Abstract
Dendritic cells (DCs) are potent antigen-presenting cells and have shown promise to function as “natural” vaccine adjuvants. Currently, most cancer vaccine trials using DCs generate autologous DCs ex vivo for each patient. Systemic treatment with Flt3 ligand (FL) results in a marked increase of DCs in tissues such as spleen and lymph nodes in mice and in the peripheral blood and skin of humans. In light of these observations, we questioned whether FL could be used systemically as a vaccine adjuvant to stimulate DC mobilization in vivo, circumventing the need to generate DCs ex vivo. Ten patients with HER-2/neu–overexpressing cancer were enrolled in a phase 1 study to receive a HER-2/neu peptide-based vaccine targeting the intracellular domain of the HER-2/neu protein. All patients received 20 μg/kg FL per day subcutaneously for 14 days. Five patients received the HER-2/neu peptide-based vaccine alone on day 7 of the 14-day cycle, and 5 patients received the vaccine admixed with 150 μg granulocyte macrophage–colony-stimulating factor (GM-CSF) on day 7 of the FL cycle. T-cell proliferative responses to HER-2/neu peptides and intracellular domain protein suggest that vaccine regimens including FL as an adjuvant were not effective in eliciting a significant HER-2/neu protein-specific T-cell proliferative response. However, including FL as a vaccine adjuvant was effective in boosting the precursor frequency of interferon-γ–secreting HER-2/neu–specific T cells. The small sample size of each group, however, did not allow a statistically significant comparison of immune responses between the FL alone and FL with GM-CSF arms. Finally, vaccine regimens including FL as a vaccine adjuvant were associated with the development of apparent autoimmune phenomena in some patients.
Introduction
Many newly defined tumor antigens are self-proteins. The development of effective cancer vaccine strategies, therefore, must focus on immunization methods that will effectively circumvent tolerance. Dendritic cells (DCs) are the most potent human antigen-presenting cells (APCs) and are uniquely qualified to act as a cancer vaccine adjuvant designed to generate immunity to self-tumor antigens. DCs have the ability to stimulate even a naive T-cell population1 and have been shown to facilitate the generation of immune responses directed against cryptic epitopes because of more effective antigen processing and presentation,2 a property critical to stimulating immunity to the self.3 DCs, however, are rare cells in humans constituting less than 1% of circulating white blood cells. Methods to expand DCs ex vivo to achieve large numbers of functional APCs are under active investigation.4
Ex vivo generation of DCs and exogenous loading of antigen is the basis of many human clinical trials designed to augment immunity to self-tumor antigens.5,6 Some significant problems, however, are associated with the in vitro generation of DCs. First, tailor-making autologous DC vaccines for each individual will limit widespread use of the vaccine. Second, the culture conditions used to expand cells ex vivo may significantly affect their function and antigen-processing capabilities. Finally, DCs generated in culture may not traffic to draining lymph nodes in great numbers, thus limiting the development of systemic immunity.7 Methods of stimulating DC mobilization and trafficking in vivo, allowing the native immune environment to naturally mature DCs, may overcome the functional limitations imposed by ex vivo culture.
Flt3-ligand (FL) is a cytokine that, when administered systemically, can increase numbers of circulating DCs more than 40-fold.8 Human DCs stimulated by the administration of FL have been shown to be functional and can stimulate T cells in vitro.8 Furthermore, the activation of DCs in vivo by FL has been shown to be an effective way of circumventing tolerance during active immunization in animal models.9 Studies have been performed in the neu transgenic mouse, immunizing the animals to a self-tumor antigen, neu, using FL as a vaccine adjuvant to mobilize DCs in vivo.10 The timing of vaccine administration corresponds to the kinetics of in vivo DC mobilization in animals11,12—early administration when few circulating DCs are present, midpoint administration when DC precursors are increasing in the peripheral blood, and vaccination at the end of the FL cycle when DCs are at peak concentrations. Thus, during a 10-day administration of FL, a HER-2/neu intercellular domain (ICD) protein vaccine was administered at 3 time points. Animals receiving the vaccine midpoint in the FL cycle generated HER-2/neu ICD-specific immunity, whereas mice immunized at the end of the FL cycle did not. In general, neu-specific immunity generated using FL resulted in T cells that predominantly secreted interferon γ (IFN-γ), a type 1–associated cytokine, rather than interleukin-4, a type 2–associated cytokine.10
We questioned whether FL could be used as a vaccine adjuvant in vivo in the human to stimulate immunity to HER-2/neu using a vaccine targeting the ICD of the HER-2/neu protein. Previous studies, by our group, had shown that granulocyte macrophage–colony-stimulating factor (GM-CSF) applied locally by the intradermal route, along with an antigen-specific vaccine, resulted in Langerhans cells, skin DCs, mobilization, and recruitment.13 Furthermore, GM-CSF, as a vaccine adjuvant, was effective in generating immunity when used with an HER-2/neu peptide-based vaccine in humans.14 Systemic administration of FL in murine models results in markedly increased numbers of DCs in skin.15 Potentially, FL administration could increase the number of DCs available for immune interaction during intradermal immunization. Our strategy was to administer FL to patients with HER-2/neu–overexpressing tumors and to vaccinate intradermally with an HER-2/neu ICD peptide-based vaccine, with or without concurrent local GM-SF administration, midpoint in the FL cycle.
Patients, materials, and methods
Patient population
Between March and December 1999, 10 subjects were enrolled in a phase 1 study designed to evaluate the immunogenicity of an HER-2/neu peptide-based vaccine administered with FL. Approval was obtained from the institutional review board at the University of Washington for these studies. Informed consent was provided according to the Declaration of Helsinki. Enrollment criteria were (1) HER-2/neu protein overexpression in the primary tumor or metastasis, (2) white blood cell (WBC) count of 3.5 × 109/L or greater, (3) no chemotherapy or other immunomodulatory therapy for a minimum of 30 days before enrollment and during the course of the study, and (4) no known history of autoimmune disease. Each patient received 20 μg/kg FL per day subcutaneously for 14 days each month. Patients were alternately assigned to receive either the ICD vaccine intradermally on day 7 of the FL cycle or the ICD vaccine admixed with 150 μg GM-CSF intradermally administered on day 7 of the FL cycle. The vaccination cycle was repeated every 28 days for a total of 6 vaccines. Patients were monitored monthly by physical examination and measured serum chemistry values for evidence of toxicity to organs expressing basal levels of HER-2/neu and for serologic evidence of autoimmune disease by serial evaluation of anti-SSA, dsDNA, and ANA antibodies. Criteria for premature study termination were defined as sufficient evidence to suggest that grade 3 or grade 4 toxicity exceeded 20% and 10%, respectively. Each arm (FL or FL–GM-CSF) was evaluated separately. Grade 3 toxicity was considered excessive if it occurred in 2 of 5 subjects or in 2 or 3 of 5 subjects. Grade 4 toxicity was considered excessive if it occurred in 1 of 5 subjects or in 1 or 2 of 5 subjects.
HER-2/neu peptide-based vaccine targeting the intercellular domain
All peptides constructed were putative helper epitopes of the ICD of the HER-2/neu protein, predicted by computer modeling and empiric testing to be immunogenic.16 The ICD vaccine included HER-2/neu peptides p776-790 (p776), p927-941 (p927), and p1166-1180 (p1166). All peptides used for in vitro immunologic monitoring were manufactured by United Biochemical (Seattle, WA) or Multiple Peptide Systems (San Diego, CA), and all were greater than 95% pure as assessed by high-performance liquid chromatography and mass spectrometric analysis. Peptides used in vaccine preparations were manufactured by Multiple Peptide Systems (kindly provided by Corixa, Seattle, WA) and were approved for use in humans. FL and GM-CSF were kindly supplied by Immunex (Seattle, WA).
Detection of HER-2/neu peptide- and protein-specific proliferative T-cell responses by tritiated thymidine incorporation
HER-2/neu-specific T-cell responses to peptide and protein were measured as previously described on freshly isolated peripheral blood mononuclear cells (PBMCs).17,18 PBMCs were analyzed for immune response at the end of a particular vaccine cycle, 14 days after the cessation of FL administration. Data are expressed as a standard stimulation index (SI) calculated from 24-well replicates. Phytohemagglutinin, incubated with patient T cells at a concentration of 5 μg/mL, was used as a positive control for the ability of T cells to respond to antigen and resulted in an SI greater than 2.0 in all patient assays reported (data not shown). PBMCs from 30 donors, all women without cancer ranging in age from 32 to 58 years, were evaluated in similar assays to establish baseline values. Means and 3 standard deviations of the T-cell response in the reference population to any of the HER-2/neu antigens tested was a maximum SI of 1.98; therefore, an SI greater than 2 was considered evidence of an immunized response. If subjects had an SI greater than 2.0 at baseline—that is, pre-existent immunity to HER-2/neu)19—a postvaccination response was defined as positive if it was greater than or equal to 2 times baseline.
FL may stimulate the autologous proliferation of T cells in culture by increasing APC numbers in PBMCs. To verify that the use of FL in vivo did not affect T-cell proliferation in vitro, we assessed whether the mean cpm values of the 24 replicate resting T-cell wells—that is, those with no antigen added—were significantly different between time points in patients when they had not yet received FL (baseline assay) and time points when the patients underwent multiple cycles of FL (after the sixth vaccine) using the Wilcoxon signed-rank test. There was no significant difference (P = .54) between these 2 values in any patient (n = 8) tested.
Determination of delayed-type hypersensitivity responses
Patients were skin-tested against their immunizing peptides 1 month after their last vaccination. Three hundred micrograms combined peptide solution, without any adjuvant, was injected intradermally on the patient's back, a site distant from the vaccine site. Induration was measured in 2 dimensions at 48 hours using calipers and was reported in cubic millimeters. All patients received 0.1 mL intradermal control test with sterile water, placed at the same time as the peptide test, with induration measured 48 hours later.
Detection of HER-2/neu–specific T-cell responses by ELISPOT
A 10-day ELISPOT assay was used to determine precursor frequencies of peptide- and protein-specific T lymphocytes as previously described on cryopreserved PBMC samples derived from patients before and after immunization.14 The assay was validated as linear and precise between 2.0 and 3.5 × 105 PBMCs per well. It had a detection limit of 1:100 000 and a detection efficiency of 93%. A positive response was defined as a precursor frequency that was significantly (P < .05) greater than the mean of control no antigen wells and detectable. The limit of detection of our assay is 1:100 000 precursors. PBMCs obtained before and after vaccination were analyzed simultaneously. Although the ELISPOT assay is sensitive and suitable for detecting low-level responses to vaccination,20-22 it is unknown whether the calculated precursor frequencies represent actual numbers of antigen-specific cytolytic T cells in the peripheral blood.
Results
Patient characteristics
Ten patients were enrolled. Nine patients had breast cancer with no evidence of disease. Five patients had stage III disease, 4 had stage IV disease, and 1 had ovarian cancer in first remission. Two patients, 1 with stage IV breast cancer and the patient with ovarian cancer, received only 2 vaccinations before having to withdraw from study to receive therapy for recurrent disease. Four of 5 subjects enrolled in the FL-alone arm completed 6 vaccines. The median age for those subjects was 46 years (range, 43-71 years), and the median time from chemotherapy was 5 months (range, 2-75 months). Four of 5 subjects enrolled in the FL and GM-CSF arm completed 6 vaccines. Their median age was 57 years (range, 46-67 years), and their median time from chemotherapy to the first vaccination was 6 months (range, 3-8 months). Immune response data are reported on the 4 patients from each treatment arm who completed 6 vaccinations. No patient enrolled had a personal or family history of autoimmune disease. Toxicity data are reported on all 10 enrolled patients.
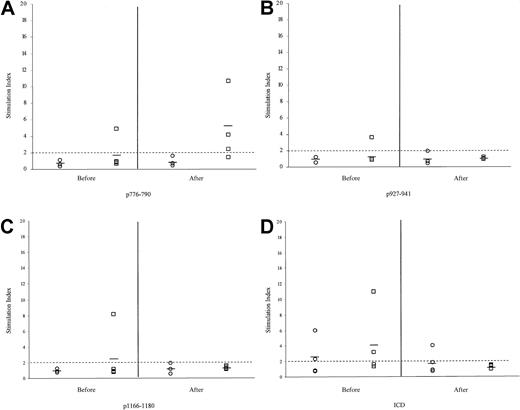
FL as a vaccine adjuvant was ineffective in generating detectable HER-2/neu protein-specific T-cell proliferative responses
Figure 1 demonstrates T-cell responses developing to HER-2/neu peptides in the ICD vaccine and the ICD protein before and after immunization courses. None of the patients in the FL alone arm had a pre-existent immune response to p776, though one patient in the FL and GM-CSF arm had an SI of 5.0 to p776 before vaccination. After immunization, no patient in the FL-alone group developed immunity to p776 (Figure 1A). The P value comparing preimmunization and postimmunization responses for this group was P = .33. Three of 4 patients in the FL and GM-CSF arm did develop detectable immunity (mean SI, 5; range, 0.5-11.8;P = .09). Figure 1B demonstrates that no patients in the FL-alone arm had a pre-existent immune response to p927, and one patient in the FL and GM-CSF arm had an SI of 4 to p927 before vaccination. After immunization, no patient in the FL-alone group or the FL and GM-CSF group developed detectable immunity to p927 (P = .48 and .23, respectively). Preimmunization evaluation of the final peptide in the immunizing mix, p1166 (Figure1C), shows no patients in the FL-alone group had evidence of pre-existent immunity to p1166 and that 1 patient in the FL and GM-CSF group had an SI of 8 before immunization. No patient in either FL arm developed detectable immunity to p1166 after completing all 6 immunizations (P = .28 [FL] and .24 [FL+GM-CSF]).
Vaccine regimens including FL as a vaccine adjuvant were ineffective in generating detectable HER-2/neu–specific T-cell proliferative responses.
Patients who received FL alone are shown as ○; patients who received FL and GM-CSF are shown as □. (A) Responses against peptide p776. (B) Responses against p927. (C) Responses against p1166. (D) Responses against the HER-2/neu ICD protein. Data are expressed as a stimulation index. Each symbol represents data on an individual patient. Bold bars indicate the mean response for that group.
Vaccine regimens including FL as a vaccine adjuvant were ineffective in generating detectable HER-2/neu–specific T-cell proliferative responses.
Patients who received FL alone are shown as ○; patients who received FL and GM-CSF are shown as □. (A) Responses against peptide p776. (B) Responses against p927. (C) Responses against p1166. (D) Responses against the HER-2/neu ICD protein. Data are expressed as a stimulation index. Each symbol represents data on an individual patient. Bold bars indicate the mean response for that group.
The demonstration of immunity to peptides developing after peptide immunization is a reflection of a patient's immune competence. However, we hypothesize that the in vitro surrogate of the ability to respond to HER-2/neu protein expressed endogenously in the major histocompatibility complex in vivo would be the detection of an immune response to ICD protein (Figure 1D). Two of 3 patients in the FL alone arm had a pre-existent immune response to ICD protein (SI 2.2 and 6.1), and 2 patients in the FL and GM-CSF arm had detectable pre-existent ICD protein-specific immune response (SI, 3 and 11.2). After completing all peptide immunizations, only 1 patient in the FL-alone arm had a detectable immune response to HER-2/neu ICD protein (SI, 4), and this patient had a pre-existent response (SI, 6.2) (P = .15). No patient in the FL and GM-CSF arm had detectable protein-specific immunity (P = .20).
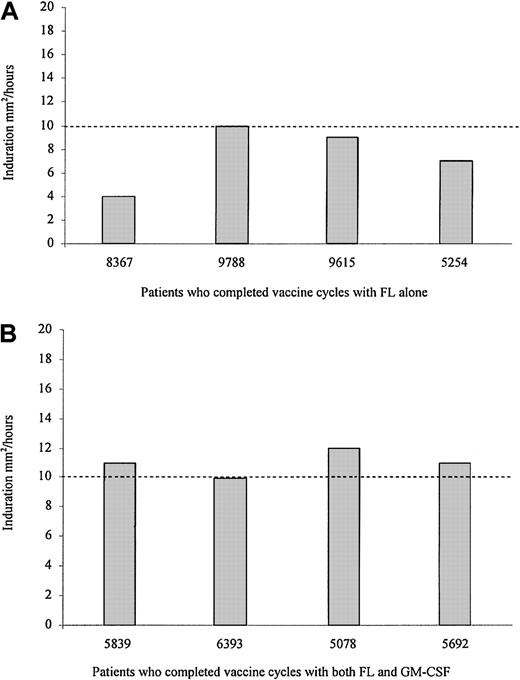
Thirty days after the last vaccination, patients were tested for a delayed-type hypersensitivity (DTH) response to the pool of immunizing peptides. Results are shown in Figure 2. Previous studies from our group have demonstrated that HER-2/neu peptide-specific DTH responses 10 mm2 or more correlate significantly to measurable (ie, SI > 2.0) peripheral blood HER-2/neu–specific T-cell responses.23 After immunization, all patients in the FL-alone arm (Figure 2A) had evidence of some measurable DTH response, though none of them had a postvaccination DTH of 10 mm2 or greater. Three of 4 patients in the FL and GM-CSF arm developed a postvaccination DTH 10 mm or more, corresponding to those who generated measurable peripheral blood T-cell responses (Figure 2B). No patient tested had a DTH to sterile water negative control greater than 0.05 mm2.
Patients immunized with HER-2/neu ICD peptides, using FL as a vaccine adjuvant, can develop peptide-specific DTH responses after completing all vaccinations.
Data represent DTH responses to a combination of the ICD peptides, without adjuvant, measured as induration in mm2 at 48 hours. (A) Results of the 4 patients who completed the vaccinations with FL alone as a vaccine adjuvant, each represented by their identification number. (B) Data derived from the 4 patients who received FL and GM-CSF as a vaccine adjuvant. The dotted line at 10 mm2 represents a DTH value previously shown to correlate with a detectable peripheral blood T-cell response.23
Patients immunized with HER-2/neu ICD peptides, using FL as a vaccine adjuvant, can develop peptide-specific DTH responses after completing all vaccinations.
Data represent DTH responses to a combination of the ICD peptides, without adjuvant, measured as induration in mm2 at 48 hours. (A) Results of the 4 patients who completed the vaccinations with FL alone as a vaccine adjuvant, each represented by their identification number. (B) Data derived from the 4 patients who received FL and GM-CSF as a vaccine adjuvant. The dotted line at 10 mm2 represents a DTH value previously shown to correlate with a detectable peripheral blood T-cell response.23
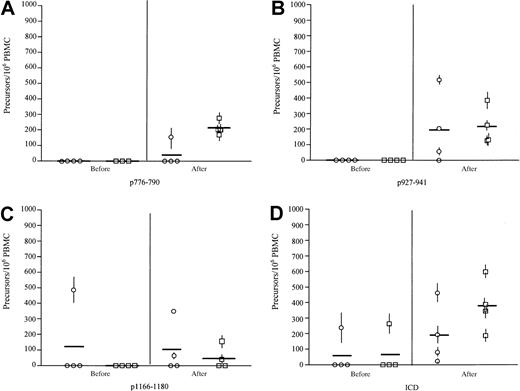
Vaccine regimens including FL as a vaccine adjuvant were effective in boosting the precursor frequency of IFN-γ–secreting HER-2/neu–specific T cells
IFN-γ precursor frequency specific for immunizing peptides and the HER-2/neu ICD protein was evaluated by ELISPOT. None of the patients had detectable pre-existent IFN-γ–secreting T cells specific for p776. After immunization, one patient in the FL-alone group developed an immune response to p776, frequency 1:5000 PBMCs (Figure 3A). The P comparing preimmunization and postimmunization responses for this group was .20. Four of 4 patients in the FL and GM-CSF arm developed detectable p776-specific T cells (mean frequency, 1:4000; range, 1:5800-1:3080;P = .001). Evaluating the IFN-γ–producing T-cell response to p927, none of the patients had detectable pre-existent IFN-γ–secreting T cells specific for p927 (Figure 3B). After immunization 3 of 4 patients in the FL-alone group developed immunity to p927 (mean frequency, 1:4000 PBMCs; range, less than 1:100 000-1:1700; P = .10) (Figure 3B). All patients in the FL and GM-CSF arm developed detectable IFN-γ–producing T cells (mean frequency, 1:3600; range, 1:8000-1:2400; P = .02). One patient in the FL-alone arm had a pre-existent, p1166-specific, IFN-γ–producing response before immunization (1:2100) (Figure 3C). After completing the vaccination cycle, 1 of 4 FL-alone patients developed a response (1:20 000). The P value comparing preimmunization and postimmunization responses for this group was .35. The patient in the FL-alone arm had a pre-existent response that did not increase to more than twice baseline and was, therefore, not considered a positive responder. Two of 4 FL and GM-CSF patients (1:30 000 and 1:7000) developed immunity specific for p1166. Preimmunization and postimmunization responses for this group wereP = .07.
Vaccine regimens including FL as a vaccine adjuvant were effective in boosting the precursor frequency of IFN-γ–secreting HER-2/neu–specific T cells.
Data are shown as the preimmunization and postimmunization IFN-γ T-cell response, as measured by 10-day ELISPOT, for the 4 patients who completed all 6 vaccines with FL alone as adjuvant (○) and the 4 patients who completed all 6 vaccines with FL and GM-CSF as a vaccine adjuvant (□). (A) T-cell responses specific for HER-2/neu peptide p776. (B) T-cell responses specific for HER-2/neu peptide p927. (C) T-cell responses specific for HER-2/neu peptide p1166. (D) Data of IFN-γ–producing T-cell responses directed against the HER-2/neu ICD protein. Data are expressed as antigen-specific T-cell precursors per 106 unfractionated PBMCs. Each symbol represents data on an individual patient as the mean and standard deviation of triplicate evaluations. Bold bars indicate the mean response for that group.
Vaccine regimens including FL as a vaccine adjuvant were effective in boosting the precursor frequency of IFN-γ–secreting HER-2/neu–specific T cells.
Data are shown as the preimmunization and postimmunization IFN-γ T-cell response, as measured by 10-day ELISPOT, for the 4 patients who completed all 6 vaccines with FL alone as adjuvant (○) and the 4 patients who completed all 6 vaccines with FL and GM-CSF as a vaccine adjuvant (□). (A) T-cell responses specific for HER-2/neu peptide p776. (B) T-cell responses specific for HER-2/neu peptide p927. (C) T-cell responses specific for HER-2/neu peptide p1166. (D) Data of IFN-γ–producing T-cell responses directed against the HER-2/neu ICD protein. Data are expressed as antigen-specific T-cell precursors per 106 unfractionated PBMCs. Each symbol represents data on an individual patient as the mean and standard deviation of triplicate evaluations. Bold bars indicate the mean response for that group.
T-cell responses to HER-2/neu ICD protein were also assessed. One patient in each arm had detectable precursor frequency to the intact protein domain (FL alone, 1:4000; FL and GM-CSF, 1:3600) (Figure 3D). After the completion of all immunizations, all 4 patients in each group developed detectable IFN-γ–producing T cell specific for the ICD protein (FL-alone arm: mean frequency, 1:5000; range, 1:30 000-1:2000;P = .07) (FL and GM-CSF arm: mean frequency, 1:2500; range, 1:5700-1:1500; P = .006).
Vaccine regimens including FL as a vaccine adjuvant were associated with the development of autoimmune phenomena in some patients
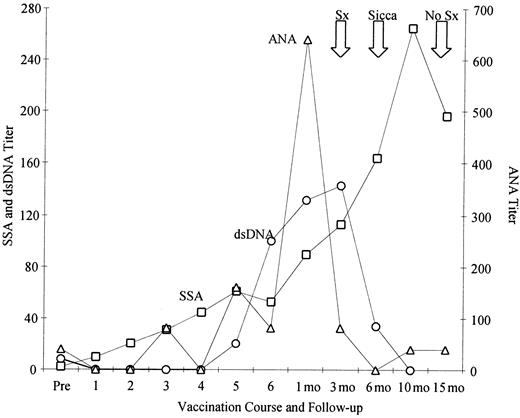
In general, vaccine regimens including FL were well tolerated. Transient monocytosis developed in all patients who received FL as part of their regimen during periods of FL administration, consistent with the mobilization of DCs to the periphery (data not shown). Toxic reactions included a grade 1 rash in a patient taking FL and autoimmune serologic abnormalities in 2 patients taking FL and GM-CSF. One patient had grade 1 serologic abnormalities (ANA, anti-SSA, anti-dsDNA). After immunization she had a detectable IFN-γ peptide-specific T-cell response to immunizing HER-2/neu peptides p927 (57 ± 12) and p1166 (80 ± 15), but not to the ICD protein. The second patient, who had stage IV breast cancer, acquired grade 2 toxicity with serologic abnormalities and Sicca syndrome, characterized by dry eyes and dry mouth not requiring immunosuppressive treatment, 3 months after the completion of the vaccine regimen (Figure4). This patient did not develop any detectable immunity to HER-2/neu peptide or protein after active immunization. In addition, this patient had no personal or family history of autoimmune disease, specifically collagen vascular, rheumatoid arthritis, lupus, Sjögren syndrome, autoimmune thyroid disease, or scleroderma. Clinical follow-up of this patient demonstrated, 18 months after her final vaccination, no clinical symptoms relating to Sicca syndrome, and her serology values became negative except for a persistently elevated SSA. No patients had evidence of autoimmune phenomena directed against tissues that expressed basal levels of HER-2/neu.
One patient, receiving systemic FL as a vaccine adjuvant, developed clinical evidence of autoimmune disease.
Sequential anti-SSA (□), dsDNA (○), and ANA (▵) are shown in one patient plotted over time. This patient received FL and GM-CSF as a vaccine adjuvant. Large arrows at the top of graph indicate the development of nonspecific symptoms (Sx) of dry eyes, diagnosis of Sicca syndrome (Sicca), and resolution of symptoms (No Sx).
One patient, receiving systemic FL as a vaccine adjuvant, developed clinical evidence of autoimmune disease.
Sequential anti-SSA (□), dsDNA (○), and ANA (▵) are shown in one patient plotted over time. This patient received FL and GM-CSF as a vaccine adjuvant. Large arrows at the top of graph indicate the development of nonspecific symptoms (Sx) of dry eyes, diagnosis of Sicca syndrome (Sicca), and resolution of symptoms (No Sx).
Discussion
In vivo mobilization of DCs for use in cancer vaccines offers an attractive alternative to ex vivo DC generation. First, cytokines that stimulate the proliferation, maturation, or migration of DCs in vivo might allow universal application of DC-based vaccine strategies. It may not be possible to generate DCs on all cancer patients in vitro.24,25 Second, avoiding the manipulation of DCs ex vivo removes the possibility of causing functional changes that occur when DCs are cultured, such as early maturation and loss of phagocytic and migratory capability before antigen loading. Third, stimulating DCs or Langerhans cells in vivo may allow the natural processing necessary to stimulate DC maturation and trafficking, which may improve antigen presentation at the level of the draining lymph node.26 27We hypothesized that increasing the number of circulating and skin DCs with FL might result in a more robust immune response and a greater percentage of patients immunized than what was seen using GM-CSF alone as an adjuvant. Studies described here demonstrate that in immunizing against the self-tumor antigen HER-2/neu, vaccine regimens that included FL as a vaccine adjuvant may not be effective in generating detectable HER-2/neu–specific T-cell proliferative responses; using FL as a vaccine adjuvant resulted in boosting the precursor frequency of IFN-γ–secreting HER-2/neu–specific T cells; and vaccine regimens with FL as a vaccine adjuvant can be associated with the development of autoimmune phenomena in some patients.
FL and GM-CSF have been used as adjuvants in a variety of vaccines in animal models. Local application of GM-CSF is associated with local Langerhans cell recruitment and the up-regulation of major histocompatibility class I and class II on APCs such as monocytes and macrophage.13 FL has been used as a systemic adjuvant in animal models. Indeed, even when administered by a route that is classically associated with the generation of tolerance—the intravenous administration of soluble protein—FL could prevent the development of tolerance to ovalbumin.9 In the trial reported here, FL, with or without GM-CSF, was not an effective vaccine adjuvant in stimulating T-cell proliferative responses. In fact, some patients showed evidence of HER-2/neu peptide and protein pre-existent immune responses at the start of the study, before receiving FL, who had no detectable T-cell response to either HER-2/neu peptide or proteins at the end of the study (Figure 1). A potential concern is that the administration of FL may actually enhance the induction of tolerance rather than circumvent tolerance. In a murine model inducing oral tolerance to ovalbumin, the systemic administration of FL actually enhanced tolerance induction.28 It is unlikely, in our study, that FL administration resulted in neu-specific tolerance. HER-2/neu antigen-specific IFN-γ–secreting precursors could be elicited during the course of vaccination. These cells would most likely be unable to be detected if antigen-specific tolerance were induced. In fact, the generation of a strongly skewed type 1 response may affect the ability of antigen-specific T cells to proliferate.29
Recent investigations have demonstrated that FL and GM-CSF may stimulate different subsets of DCs in vivo and that the cytokine microenvironment elicited, either type 1 or type 2, is markedly influenced by the particular DC subset generated. Evaluating a murine model of cancer, using tumors engineered to express either GM-CSF or FL demonstrated that GM-CSF–engineered cells were more potent in inducing an antitumor response.30 GM-CSF elicited a diverse cytokine environment consisting of Th1 and Th2 immune effectors. In contrast, immune responses generated with FL-expressing tumor cells were specifically restricted to a Th1 phenotypic response.30 Our data support that FL is associated with the development of a strong type 1 response. Significant HER-2/neu antigen-specific precursor frequencies could be detected after immunization only with assays specifically designed to evaluate IFN-γ–producing T cells. An unexpected finding was the observation that though measurement of the T-cell proliferative response and the DTH response correlated well with each other, the detection of IFN-γ–secreting cells would predict a greater number of successfully immunized patients when FL was used as an adjuvant. The detection of antigen-specific cytokine production without concomitant measurable clonal proliferation has been reported and is potentially a reflection of a strongly restricted type 1 environment. Nitric oxide (NO) is produced during the inflammatory response, and it is proposed that it acts as a mediator to limit tissue destruction caused by inflammatory cytokines.29 Studies evaluating the effects of NO on the function of Th1 and Th2 antigen-specific clones demonstrated that the addition of NO to clones after stimulation did not inhibit cytokine production or induce apoptosis of cells but that it markedly inhibited proliferation of the antigen-specific population.29Subsequent studies have suggested activated macrophages may mediate this effect31 and have determined that macrophages produce NO after antigen presentation to Th1 T-cell clones. Furthermore, macrophage-derived NO inhibits measurable proliferation of these clones without affecting IFN-γ production.31 More recent studies have demonstrated that DCs can express NO in response to self-antigens and that the secretion of NO may have a role in regulating the immune response strongly induced by such potent APCs.32
The association of autoimmune phenomena occurring in patients who received FL as a vaccine adjuvant suggests the APCs generated are functional. Autoimmune phenomena have been demonstrated with the use of DCs as a vaccine adjuvant in animal models.33 In vivo mobilization of DCs using FL will cause the peripheralization of DC precursors systemically. The antigen-presenting capabilities of these cells as they circulate to target organs is unknown. FL increases the number of circulating bone marrow–derived DCs. These DCs are, most likely, not fully matured and are capable of taking up antigen derived from the environment. It is possible that circulating DC precursors could respond to self-proteins in the environment and could elicit immunity to self. The in vivo kinetics or initiation process involved in generating this immune response is not defined. Although serologic abnormalities could be detected during active FL administration, the clinical syndrome associated with those abnormalities developed only after the study was completed, suggesting a more extended follow-up may be appropriate for evaluating the potential of an immune-mediated toxicity when evaluating immunotherapeutic strategies.
Studies such as the one presented here demonstrate that FL, given systemically, can influence immune responses generated in vivo. DC precursors can be effectively mobilized by FL and are able to present antigen, as evidenced by the demonstration not only of HER-2/neu–specific T-cell responses inducing IFN-γ–producing T cells but also of the development of apparent autoimmune phenomena. Although FL alone may not be optimal as a vaccine adjuvant, preclinical studies further evaluating the role of additional cytokines used in conjunction with FL to augment DC maturation and function34 provide for the successful application of FL in mobilizing DCs in vivo for use in therapeutic strategies designed to augment the tumor-specific immune response.
We thank Chalie Livingston for assistance in manuscript preparation and Dr Kathleen Ruffner for assistance in the statistical analysis of laboratory data. Our heartfelt thanks go to all the patients who agreed to participate in this study.
Supported by grants from the National Institutes of Health/National Cancer Institute (K08 CA61834 and R01 CA75163), the Department of Defense (DOD) Breast Cancer Program, and the Cancer Research Treatment Foundation (M.L.D.). Patient care was conducted through the Clinical Research Center Facility at the University of Washington, which is supported by NIH grant MO1-RR-00037. Supported in part by a grant from the Immunex Corporation (M.L.D.). K.L.K. was supported by a DOD Breast Cancer Program Fellowship Award.
One of the authors (D.C.) is employed by Immunex Corporation, Seattle, WA.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mary L. Disis, Division of Oncology, University of Washington, Box 356527, Seattle, WA 98195-6527; e-mail:ndisis@u.washington.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal