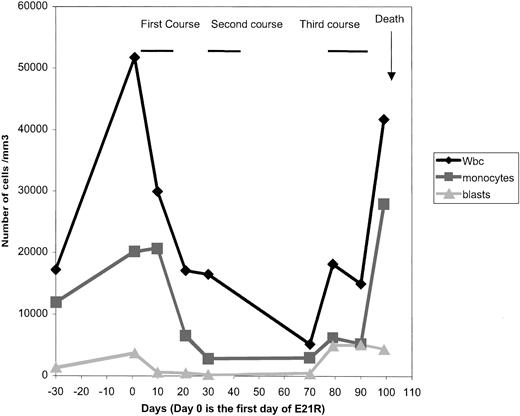
E21R was safe and well tolerated, based on standard blood and urine chemistry analysis, as well as clinical cardiac and pulmonary functions. The only clinical side effect was minor local pain at the site of injection. The response to the first 2 courses was clear and unexpectedly favorable. Clinically, we observed a dramatic improvement a few days after the end of the first course, with an increase in the Karnofsky index from 50% to 90% (for one month, this boy was able to return to school), and diminution of the size of splenomegaly (to 2 cm below the left costal margin). Laboratory tests showed a decrease in WBC, monocyte, and blast counts (Figure1), and in bone marrow blasts (8% at day 21 and 13% at day 70). The only adverse hematologic effect noted during the 3 courses was persistent thrombocytopenia. At the beginning of the third course, the clinical situation was clearly bad, with bone pain, skin nodules, and a low Karnofsky index. During and after the third course, monocytosis, and blast cell counts, blast cell percentage increased, and the disease appeared to have become completely refractory to E21R. Our patient died one month after the commencement of the third course, from bleeding and multiorgan failure.
CBC evolution and outcome.
A dramatic response was observed after the end of the first course. During and after the third course, WBC, monocytosis, and blast cell counts increased.
CBC evolution and outcome.
A dramatic response was observed after the end of the first course. During and after the third course, WBC, monocytosis, and blast cell counts increased.
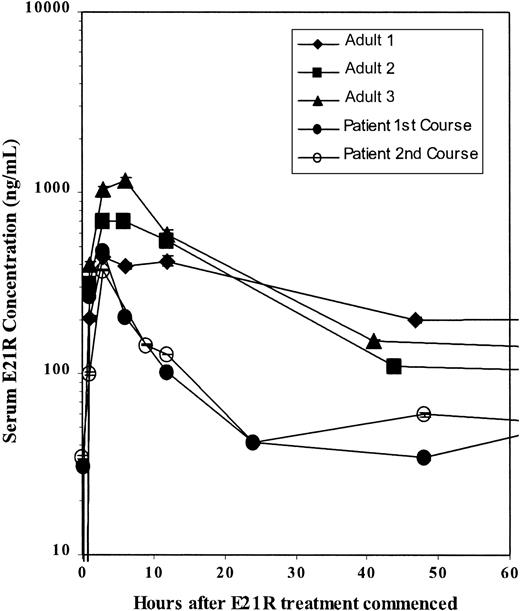
The serum level of E21R was monitored through the 3 courses of treatment. A maximum E21R concentration was observed 3 hours after E21R injection. This is comparable with the levels observed in the adult phase I study (Olver et al, unpublished data, December 2001). Further levels are lower than those observed in the phase I study (Figure 2). A modest IgG and IgM response toward the injected E21R was observed at the beginning of the third course (data not shown) and may have resulted in the decreased effect of the drug during this course.
Kinetics of E21R.
The serum level of E21R was monitored through the first and second courses of treatment and compared with the levels observed in 3 adult solid tumor patients during the phase I study.
Kinetics of E21R.
The serum level of E21R was monitored through the first and second courses of treatment and compared with the levels observed in 3 adult solid tumor patients during the phase I study.
This is the first time that E21R, a specific GM-CSF inhibitor, has been used for the treatment of JMML. The schedule and the dose were based on the phase I study previously performed on solid tumor patients in Australia. The patient had end-stage disease, relapsing after 2 bone marrow transplants. Despite all these difficulties, clear in vivo efficacy was observed after only 2 courses of E21R, and lasted for approximately 60 days. The third course commenced 40 days after the end of the second, at which time the clinical status was poor (although not reflected by the CBC performed, with only a moderate increase in blast cell count compared with the previous counts). In JMML, especially in an acute transformation, such a delay is likely to preclude cumulative benefit following the first 2 courses. Moreover, during this time the sensitivity of the disease may have changed. The overall tolerance of E21R appeared to be very good, without the side effects of chemotherapy regimens usually employed in such situations. More studies are necessary in order to investigate the dose and schedule of administration of the drug and to study its pharmacokinetics, but this novel therapeutic approach clearly deserves further evaluation in JMML, and may be of potential interest in myeloid malignancy in general.
The authors thank Bresagen Ltd for kindly providing E21R for compassionate use, and Dr Naomi Taylor for valuable comments on the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Frédéric Bernard, Service de Pédiatrie III, Unité d'Hémato Oncologie, Hôpital Arnaud de Villeneuve, Avenue du Doyen Giraud, F-34000 Montpellier, France; e-mail: f-bernard@chu-montpellier.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal