Chronic lymphocytic leukemia (CLL) is a neoplastic disease characterized by the accumulation of small mature-appearing lymphocytes in the blood, bone marrow, and lymphoid tissues.1 Current therapy has not been shown to prolong survival.2
Etodolac is a racemic mixture of R-(-) and S-(+)-1,8-diethyl-1,3,4,9,-tetrahydropyrano-(3,4-b)indole-1-acetic acid.3 Etodolac selectively inhibits cyclooxygenase-2,4 and it is approved for use in various parts of the world for treatment of degenerative joint disease and rheumatoid arthritis and for use as an analgesic.5
We report the chance observation that racemic etodolac lowers the lymphocyte count in a patient with B-CLL and show that challenges with 13 other nonsteroidal anti-inflammatory drugs (NSAIDs) produced no significant effect. We also present studies to show that at standard anti-inflammatory doses, racemic etodolac achieves this action by enhancing the selective clearance rather than by the direct killing of leukemic lymphocytes.
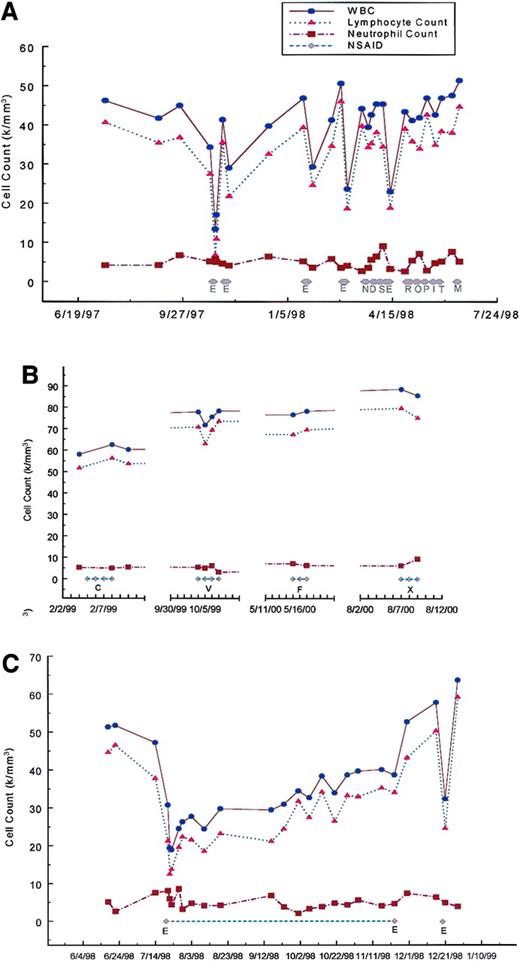
The patient's condition was diagnosed as B-CLL in 1994 at age 57. Her leukemic lymphocyte phenotype was CD5+, CD19+, CD20+, CD23+, CD25+, FMC7+, and λ dim+. She was clinically staged at Rai/Binet 0/A. On October 23, 1997, her white blood cell count (WBC) was 34 300 and her lymphocyte count was 27 440. From October 25, 1997, to October 27, 1997, she took etodolac 300 mg PO twice a day for neck pain. On October 28, 1997, because of the development of petechiae etodolac was discontinued and a complete blood count was performed. Her platelet count was normal but her WBC had dropped to 13 400 with a lymphocyte count of 6 700. To determine whether this was a reproducible effect, etodolac at the same dose of 300 mg was administered twice a day from November 5, 1997, to November 11, 1997; January 21, 1998, to January 25, 1998; February 26, 1998, to March 2, 1998; and April 8, 1998, to April 13, 1998, all with similar effects (Figure 1A). No clinically significant changes in the hemoglobin level, neutrophil count, or platelet count were seen with etodolac (platelet data not shown). Also seen in Figure 1A and B are the results of the administration of 13 other NSAIDs. None produced any significant decrease in the lymphocyte count. The effect of etodolac appears not to be due to its anti-inflammatory property since none of the other NSAIDs elicited this response. Furthermore, the effect does not appear to be related to cyclooxygenase specificity since celecoxib, rofecoxib, and meloxicam, selective cylooxygenase-2 inhibitors, did not significantly affect the lymphocyte count. A 4-month course of etodolac was then administered between July 20, 1998, and November 23, 1998 (Figure 1C). Taken together with the previous data, the reduction of lymphocyte count in response to etodolac administration was prompt, with a nadir occurring after 2 days of administration. The duration of the effect after drug withdrawal was short, with a return of lymphocyte count to baseline within 2 weeks of drug cessation independent of the duration of therapy. The effect was also reproducible and did not appear to be lessened with repeated or prolonged drug challenges.
Cell counts as a function of NSAID administration.
(A) Effects of E, etodolac, 300 mg twice a day for 3 to 6 days, compared with 9 other NSAIDs: N, naproxen, 250 mg twice a day for 4 days; D, diclofenac, 50 mg 3 times a day for 4 days; S, sulindac, 200 mg twice a day for 4 days; R, nabumetone 500 mg twice a day for 6 days; O, oxaprozin, 600 mg twice a day for 4 days; P, piroxicam, 20 mg every day for 3 days; I, indomethacin, 25 mg 3 times a day for 3 days; T, tolmetin, 400 mg twice a day for 3 days; and M, ibuprofen, 400 mg 3 times a day for 3 days. (B) Effects of C, celecoxib, 100 mg twice a day for 4 days; V, rofecoxib, 25 mg every day for 4 days; F, flurbiprofen, 100 mg 3 times a day for 3 days; and X, meloxicam, 7.5 mg every day for 3 days. (C) Effect of a 4-month course of etodolac. A 400 mg sustained release preparation was administered twice a day from July 20, 1998 until July 28, 1998, followed by 300 mg twice a day of the standard release preparation until September 8, 1998, followed by reinstitution of the 400 mg sustained release preparation twice a day. There was a rapid decline in the lymphocyte count in the first several days to a nadir of 12 510 followed by rebound and gradual increase to 33 968 on November 23, 1998, the last day of etodolac administration. After discontinuation of etodolac, there was a rapid rise of the lymphocyte count to 43 132 on November 30, 1998, and to 50 199 on December 16, 1998. To determine if a full response to etodolac was still possible after a prolonged course, etodolac was administered at 400 mg sustained release twice per day for 2 days from December 19, 1998, to December 21, 1998. The lymphocyte count on December 21, 1998, dropped to 24 548 with a rapid return to 59 148 by December 28, 1998.
Cell counts as a function of NSAID administration.
(A) Effects of E, etodolac, 300 mg twice a day for 3 to 6 days, compared with 9 other NSAIDs: N, naproxen, 250 mg twice a day for 4 days; D, diclofenac, 50 mg 3 times a day for 4 days; S, sulindac, 200 mg twice a day for 4 days; R, nabumetone 500 mg twice a day for 6 days; O, oxaprozin, 600 mg twice a day for 4 days; P, piroxicam, 20 mg every day for 3 days; I, indomethacin, 25 mg 3 times a day for 3 days; T, tolmetin, 400 mg twice a day for 3 days; and M, ibuprofen, 400 mg 3 times a day for 3 days. (B) Effects of C, celecoxib, 100 mg twice a day for 4 days; V, rofecoxib, 25 mg every day for 4 days; F, flurbiprofen, 100 mg 3 times a day for 3 days; and X, meloxicam, 7.5 mg every day for 3 days. (C) Effect of a 4-month course of etodolac. A 400 mg sustained release preparation was administered twice a day from July 20, 1998 until July 28, 1998, followed by 300 mg twice a day of the standard release preparation until September 8, 1998, followed by reinstitution of the 400 mg sustained release preparation twice a day. There was a rapid decline in the lymphocyte count in the first several days to a nadir of 12 510 followed by rebound and gradual increase to 33 968 on November 23, 1998, the last day of etodolac administration. After discontinuation of etodolac, there was a rapid rise of the lymphocyte count to 43 132 on November 30, 1998, and to 50 199 on December 16, 1998. To determine if a full response to etodolac was still possible after a prolonged course, etodolac was administered at 400 mg sustained release twice per day for 2 days from December 19, 1998, to December 21, 1998. The lymphocyte count on December 21, 1998, dropped to 24 548 with a rapid return to 59 148 by December 28, 1998.
To determine whether cell killing could be responsible for the drop in circulating lymphocytes, mononuclear cells from the patient were cultured for 72 hours in presence of increasing concentrations of naproxen or etodolac up to 240 μM and analyzed at 24, 48, and 72 hours for the binding of monoclonal antibody to internucleosomal DNA by enzyme-linked immunosorbent assay (ELISA) as a measure of apoptosis using a standard kit assay (Cell Death Detection ELISA Plus, Boerhinger-Mannheim, Indianapolis, IN). No significant enhancement of apoptosis was achieved by etodolac compared with naproxen (data not shown). The same results were obtained in a separate experiment when assayed by analysis of the binding of FITC annexin V to and the uptake of propidium iodide by the patient's mononuclear cells by flow cytometry (data not shown). The fact that an increase in apoptosis or necrosis could not be detected for etodolac compared with naproxen in vitro did not exclude the possibility that a metabolite of etodolac or a serum factor might be necessary to achieve the effect in vivo. To examine this possibility, the percentages of viable, apoptotic, and late apoptotic/necrotic cells were measured by analysis of the binding of FITC annexin V to and the uptake of propidium iodide by isolated mononuclear cells after administration of etodolac to the patient (data not shown). As the lymphocyte count dropped after administration of etodolac, the percentage of apoptotic cells remained the same. These data suggested that etodolac at standard anti-inflammatory and analgesic concentrations did not achieve reduction of leukemic lymphocyte count by direct killing but by increasing the clearance of leukemic cells into other compartments.
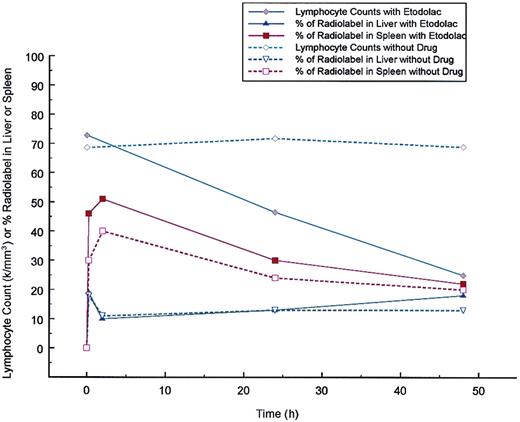
To directly determine whether etodolac was enhancing the clearance of the patient's leukemic lymphocytes, a WBC scan was performed in the standard fashion with and without the administration of etodolac. As shown in Figure 2, an increase in the radiolabel appeared in the spleen with the administration of etodolac. No difference was detected in the amount of radiolabel that appeared in the liver.
Effect of etodolac on the clearance of Indium 111 labeled patient leukocytes.
Two experiments are superimposed. One experiment without drug begun on March 21, 2000 and one with etodolac extended release administered 400 mg twice a day beginning at 7:30 am after blood collection for radiolabeling on November 8, 1999 and continued for 48 hours. Patient leukocytes were Indium 111 labeled according to supplier's instructions (Mediphysics, Amersham Healthcare, Arlington Heights, IL). Radiolabeled material left in the syringe after injection into the patient was submitted to the clinical laboratory at Scottsdale Healthcare Shea, Scottsdale, AZ for manual WBC differential counting. For the study of November 8, 1999 the radiolabeled cells were 100% lymphocytes and for the study of March 21, 2000, 67% were lymphocytes, 29% neutrophils, 3% eosinophils, and 1% bands. Indium-labeled patient leukocytes were injected at 11 am in both experiments. Total body WBC scans were performed in the Department of Radiology at Scottsdale Healthcare Shea using an ADAC Vertex Plus gamma camera (Milpitos, CA) under the supervision of Ronald Korn. The same camera was used for both studies. Scans were taken at 15 minutes, 2 hours, 24 hours, and 48 hours after injections and the percent of counts in the liver and spleen after background subtraction were determined at each time point. As the lymphocyte count dropped in response to etodolac administration, the percent of radiolabel in the spleen was increased in comparison to the percent of radiolabel in the spleen found without drug. No significant change in the percent of radiolabel in the liver was found with etodolac administration.
Effect of etodolac on the clearance of Indium 111 labeled patient leukocytes.
Two experiments are superimposed. One experiment without drug begun on March 21, 2000 and one with etodolac extended release administered 400 mg twice a day beginning at 7:30 am after blood collection for radiolabeling on November 8, 1999 and continued for 48 hours. Patient leukocytes were Indium 111 labeled according to supplier's instructions (Mediphysics, Amersham Healthcare, Arlington Heights, IL). Radiolabeled material left in the syringe after injection into the patient was submitted to the clinical laboratory at Scottsdale Healthcare Shea, Scottsdale, AZ for manual WBC differential counting. For the study of November 8, 1999 the radiolabeled cells were 100% lymphocytes and for the study of March 21, 2000, 67% were lymphocytes, 29% neutrophils, 3% eosinophils, and 1% bands. Indium-labeled patient leukocytes were injected at 11 am in both experiments. Total body WBC scans were performed in the Department of Radiology at Scottsdale Healthcare Shea using an ADAC Vertex Plus gamma camera (Milpitos, CA) under the supervision of Ronald Korn. The same camera was used for both studies. Scans were taken at 15 minutes, 2 hours, 24 hours, and 48 hours after injections and the percent of counts in the liver and spleen after background subtraction were determined at each time point. As the lymphocyte count dropped in response to etodolac administration, the percent of radiolabel in the spleen was increased in comparison to the percent of radiolabel in the spleen found without drug. No significant change in the percent of radiolabel in the liver was found with etodolac administration.
These data show that at standard anti-inflammatory doses, etodolac reproducibly lowers the lymphocyte count in a patient with B-CLL. Furthermore, data is presented to show that etodolac achieves this action by affecting changes in leukemic cell compartmentalization. Further work should clarify the prevalence of this effect in B-CLL, the molecular mechanisms involved, and whether or not racemic etodolac, one of its chiral isomers, or similar compounds could be useful in B-CLL or other B-cell neoplasms.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal