Abstract
Dendritic cells (DCs) are rare antigen-presenting cells that play a central role in stimulating immune responses. The combination of recombinant granulocyte macrophage–colony-stimulating factor (rGM-CSF) and recombinant interleukin-4 (rIL-4) provides an important stimulus for generating DCs from murine bone marrow precursors in vitro. Using miniature osmotic pumps, we now demonstrate that continuous infusion of these cytokines for 7 days had a similar effect in vivo, increasing the number and function of splenic DCs. Administration of rGM-CSF/rIL-4 (10 μg/d each) increased the concentration of CD11+ DCs by 2.7-fold and the absolute number of splenic DCs by an average of 5.7-fold. DC number also increased in peripheral blood and lymph nodes. The resultant DCs exhibited a different phenotype and function than those in control mice or mice treated with rGM-CSF alone. rGM-CSF/IL-4 increased both the myeloid (CD11c+/CD11b+) and the lymphoid (CD11c+/CD8α+) subpopulations, whereas rGM-CSF increased only myeloid DCs. DCs were highly concentrated in the T-cell areas of white pulp after rGM-CSF/IL-4 administration, whereas they were diffusely distributed throughout white pulp, marginal zones, and red pulp in mice treated with rGM-CSF alone. rGM-CSF/rIL-4 also significantly increased the expression of major histocompatibility complex (MHC) class I and MHC class II on CD11c+ cells and increased their capacity to take up antigens by macropinocytosis and receptor-mediated endocytosis. Splenic DCs generated in response to rGM-CSF/rIL-4 were functionally immature in terms of allostimulatory activity, but this activity increased after short-term in vitro culture. Systemic treatment with rGM-CSF/rIL-4 enhanced the response to an adenoviral-based vaccine and led to antigen-specific retardation in the growth of established tumor. We conclude that systemic therapy with the combination of rGM-CSF/rIL-4 provides a new approach for generating DCs in vivo.
Introduction
Dendritic cells (DCs) develop from bone marrow precursors and are distributed in limited numbers throughout peripheral tissues and lymphoid organs. Although they represent only a small percentage of mononuclear leukocytes, they play a sentinel role in initiating and regulating immune responses.1,2 The use of cytokines (granulocyte macrophage–colony-stimulating factor [GM-CSF], interleukin-3 [IL-3], IL-4, tumor necrosis factor-α [TNF-α]) and receptor ligands (flt3-ligand, CD40-ligand) to promote DC differentiation has become an important strategy for enhancing immune responsiveness and stimulating antigen-specific immunity.3-9 Of these approaches, treating DC precursors with the combination of GM-CSF/IL-4 has been the most widely studied. With human cells, neither GM-CSF nor IL-4 generates DCs when used alone in vitro.10-12 Despite the expression of GM-CSF receptors on myeloid precursors and the activity of this cytokine as a DC survival factor, GM-CSF does not promote functional differentiation into DCs. In contrast, the combination of GM-CSF with IL-4 produces a coordinated down-regulation of myeloid features and an up-regulation of major histocompatibility complex (MHC) expression, costimulatory molecules, antigen processing, and T-cell stimulatory activity.7,11 These effects are similarly observed in vivo when cancer patients are treated with a combination of systemic GM-CSF and IL-4 but not with GM-CSF alone.13-15
In contrast to results obtained with human cells, murine studies suggest a more independent role for GM-CSF as a DC growth and differentiation factor.16-18 Inaba et al16cultured bone marrow progenitors in GM-CSF and identified budding clusters of cells expressing dendritic morphology, MHC class I, MHC class II, CD11c, and DEC-205. These cells exhibited potent allostimulatory activity consistent with their identification as DCs. Similarly, the systemic administration of GM-CSF to mice increases DC number in vivo. Hanada et al19 implanted GM-CSF–secreting tumors into mice and observed increased numbers of splenic DCs when compared to controls. The direct administration of GM-CSF alone, in the form of a polyethylene glycol–modified molecule, was also recently shown to increase the number of splenic DCs, but only the myeloid subpopulation expressing CD11c and CD11b.20
Despite these independent effects of GM-CSF, there is evidence that IL-4 still plays an important synergistic role in generating mouse DCs. Labeur et al8 compared bone marrow progenitors cultured in GM-CSF alone to those cultured with the combination of GM-CSF/IL-4. The presence of IL-4 increased the expression of MHC, CD40, CD80, CD86, and DEC-205. Cells cultured with GM-CSF/IL-4 produced 3-fold more IL-12 than those cultured in GM-CSF, and they stimulated greater T-cell proliferation in response to alloantigens or OVA peptide. Others have reported that DCs grown in low concentrations of GM-CSF alone are tolerogenic, whereas those generated in response to GM-CSF/IL-4 are not.20 Gunji et al22 23 failed to observe tumor rejection in mice inoculated with tumor cells producing GM-CSF or IL-4 alone, but they noted tumor rejection and the development of tumor-specific immunity in mice injected with a combination of GM-CSF– and IL-4–producing tumors.
To clarify the role of IL-4 on the differentiation and expansion of mouse DCs in vivo, we used miniature osmotic pumps to deliver continuous infusions of GM-CSF, either alone or in combination with IL-4. Spleens and lymph nodes from cytokine-treated mice were evaluated for evidence of DC differentiation and function. The combination of GM-CSF/IL-4 increased the number of myeloid (CD11c+/CD11b+) and lymphoid (CD11c+/CD8α+) DCs, whereas GM-CSF alone expanded only on the myeloid subset. Immunohistology revealed a focal concentration of DCs within the white pulp of splenic follicles in mice treated with GM-CSF/IL-4, whereas GM-CSF by itself produced a more diffuse response. DCs generated in vivo with rGM-CSF/rIL-4 expressed higher levels of MHC class I and class II and a greater capacity for endocytosis, macropinocytosis, and allostimulatory activity. Finally, in an immunotherapy model, combining systemic rGM-CSF/rIL4 with an adenoviral-based vaccine significantly slowed tumor growth in an antigen-specific manner, whereas systemic administration of rGM-CSF had no effect. Our results demonstrate important synergistic effects on DC differentiation and function when GM-CSF and IL-4 are administered together in vivo.
Materials and methods
Mice
Male C57BL/6 and BALB/c mice, 8 to 12 weeks old (Charles River Laboratories, Wilmington, MA) were housed in a pathogen-free vivarium and fed ad libitum. Procedures involving mice were approved by the UCLA and the West Los Angeles VA animal research committees.
Cytokines
Recombinant mouse GM-CSF (rGM-CSF; 2.4 × 108U/mg) and IL-4 (rIL-4; 1 × 108 U/mg) were provided by the Schering-Plough Research Institute (Kenilworth, NJ).
In vivo generation of DCs
C57BL/6 mice were treated with a 7-day continuous infusion of rGM-CSF, alone or in combination with rIL-4, administered by mini-osmotic pump (model 1007D; Alza, Palo Alto, CA). In brief, osmotic pumps were loaded under sterile conditions with rGM-CSF (2-20 μg/mL), rIL-4 (2-20 μg/mL), or both and were implanted in subcutaneous tissue over the mid-back. Control animals received pumps loaded with diluent alone (0.9% saline). The presence of circulating rGM-CSF and rIL-4 was determined on day 7 serum samples by specific enzyme-linked immunosorbent assay, as described by the manufacturer (BioSource International, Camarillo, CA).
Spleen cell preparation and phenotyping
Single-cell suspensions from control and experimental spleens and lymph nodes were prepared by cutting the organs into small pieces and then digesting them with 1 mg/mL type II collagenase (Worthington Biochemical, Freehold, NJ) and 0.02 mg/mL bovine pancreatic DNAse (Boerhinger Mannheim, Mannheim, Germany) for 30 minutes at room temperature. Digestion mixtures were then treated with 0.1 M EDTA (Sigma, St Louis, MO) for 5 minutes and were centrifuged to remove tissue debris, and cells were washed in RPMI 1640 (Irvine Scientific, Santa Ana, CA) containing 2% fetal calf serum (FCS; Omega Scientific, Tarazana, CA). Red blood cells were depleted by hypotonic shock. For fluorescence-activated cell sorter (FACS) analysis, cell surface Fc receptors (FcR) were first blocked by incubation with anti-FcRIIγ monoclonal antibody (mAb) (clone 2.4G2; ATCC, Rockville, MD) for 30 minutes at 4°C. T cells, B cells, and NK cells were identified by incubation with biotinylated Thy1.2, B220, or NK1.1 mAb (Caltag Laboratories, Burlingame, CA) for 30 minutes at 4°C and labeling with fluorescein isothiocyanate (FITC)–conjugated streptavidin (Caltag Laboratories). DC subsets were identified by incubation with FITC, allophycocyanin (APC)–, or phycoerythrin (PE)–conjugated mAb directed against CD11c, CD11b, CD8α, MHC class I, or MHC class II (PharMingen, San Diego, CA). After washing in phosphate-buffered saline (PBS) with 2% FCS, cells were fixed in 1% paraformaldehyde. The acquisition of 103 to 105 events was performed on a FACStar cytometer (Becton Dickinson, San Jose, CA), and results were analyzed using Cellquest software (Becton Dickinson).
In some experiments, DCs were further enriched by the depletion of T and B cells. Single-cell suspensions of spleen cells were depleted of RBCs and incubated with purified mAbs against Thy-1.2 and B220 for 30 minutes at 4°C (PharMingen). Cells were then incubated for 30 minutes at 37°C in serum-free RPMI 1640 medium containing 10% rabbit complement (Sigma, St Louis, MO). After washing, the remaining cells were used as an enriched population of DCs.
Histology and immunohistology
Spleens from control, rGM-CSF, and rGM-CSF/rIL-4–treated mice were embedded in Tissue-Tek OCT compound (Miles, Elkhart, IN) by freezing in liquid N2 and stored at −80°C. Six-micrometer sections were cut on a cryostat (Reichert Jung, Cambridge Instruments GmbH, Germany) and were mounted on poly-L-lysine–coated slides. Sections were air dried overnight, fixed in 10% formalin (Sigma) for 10 minutes, and stained with hematoxylin (Fisher Scientific) and eosin (Sigma) or for immunohistology as described for each antibody combination.
Dual detection of CD11c and CD11b was performed by washing slides with PBS and blocking endogenous peroxidase activity with 0.3% H2O2 (10 minutes). Sections were blocked for 20 minutes with PBS containing 5% bovine serum albumin (Fisher Scientific, Springfield, NJ) and 1% goat serum (Jackson Immuno Research, West Grove, PA), rinsed, and stained with anti-CD11c (PharMingen) for 1 hour. After washing, biotinylated goat antihamster antibody (PharMingen) was added for 30 minutes. Specific antibody binding was visualized by treating with a peroxidase substrate for 30 minutes using the Vectastain Elite ABC Kit (Vector Laboratories, Burlingame, CA). Sections were again treated with H2O2 and were blocked with 1% goat serum before incubation with rat anti-mouse CD11b (PharMingen) for 1 hour. Sections were stained with biotinylated donkey anti-rat antibody (Jackson Immuno Research) and were visualized with an alkaline-phosphatase, fast-blue substrate using the Vectastain ABC-AP kit (Vector Laboratories).
Dual detection of DEC-205 (rat antimouse DEC-205; Serotech, Raleigh, NC) and immunoglobulin Igβ (hamster antimouse Igβ; PharMingen) was performed in an analogous manner using species-appropriate blocking serum and secondary antibody.
Measurement of endocytosis and pinocytosis
Enriched DC populations (1 × 106) prepared from spleens of control and cytokine-treated mice were incubated with 1 mg/mL FITC-dextran (70 kd) or Lucifer yellow (both from Molecular Probes, Eugene, OR) for 1 hour at 37°C. Control cells were treated in the same manner but were maintained on ice to block energy-dependent uptake. Antigen uptake was terminated by the addition of ice-cold PBS containing 0.1% azide. Cells were washed 3 times in PBS/1% FCS/0.1% azide, and extracellular antigen was removed by incubation with a 1% trypsin–PBS solution (Sigma) for 3 minutes at 37°C. Cells were counterstained with PE–anti-CD11c and APC–anti-CD11b, and intracellular uptake of FITC-dextran and Lucifer yellow was determined by flow cytometry.
Mixed-lymphocyte reaction
Splenocytes from control and cytokine-treated C57BL/6 mice were depleted of B and T cells, as described above, to prepare DC-enriched populations. Either they were directly tested as stimulators in mixed-lymphocyte reaction (MLR) assays (fresh) or they were cultured for 36 hours in the presence of 20 ng/mL of rGM-CSF and rIL-4 before testing (cultured). Allogenic T cells were prepared from the lymph nodes of 8- to 12-week-old BALB/c mice. In brief, single-cell suspensions from inguinal and axillary lymph nodes were incubated with mAbs to B220, NK1.1, and Gr-1 for 45 minutes at 4°C. Cells were then incubated at 37°C for 30 minutes in serum-free RPMI 1640 medium containing 10% rabbit complement (Sigma). T cells prepared by this method were at least 90% pure, as determined by FACS analysis. MLR assays were performed in 96-well, round-bottomed culture plates. Allogenic T cells (1 × 105 BALB/c) were incubated with varying numbers of irradiated DC-enriched spleen cells (20 Gy) from control or cytokine-treated mice. Cells were cultured in 0.2 mL RPMI 1640 containing 10% FCS and 10−4 M 2-ME (Sigma) in a humidified CO2 incubator for 3 days. Culture wells were pulsed with 1.25 μCi (46.2 kBq) [3H] thymidine for 12 hours, and the cells were harvested onto glass fiber sheets using an automated harvester. Proliferation was determined by counting each sample in a liquid scintillation β-counter. Background counts for T cells or DCs alone were always less than 200 cpm.
Tumor cell line
The murine cell line E-22 (kindly provided by Dr S. Restifo, National Cancer Institute, Bethesda, MD), a clone of the EL4 mouse thymoma line stably expressing the LacZ gene, was used for in vivo tumor studies. Cells were maintained in RPMI 1640, 10% heat-inactivated FCS, 0.03% L-glutamine, 100 μg/mL streptomycin, 100 μg/mL penicillin, and 50 μg/mL gentamicin sulfate (Life Technologies, Rockville, MD) in the presence of 400 μg/mL G418 (Life Technologies).
Recombinant adenoviral vectors
Recombinant adenovirus Ad5.CMV-LacZ (AdV/β-gal) was obtained from Quantum Biotechnologies (Montreal, Quebec, Canada). Ad5.CMV-LacZ is a first-generation, E-1–deleted adenovirus serotype 5 expressing the bacterial LacZ gene under the control of the CMV-IE promoter/enhancer. The control adenovirus, AdV/RR5 (kindly provided by Dr L. Butterfield, University of California Los Angeles) is an E-1–deleted type 5 vector that carries no reporter gene construct.24 Viral stocks were amplified on 293 cells. This was followed by CsCl purification, dialysis, and storage at −80°C. The titer of viral stock was between 109 and 1013 plaque-forming units (PFU)/mL by plaque assay on 293 cells.
In vivo adenoviral immunization and tumor immunotherapy model
Eight- to 12-week-old C57BL/6 mice (n = 5/group) were injected subcutaneously with E-22 tumor cells (1 × 105 in 100 μL saline) in the right flank. The following day, mice were implanted with osmotic pumps and were treated with 7-day infusions of saline, rGM-CSF, or the combination of rGM-CSF/rIL-4 (10 μg each per day) to increase the number of DCs in vivo. Fourteen days after tumor inoculation, mice were immunized by intraperitoneal injection with 1 × 108 PFU of either ADV/β-gal, expressing theLacZ transgene, or the control vector, AdV/RR5. Tumor volumes were measured biweekly in mm3 (maximal length × width × height) with an electronic caliper (Stoelting, Wheat Lake Wood Dale, IL).
Statistics
Data from individual representative experiments were presented as mean values ± SD, and data from multiple experiments were represented as mean group values ± SE. Differences between groups were determined by paired or unpaired Student t test, as applicable to the assay conditions, with P values as indicated.
Results
Systemic rGM-CSF and combined rGM-CSF/IL-4 increased spleen and lymph node sizes and the concentration of CD11c+DCs
Continuous infusions of rGM-CSF and/or rIL-4 were delivered to mice through miniature osmotic pumps, and dosing was initially evaluated by changes in spleen size and the presence of CD11c+ DCs. Cytokine effects reached a plateau by day 7, and serum levels reached 158.3/0 pg/mL (GM-CSF/IL-4) for rGM-CSF–treated mice and 52.8/410 pg/mL (GM-CSF/IL-4) for rGM-CSF/rIL-4–treated mice at the 10 μg/d dosing (data represent pooled serum from 5 mice). When used alone, rGM-CSF increased spleen cellularity and DC percentage in a dose-dependent manner up to 10 μg/d. In contrast, rIL-4 produced minimal effects when infused by itself but induced specific changes in spleen size and composition when given in combination with rGM-CSF (Figure1). At 10 μg/d, rGM-CSF increased cellularity by 4-fold and the concentration of CD11c+ DC by 2.1-fold compared with control mice (P ≤ .05). In contrast, the combination of rGM-CSF/rIL-4 increased spleen cell number by only 2.1-fold, yet it enriched the percentage of CD11c on average by 2.7-fold to as much as 25% of total spleen cells (P ≤ .05). Although rGM-CSF significantly increased the percentage of DCs, the response to combined rGM-CSF/rIL-4 was greater in all experiments (P ≤ .05).
Continuous infusion of rGM-CSF alone, or in combination with rIL-4, increased spleen cellularity and the percentage of CD11c+ DCs.
C57BL/6 mice were treated for 7 days with rGM-CSF (GM, 10 μg/d) or the combination of rGM-CSF and rIL-4 (GM/IL-4, 10 μg each per day) by subcutaneous osmotic pump. (A) Total number of viable splenic leukocytes was determined by hemocytometer count on day 7. Treatment with GM alone produced an average 4.4-fold increase in cell number, whereas combination rGM/rIL-4 produced an average 2.0-fold increase in cellularity. Values represent mean ± SE of 3 experiments. (B) Single-cell spleen suspensions were stained with anti-CD11c-FITC mAbs, and the effects of cytokine treatment on the percentage of CD11c+ DC were determined by FACS analysis. Three percent to 7% of control cells expressed CD11c, whereas 6% to 22% of cells from GM-treated mice expressed CD11c and 8% to 25% of cells from rGM/rIL-4–treated mice expressed CD11c. Values represent mean ± SE of 3 experiments. *P ≤ .05 compared with control; †P ≤ .05 compared with GM; ‡P ≤ .1 compared with control.
Continuous infusion of rGM-CSF alone, or in combination with rIL-4, increased spleen cellularity and the percentage of CD11c+ DCs.
C57BL/6 mice were treated for 7 days with rGM-CSF (GM, 10 μg/d) or the combination of rGM-CSF and rIL-4 (GM/IL-4, 10 μg each per day) by subcutaneous osmotic pump. (A) Total number of viable splenic leukocytes was determined by hemocytometer count on day 7. Treatment with GM alone produced an average 4.4-fold increase in cell number, whereas combination rGM/rIL-4 produced an average 2.0-fold increase in cellularity. Values represent mean ± SE of 3 experiments. (B) Single-cell spleen suspensions were stained with anti-CD11c-FITC mAbs, and the effects of cytokine treatment on the percentage of CD11c+ DC were determined by FACS analysis. Three percent to 7% of control cells expressed CD11c, whereas 6% to 22% of cells from GM-treated mice expressed CD11c and 8% to 25% of cells from rGM/rIL-4–treated mice expressed CD11c. Values represent mean ± SE of 3 experiments. *P ≤ .05 compared with control; †P ≤ .05 compared with GM; ‡P ≤ .1 compared with control.
A similar increase in total cell number was noted in the lymph nodes of mice treated with rGM-CSF and rGM-CSF/rIL-4, with the response always greater in mice receiving rGM-CSF/rIL-4 (1.7-fold vs 2.7-fold increase compared to control, respectively; Tables1 and 2). An increase in the percentage of CD11c cells in lymph nodes also occurred, though the effects were smaller in magnitude compared with those observed in spleen (1.3- to 2.3-fold increase compared with control). The overall effect of cytokine treatment was an increase in total cell number and CD11c content in spleen and lymph nodes.
Changes in spleen cell type and number after treatment with rGM-CSF and rGM-CSF/rIL-4
| . | Cells (%) . | Total cells (× 106) . | ||||
|---|---|---|---|---|---|---|
| Control . | GM . | GM/IL-4 . | Control . | GM . | GM/IL-4 . | |
| B220 | 47.6 ± 14.8 | 42.5 ± 16 | 32.4 ± 5.6 | 25.1 ± 12.6 | 55.9 ± 22.6 | 22.1 ± 1.9 |
| Thy1.2 | 25.8 ± 3.4 | 24.7 ± 3.8 | 21.2 ± 1.2 | 13.0 ± 2.9 | 32.8 ± 5.2 | 14.6 ± 2.0 |
| NK1.1 | 2.2 ± 1.7 | 1.2 ± 1.0 | 5.2 ± 5.9 | 1.0 ± 0.6 | 1.5 ± 0.9 | 3.7 ± 4.6 |
| CD11b+ | 18.2 ± 7.5 | 23.9 ± 2.1 | 31.3 ± 11.9 | 9.2 ± 4.6 | 33.5 ± 6.2 | 21.7 ± 9.2 |
| Total | 93.8 ± 8.5 | 92.4 ± 18.4 | 90.1 ± 14.9 | 48.2 ± 14.2 | 123.7 ± 25.3 | 62.2 ± 13.6 |
| . | Cells (%) . | Total cells (× 106) . | ||||
|---|---|---|---|---|---|---|
| Control . | GM . | GM/IL-4 . | Control . | GM . | GM/IL-4 . | |
| B220 | 47.6 ± 14.8 | 42.5 ± 16 | 32.4 ± 5.6 | 25.1 ± 12.6 | 55.9 ± 22.6 | 22.1 ± 1.9 |
| Thy1.2 | 25.8 ± 3.4 | 24.7 ± 3.8 | 21.2 ± 1.2 | 13.0 ± 2.9 | 32.8 ± 5.2 | 14.6 ± 2.0 |
| NK1.1 | 2.2 ± 1.7 | 1.2 ± 1.0 | 5.2 ± 5.9 | 1.0 ± 0.6 | 1.5 ± 0.9 | 3.7 ± 4.6 |
| CD11b+ | 18.2 ± 7.5 | 23.9 ± 2.1 | 31.3 ± 11.9 | 9.2 ± 4.6 | 33.5 ± 6.2 | 21.7 ± 9.2 |
| Total | 93.8 ± 8.5 | 92.4 ± 18.4 | 90.1 ± 14.9 | 48.2 ± 14.2 | 123.7 ± 25.3 | 62.2 ± 13.6 |
Spleen cells were isolated and counted from mice treated with rGM-CSF or rGM-CSF/rIL-4 (10 μg/mouse per day for 7 days). Cells were analyzed by FACS for surface expression of various markers of B cells (B220), T cells (Thy1.2), NK cells (NK1.1), and myeloid cells (CD11b). Data represent the mean ± SD (n = 3 experiments).
Changes in lymph node cell type and number after treatment with rGM-CSF and rGM-CSF/rIL-4
| . | Cells (%) . | Total cells (× 106) . | ||||
|---|---|---|---|---|---|---|
| Control . | GM . | GM/IL-4 . | Control . | GM . | GM/IL-4 . | |
| B220 | 14.3 ± 1.9 | 20.8 ± 1.9 | 16.0 ± 3.2 | 1.0 ± 0.7 | 1.8 ± 0.6 | 2.2 ± 0.8 |
| Thy1.2 | 78.5 ± 3.6 | 69.3 ± 1.0 | 71.2 ± 4.0 | 5.4 ± 3.3 | 6.8 ± 1.9 | 10.0 ± 4.3 |
| NK1.1 | 0.5 ± 0.0 | 0.7 ± 0.0 | 1.1 ± 0.2 | 0.03 ± 0.03 | 0.5 ± 0.4 | 0.1 ± 0.1 |
| CD11b+ | 5.1 ± 0.6 | 7.9 ± 0.8 | 5.6 ± 0.4 | 0.3 ± 0.2 | 0.7 ± 0.2 | 0.8 ± 0.8 |
| Total | 100.5 ± 3.6 | 97.4 ± 3.8 | 95.0 ± 4.3 | 6.8 ± 4.4 | 10.0 ± 3.0 | 13.2 ± 5.7 |
| . | Cells (%) . | Total cells (× 106) . | ||||
|---|---|---|---|---|---|---|
| Control . | GM . | GM/IL-4 . | Control . | GM . | GM/IL-4 . | |
| B220 | 14.3 ± 1.9 | 20.8 ± 1.9 | 16.0 ± 3.2 | 1.0 ± 0.7 | 1.8 ± 0.6 | 2.2 ± 0.8 |
| Thy1.2 | 78.5 ± 3.6 | 69.3 ± 1.0 | 71.2 ± 4.0 | 5.4 ± 3.3 | 6.8 ± 1.9 | 10.0 ± 4.3 |
| NK1.1 | 0.5 ± 0.0 | 0.7 ± 0.0 | 1.1 ± 0.2 | 0.03 ± 0.03 | 0.5 ± 0.4 | 0.1 ± 0.1 |
| CD11b+ | 5.1 ± 0.6 | 7.9 ± 0.8 | 5.6 ± 0.4 | 0.3 ± 0.2 | 0.7 ± 0.2 | 0.8 ± 0.8 |
| Total | 100.5 ± 3.6 | 97.4 ± 3.8 | 95.0 ± 4.3 | 6.8 ± 4.4 | 10.0 ± 3.0 | 13.2 ± 5.7 |
Axillary and inguinal lymph nodes were recovered from mice and were processed in a manner identical to that used for spleen cells. Data represent the mean ± SD (n = 3 experiments).
rGM-CSF increased all lineage-specific subsets, whereas rGM-CSF/rIL-4 preferentially increased natural killer cells and myeloid cells in spleen
Cytokine treatments increased spleen and lymph node cellularity, and flow cytometry was used to determine changes in the B cell (B220+), T cell (Thy1.2+), NK cell (NK1.1+), and myeloid (CD11b+) subsets (Tables1, 2). In spleen, treatment with rGM-CSF produced a proportional increase in all these subsets. In contrast, rGM-CSF/rIL-4 selectively increased the percentage and number of NK cells and myeloid cells with little effect on the total number of B cells and T cells. These differences suggest a more generalized proliferative response to rGM-CSF and a more targeted effect of rGM-CSF/rIL-4 on spleen cell populations. The response in lymph node was slightly different, with both rGM-CSF and rGM-CSF/rIL-4 resulting in a proportional increase in all cell subsets.
rGM-CSF/rIL-4 increased the number of myeloid (CD11c+/CD11b+) and lymphoid (CD11c+/CD8α+) DCs
Murine CD11c+ DCs were divided into 2 primary subsets: lymphoid DCs identified by the concurrent expression of CD8α and myeloid DCs delineated by the expression of CD11b. Consistent with prior reports,17,20,21 25 treatment with 10 μg/d rGM-CSF for 7 days increased the percentage of myeloid DCs by 3- to 5-fold, making them the predominant DC population in rGM-CSF–treated mice. The addition of rIL-4 at 10 μg/d increased this response further, boosting the concentration of CD11c+/CD11b+cells up to 7 times that found in control mice (Figure2). The effects of cytokine treatment on the lymphoid subset in spleen were different. rGM-CSF, when used as a single agent, did not increase the percentage of CD11c+/CD8α+ cells. However, when combined with rIL-4 (both at 10 μg/d), the concentration of CD8α+ DCs increased by 2- to 3-fold over the concentration found in control mice (Figure 2). These results were confirmed by 3-color analysis demonstrating that CD11c+/CD8α+ cells in rGM-CSF/rIL-4–treated mice also stained for DEC-205, another lymphoid DC marker. The percentage of cells expressing both DEC-205 and CD11c increased 2- to 3-fold in the rGM-CSF/rIL-4 treatment group (Figure 2). The effects on DC composition were further magnified by the overall increase in spleen size. On average, the number of myeloid DCs in the spleens of mice treated with rGM-CSF increased approximately 8-fold compared with control mice, averaging 7.1 × 106CD11c+/CD11b+ cells per spleen (Figure3). In mice treated with rGM-CSF/rIL-4, the number of myeloid DCs increased by approximately 5-fold, and the number of lymphoid DCs increased by 3.7-fold. Similar, but not identical, trends were observed in axillary and inguinal lymph nodes. Mice treated with rGM-CSF exhibited a 2.2-fold increase in the number of myeloid DCs in lymph nodes and a 3-fold increase in the number of lymphoid DC. In rGM-CSF/rIL-4–treated mice, there was a similar 2-fold increase in myeloid DCs in lymph nodes and a 4- to 6-fold increase in lymphoid DCs (Figure 3). Dual-staining demonstrated that DEC-205 was primarily expressed on the CD8α+ subset in spleen and lymph nodes, consistent with its established expression on lymphoid DCs. In total, the response to rGM-CSF was clearly biased toward increasing primarily myeloid DCs, whereas the response to rGM-CSF/rIL-4 was more balanced, significantly increasing myeloid and lymphoid subsets in spleen and lymph node.
rGM-CSF and rGM-CSF/rIL-4 increased the percentage of myeloid DCs (CD11c+/CD11b+) in spleen, but only rGMCSF/rIL-4 increased the percentage of lymphoid DCs (CD11c+/CD8α+).
Mice were treated with rGM-CSF (GM) or the combination of rGM-CSF/rIL-4 (GM/IL-4), as described for Figure 1. Spleen cells were isolated on day 7, and FACS analysis was performed to determine the percentage of total spleen cells expressing either a myeloid DC phenotype (upper panel, stained with CD11b-PE and CD11c-FITC) or a lymphoid phenotype (middle panel, stained with CD8α-PerCP and CD11c-FITC). The percentage of cells expressing DEC-205, another lymphoid DC marker, were also determined (lower panel, stained with CD11c-PE and DEC-205-FITC). The percentage of double-stained cells for each marker set are identified. Results are from a single representative experiment (n > 20).
rGM-CSF and rGM-CSF/rIL-4 increased the percentage of myeloid DCs (CD11c+/CD11b+) in spleen, but only rGMCSF/rIL-4 increased the percentage of lymphoid DCs (CD11c+/CD8α+).
Mice were treated with rGM-CSF (GM) or the combination of rGM-CSF/rIL-4 (GM/IL-4), as described for Figure 1. Spleen cells were isolated on day 7, and FACS analysis was performed to determine the percentage of total spleen cells expressing either a myeloid DC phenotype (upper panel, stained with CD11b-PE and CD11c-FITC) or a lymphoid phenotype (middle panel, stained with CD8α-PerCP and CD11c-FITC). The percentage of cells expressing DEC-205, another lymphoid DC marker, were also determined (lower panel, stained with CD11c-PE and DEC-205-FITC). The percentage of double-stained cells for each marker set are identified. Results are from a single representative experiment (n > 20).
rGM-CSF increased the total number of myeloid DCs in mouse spleen, whereas the combination of rGM-CSF/rIL-4 increased the number of myeloid and lymphoid DCs in spleen and lymph nodes.
Mice were treated with rGM-CSF (GM) or the combination of rGM-CSF/rIL-4 (GM/IL-4), as described for Figure 1. Spleen cells (A) and pooled axillary and inguinal lymph nodes cells (B) cells were isolated, and FACS analysis was performed to determine the percentage of cells expressing the myeloid DC (CD11c+/CD11b+) or the lymphoid DC (CD11c+/CD118α+) phenotype. Percentages were multiplied by the number of total spleen or lymph node cells to determine the total spleen or lymph node cell number expressing each phenotype. Data represent the mean ± SE of 3 experiments. *P ≤ .05 compared with control.
rGM-CSF increased the total number of myeloid DCs in mouse spleen, whereas the combination of rGM-CSF/rIL-4 increased the number of myeloid and lymphoid DCs in spleen and lymph nodes.
Mice were treated with rGM-CSF (GM) or the combination of rGM-CSF/rIL-4 (GM/IL-4), as described for Figure 1. Spleen cells (A) and pooled axillary and inguinal lymph nodes cells (B) cells were isolated, and FACS analysis was performed to determine the percentage of cells expressing the myeloid DC (CD11c+/CD11b+) or the lymphoid DC (CD11c+/CD118α+) phenotype. Percentages were multiplied by the number of total spleen or lymph node cells to determine the total spleen or lymph node cell number expressing each phenotype. Data represent the mean ± SE of 3 experiments. *P ≤ .05 compared with control.
Localization of DCs by immunohistology
Spleens from control and cytokine-treated mice were stained with hematoxylin and eosin or 2-color immunocytochemistry for evaluation of cytokine-related changes on DC number and distribution (Figure4). Anti-CD11b was used to identify cells of myeloid origin, anti-DEC-205 to identify lymphoid DCs, anti-CD11c to detect total DCs, and anti-Igβ to detect B cells.
Histology and immunohistology of DC subsets.
Magnification, × 20. (top panel) Hematoxylin and eosin. Cytokine therapy resulted in a mixed cellular infiltrate and distortion of normal follicle structure. (middle panel) CD11c (brown) and CD11b (blue) immunostain. Treatment with either GM alone or with GM/IL-4 resulted in enlarged and somewhat disorganized follicles (arrows). GM increased the number of CD11b+ and CD11c+ cells distributed throughout the white pulp (W) and marginal zones. GM/IL-4 preferentially increased the CD11c+ population with a marked localization to the white pulp. (bottom panel) DEC-205 (brown) and Igβ (blue) immunostain. Igβ identifies B cells and highlights the enlarged follicles in cytokine-treated mice. Control mice demonstrated light staining for DEC-205 within follicle centers. GM therapy was associated with an increase in DEC-205 staining, consistent with the increase in follicle size. In contrast, rGM/rIL-4 resulted in intense staining of tightly packed DEC-205+ cells within the centers of follicles.
Histology and immunohistology of DC subsets.
Magnification, × 20. (top panel) Hematoxylin and eosin. Cytokine therapy resulted in a mixed cellular infiltrate and distortion of normal follicle structure. (middle panel) CD11c (brown) and CD11b (blue) immunostain. Treatment with either GM alone or with GM/IL-4 resulted in enlarged and somewhat disorganized follicles (arrows). GM increased the number of CD11b+ and CD11c+ cells distributed throughout the white pulp (W) and marginal zones. GM/IL-4 preferentially increased the CD11c+ population with a marked localization to the white pulp. (bottom panel) DEC-205 (brown) and Igβ (blue) immunostain. Igβ identifies B cells and highlights the enlarged follicles in cytokine-treated mice. Control mice demonstrated light staining for DEC-205 within follicle centers. GM therapy was associated with an increase in DEC-205 staining, consistent with the increase in follicle size. In contrast, rGM/rIL-4 resulted in intense staining of tightly packed DEC-205+ cells within the centers of follicles.
Hematoxylin and eosin sections from control mice demonstrated well-organized follicular structures with normal distributions of red and white pulp. In cytokine-treated mice, these structures were disrupted by a diffuse infiltration of mononuclear leukocytes. High-power examination also revealed increased stromal tissue in cytokine-treated mice (not shown).
Dual staining for CD11b and CD11c (Figure 4, middle panel) demonstrated several important features. First, it suggested the presence of enlarged follicular structures in the spleens of cytokine-treated mice that were not discerned by simple hematoxylin and eosin stain. This was confirmed by staining for Igβ (Figure 4, lower panel). In control mice, CD11c+ DCs were present in small numbers and were distributed in the peri-arteriolar lymphoid sheaths (PALS) of the white pulp (W) and in the marginal zones. In response to rGM-CSF, there was a striking increase in the CD11c+ population at these sites and extension of cells into the red pulp. The pattern in mice treated with rGM-CSF/rIL4 was distinct. Cells intensely stained for CD11c+ were concentrated within the center of follicular structures, with only rare CD11c+ cells observed in the marginal zones. In contrast, CD11b+ cells were predominantly distributed within the marginal zones of all mice, regardless of cytokine treatment, but were increased in number in response to rGM-CSF. The DEC-205 marker was observed within the PALS area of white pulp and, to a lesser degree, in the B-cell areas of control spleen. Tissue sections from rGM-CSF–treated mice demonstrated hypertrophic PALS containing DEC-205+ cells and the infiltration of DEC205+ cells into B-cell regions. The changes observed in response to rGM-CSF alone were magnified by the addition of rIL-4, resulting in intense staining and clustering of DEC-205+ cells in PALS and within B-cell areas. As such, immunohistology supported the findings obtained by FACS analysis and demonstrated distinct effects of rIL-4 on the distribution of DC within T- and B-cell areas of the spleen.
Combined rGM-CSF/rIL-4 up-regulated MHC expression and antigen uptake by splenic DCs
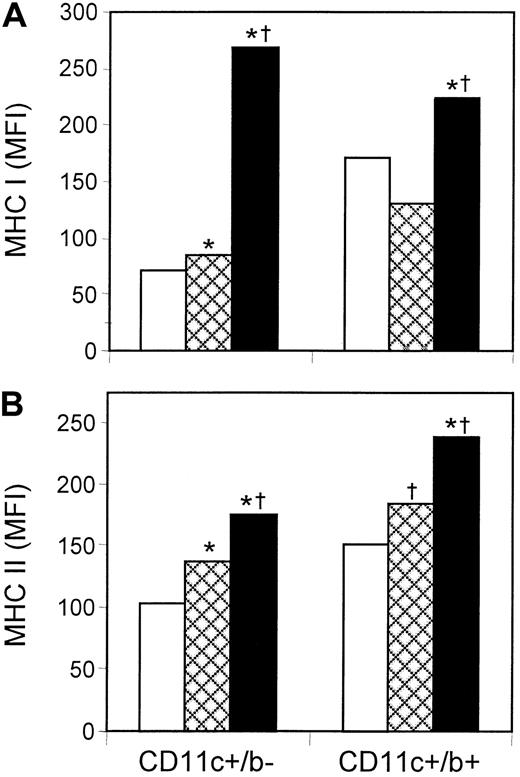
Expression of MHC class I and class II is essential for antigen-presenting activity, and it increases as DCs mature. Flow cytometry was used to determine the mean florescence intensity (MFI) of MHC class I and class II expression on lymphoid (CD11c+/CD8α+) and myeloid (CD11c+/CD11b+) DCs from control and cytokine-treated mice (Figure 5). The most dramatic effects occurred with MHC class I, which did not increase after treatment with rGM-CSF alone but increased significantly after treatment with rGM-CSF/rIL-4. These effects were most prominent on lymphoid DCs, where MHC class I expression increased by 3- to 4-fold compared with myeloid DC, where expression increased by only 30% to 75%. The response pattern was different with respect to MHC class II, which increased moderately in both DC subsets in response to rGM-CSF and increased further in response to rGM-CSF/rIL-4. The up-regulation of MHC class I and MHC class II, in response to systemic rGM-CSF/rIL-4, suggests cytokine-induced maturation in vivo.
rGM-CSF/rIL-4 increased the expression of MHC class I, whereas rGM-CSF and rGM-CSF/IL-4 increased the expression of MHC class II on splenic DCs.
Mice were treated with rGM-CSF (GM) or the combination of rGM-CSF/rIL-4 (GM/IL-4), as described for Figure 1. Spleen cells were stained with fluorescent mAb to CD11c and CD8α (lymphoid DCs) or CD11c and CD11b (myeloid DCs) and were examined for the expression of MHC class I (A) or MHC class II (B). Only GM/IL-4 significantly increased the expression of MHC class I on lymphoid and myeloid DC subsets. In contrast, both GM and GM/IL-4 significantly increased the expression of MHC class II, with the highest expression on cells from mice treated with GM/IL-4. Data from a representative experiment (n = 5). *P ≤ .05 compared with control; †P ≤ .05 compared with GM. □ indicates control; , GM; ■, GM/IL-4.
rGM-CSF/rIL-4 increased the expression of MHC class I, whereas rGM-CSF and rGM-CSF/IL-4 increased the expression of MHC class II on splenic DCs.
Mice were treated with rGM-CSF (GM) or the combination of rGM-CSF/rIL-4 (GM/IL-4), as described for Figure 1. Spleen cells were stained with fluorescent mAb to CD11c and CD8α (lymphoid DCs) or CD11c and CD11b (myeloid DCs) and were examined for the expression of MHC class I (A) or MHC class II (B). Only GM/IL-4 significantly increased the expression of MHC class I on lymphoid and myeloid DC subsets. In contrast, both GM and GM/IL-4 significantly increased the expression of MHC class II, with the highest expression on cells from mice treated with GM/IL-4. Data from a representative experiment (n = 5). *P ≤ .05 compared with control; †P ≤ .05 compared with GM. □ indicates control; , GM; ■, GM/IL-4.
Flow cytometry was also used to examine the effects of cytokine therapy on endocytosis (uptake of FITC-dextran) and macropinocytosis (uptake of Lucifer yellow). Similar to the effects on MHC class I, the systemic administration of rGM-CSF did not increase the pinocytotic activity of either myeloid (CD11c+/CD11b+) or nonmyeloid (CD11c+/CD11b−) DCs, but rGM-CSF/rIL-4 increased uptake by both subsets (Figure6). Similar to the effects of in vivo cytokines on the expression of MHC class II, there was a modest but significant increase in endocytotic activity by all DC subsets in response to rGM-CSF and another increase after the addition of rIL-4. This up-regulation of endocytosis and pinocytosis after systemic rGM-CSF/rIL-4 suggests cytokine-induced differentiation in vivo.
rGM-CSF and rGM-CSF/rIL-4 increased antigen capture by splenic DCs.
DC-enriched splenocytes from control and cytokine-treated mice were incubated with 1 mg/mL FITC-dextran or Lucifer yellow for 1 hour at 37°C, stained with CD11c and CD11b to identify DC subsets, and analyzed by FACS for receptor-mediated endocytosis of FITC-dextran (A) or pinocytosis of Lucifer yellow (B), as determined by mean fluorescence intensity (MFI). Uptake at 37°C is shown in the upper bars, and uptake of cells incubated at 4°C is demonstrated by the lower bars. Both DC subsets responded to in vivo GM and GM/IL-4 with significant increases in endocytosis; the greatest response was observed in GM/IL-4–treated mice. In contrast, only DCs from GM/IL-4–treated mice demonstrated increased pinocytosis. Data are from a representative experiment (n = 5). *P ≤ 0.05 compared with control; †P ≤ .05 compared with GM. □ indicates control; , GM; ■, GM/IL-4.
rGM-CSF and rGM-CSF/rIL-4 increased antigen capture by splenic DCs.
DC-enriched splenocytes from control and cytokine-treated mice were incubated with 1 mg/mL FITC-dextran or Lucifer yellow for 1 hour at 37°C, stained with CD11c and CD11b to identify DC subsets, and analyzed by FACS for receptor-mediated endocytosis of FITC-dextran (A) or pinocytosis of Lucifer yellow (B), as determined by mean fluorescence intensity (MFI). Uptake at 37°C is shown in the upper bars, and uptake of cells incubated at 4°C is demonstrated by the lower bars. Both DC subsets responded to in vivo GM and GM/IL-4 with significant increases in endocytosis; the greatest response was observed in GM/IL-4–treated mice. In contrast, only DCs from GM/IL-4–treated mice demonstrated increased pinocytosis. Data are from a representative experiment (n = 5). *P ≤ 0.05 compared with control; †P ≤ .05 compared with GM. □ indicates control; , GM; ■, GM/IL-4.
Allostimulatory capacity is primed by in vivo exposure to rGM-CSF and rGM-CSF/rIL-4
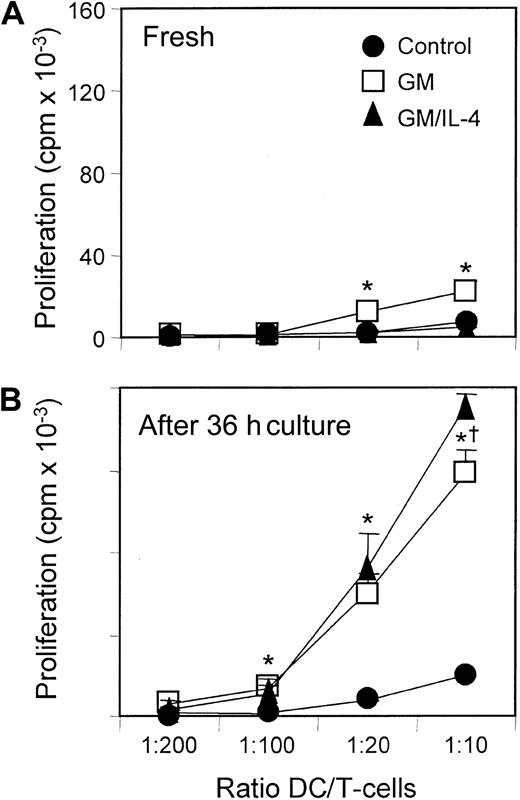
Purified DCs from the spleens of control and cytokine-treated mice were tested for their ability to stimulate allogenic T cells in a one-way MLR (Figure 7). When tested fresh, without a period of in vitro culture, only DCs from rGM-CSF–treated mice produced significant T-cell proliferation above that of DCs from control spleens. However, when DCs from the different groups were placed in culture for 24 to 36 hours with 20 ng/mL rGM-CSF and rIL-4, there was a dramatic increase in stimulatory activity from those previously exposed to cytokines in vivo. T-cell proliferation increased approximately 8-fold in response to in vivo rGM-CSF and 10-fold in response to in vivo rGM-CSF/rIL-4, with the response consistently higher in the rGM-CSF/rIL-4 group (P ≤ .05). Taken together, these results suggest that rGM-CSF and the combination of rGM-CSF/rIL-4 increased the number of DCs and primed them in vivo for enhanced T-cell stimulatory activity.
Ability to stimulate allogeneic T-cell proliferation increased in spleen cells from mice treated with either rGM-CSF or rGM-CSF/rIL-4.
Spleen cells from control and cytokine-treated C57BL/6 mice were enriched for DCs by depleting B cells and T cells with antibody and complement. DC-enriched populations were added directly to microwells containing 1 × 105 allogeneic T cells (A) or were cultured for 36 hours in vitro with rGM-CSF and rIL-4 (20 ng/mL each) before testing in the MLR assay (B). DCs from GM mice stimulated greater T-cell proliferation, and this capacity increased with in vitro culture. In contrast, DCs from GM/IL-4 mice demonstrated control levels of allostimulatory activity when directly added to the MLR assay but dramatically higher activity after in vitro culture. Data are the mean ± SE of triplicate cultures and are representative of 5 separate experiments. *P ≤ .05 compared with control. †P ≤ .05 compared with GM.
Ability to stimulate allogeneic T-cell proliferation increased in spleen cells from mice treated with either rGM-CSF or rGM-CSF/rIL-4.
Spleen cells from control and cytokine-treated C57BL/6 mice were enriched for DCs by depleting B cells and T cells with antibody and complement. DC-enriched populations were added directly to microwells containing 1 × 105 allogeneic T cells (A) or were cultured for 36 hours in vitro with rGM-CSF and rIL-4 (20 ng/mL each) before testing in the MLR assay (B). DCs from GM mice stimulated greater T-cell proliferation, and this capacity increased with in vitro culture. In contrast, DCs from GM/IL-4 mice demonstrated control levels of allostimulatory activity when directly added to the MLR assay but dramatically higher activity after in vitro culture. Data are the mean ± SE of triplicate cultures and are representative of 5 separate experiments. *P ≤ .05 compared with control. †P ≤ .05 compared with GM.
Administration of rGM-CSF/rIL4, but not rGM-CSF alone, enhances the response to an adenoviral-based vaccine and promotes antigen-specific tumor responses in vivo
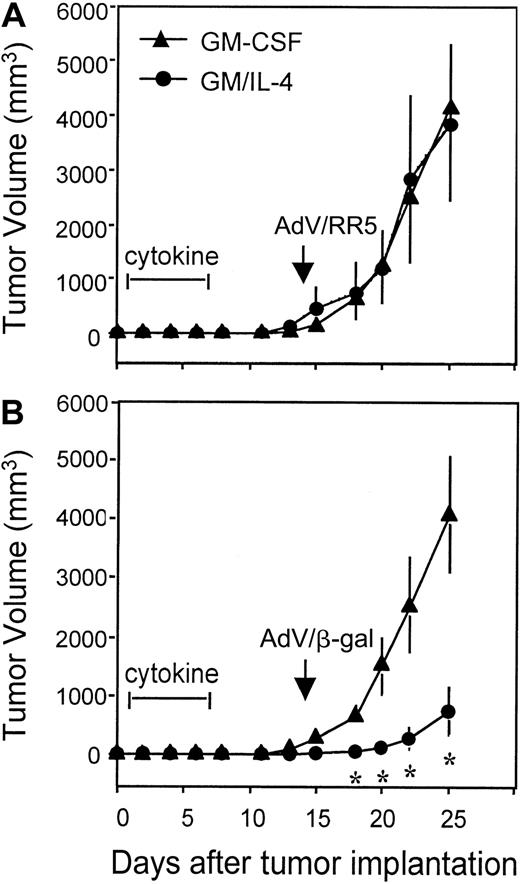
To access the functional impact of cytokine therapy (rGM/CSF vs rGM-CSF/rIL-4) on antigen-presentation and antitumor immunity in vivo, an established tumor immunotherapy model was developed. E-22 tumor cells, expressing β-gal as a model tumor antigen, developed into solid tumors in 100% of mice treated with subcutaneous injection of 1 × 105 cells. Pre-immunization with a single intraperitoneal administration of 109 PFU of AdV/β-gal, but not the control vector (AdV/RR5), imparted 100% protection from subsequent tumor challenge and generated tumor-specific immunity (data not shown). However, in mice with established tumors, vaccination with 109 PFU of AdV/β-gal only slowed tumor progression, and vaccination with 108 PFU had no detectable effect (data not shown). To evaluate the role of cytokines in enhancing the vaccine response, mice were injected with tumor and, 1 day later, were treated with saline, rGM-CSF, or rGM-CSF/rIL-4 by mini-osmotic pump. Subsequent vaccination with 108 PFU of AdV/β-gal significantly reduced the rate of tumor growth only in mice that had been treated with rGM-CSF/IL-4 (Figure 8B). The response in mice pretreated with rGM-CSF was identical to that in saline-treated controls (not shown), yielding no detectable effect on tumor growth. The antigen-specific nature of the response was confirmed in animals immunized with AdV/RR5 (Figure 8A). Vaccination with this control vector, lacking β-gal, produced no effect on the growth of E-22. In vitro cocultures of cytokines and tumor cells were carried out to evaluate whether combined rGM-CSF/rIL-4 had a direct cytotoxic or cytostatic effect on the growth of E-22. No effect on cell growth (as assessed by cell number and thymidine uptake) was observed in the presence of cytokines in the range of 0.1 ng/mL to 1μg/mL. These results suggest that pretreatment with rGM-CSF/rIL-4 enhances the vaccine response and stimulates antigen-specific, antitumor immunity in vivo.
Systemic rGM-CSF/rIL-4 enhanced the response to an adenoviral-based vaccine and led to antigen-specific retardation in tumor growth.
Mice were inoculated subcutaneously in the flank with 1 × 105 E-22 mouse thymoma cells that expressed theLacZ gene. Twenty-four hours later, osmotic pumps were implanted to deliver 7-day administration of either saline, rGM-CSF, or the combination of rGM-CSF/rIL-4 (10 μg each cytokine per day). Fourteen days after tumor inoculation, mice were immunized by intraperitoneal injection with 1 × 108 PFU of an adenoviral vector expressing either the LacZ transgene (ADV/β-gal) (B) or no transgene (AdV/RR5) (A). Tumor sizes (mm3) were measured twice a week, and data were presented as mean tumor size ± SE for 5 mice per group. *P < .05 compared with mice treated with either saline (not shown) or rGM-CSF. Tumor growth in mice treated with saline (not shown) was identical to that in mice receiving rGM-CSF. Representative experiment (n = 2).
Systemic rGM-CSF/rIL-4 enhanced the response to an adenoviral-based vaccine and led to antigen-specific retardation in tumor growth.
Mice were inoculated subcutaneously in the flank with 1 × 105 E-22 mouse thymoma cells that expressed theLacZ gene. Twenty-four hours later, osmotic pumps were implanted to deliver 7-day administration of either saline, rGM-CSF, or the combination of rGM-CSF/rIL-4 (10 μg each cytokine per day). Fourteen days after tumor inoculation, mice were immunized by intraperitoneal injection with 1 × 108 PFU of an adenoviral vector expressing either the LacZ transgene (ADV/β-gal) (B) or no transgene (AdV/RR5) (A). Tumor sizes (mm3) were measured twice a week, and data were presented as mean tumor size ± SE for 5 mice per group. *P < .05 compared with mice treated with either saline (not shown) or rGM-CSF. Tumor growth in mice treated with saline (not shown) was identical to that in mice receiving rGM-CSF. Representative experiment (n = 2).
Discussion
Various differentiation and maturation signals have been used to generate DCs in vitro, including GM-CSF for stimulating murine progenitors from bone marrow or spleen,16-18 GM-CSF in combination with IL-4 for stimulating human and murine precursors,7,8,10-12,26,27 or these cytokines in combination with a variety of accessory stimuli, such as tumor necrosis factor-α (TNF-α), lipopolysaccharide (LPS), CD40-ligand, or flt3-ligand.2-9 Of these approaches, the combination of rGM-CSF/rIL-4 has emerged as one of the most effective and frequently used. In vitro, rGM-CSF plays a primary role in precursor survival, proliferation, and early differentiation, whereas IL-4 promotes final differentiation along a DC pathway.28,29 IL-4 promotes the loss of macrophage features, increases MHC expression, up-regulates costimulatory molecules, enhances antigen uptake and processing, and synergizes with other factors to increase IL-12 production.7,8,29 30 In the current study, we expanded on these in vitro observations and demonstrated similar synergism between rGM-CSF and rIL-4 when they were used to generate DCs in mice in vivo.
An initial challenge in performing these studies was the short half-life of rGM-CSF and rIL-4 when administered to mice in vivo. In contrast to humans, in whom sustained cytokine levels persist for up to 8 to 12 hours after a single subcutaneous injection,14,15,31 the half-lives for rGM-CSF and rIL-4 in the mouse are only 11 minutes20 and 5 minutes,32 respectively. These pharmacokinetics likely explain why an earlier study, in which mice were treated with 10 μg/d GM-CSF and IL-4 as a single daily injection, failed to elicit significant increases in DC.5 Others have approached this problem by implanting gene-modified tumor cells secreting one or both cytokines.19,22,23 These models, though delivering a complex mixture of cytokines and other tumor-derived factors, confirmed that in vivo exposure to GM-CSF alone, or in combination with IL-4, can increase the number of functional DCs. In fact, several studies demonstrated greater induction of antitumor immunity when both cytokines were administered together. Recently, Daro et al20 successfully administered polyethylene-glycol modified GM-CSF (pegGM-CSF) as a mechanism for generating DCs in vivo. To extend this line of investigation and to study the combined effects of GM-CSF/IL-4, we administered unmodified cytokines by miniature osmotic pumps. When implanted subcutaneously, these pumps release cytokine(s) at a continuous rate for up to 1 to 2 weeks.33 34 This technique allowed us to examine the effects of GM-CSF and IL-4 in the absence of other factors, such as tumor burden.
Using this approach, systemic rGM-CSF–produced marked splenic hypertrophy with increases in most cell lineages including DCs, monocytes, granulocytes, B cells, and T cells. Within the DC population, rGM-CSF expanded only myeloid DCs, identified by their co-expression of CD11c and CD11b and their lack of expression of CD8α.5,35 These DCs demonstrated modest increases in MHC expression and endocytotic activity in comparison with myeloid DCs from control mice, and they expressed significantly more of these features than did lymphoid DCs. These findings are essentially identical to those reported in mice treated with pegGM-CSF or implanted with irradiated tumor cells secreting rGM-CSF.19,20 However, it is interesting that these results differ from those reported in cancer patients treated with rGM-CSF.13 In humans, the systemic administration of rGM-CSF increases the number of circulating monocytes but does not increase DC number or promote circulating monocytes to acquire a DC phenotype or function in vivo.13 Although these human studies did not examine GM-CSF for its effects on spleen, mouse studies suggest that cytokine-induced changes in DC should be observed in the circulation.20 In vitro studies also suggest that GM-CSF alone can be used to generate murine DC,17,18,26 whereas human DCs require additional differentiation factors such as IL-4.3,7,11 36
In contrast to rGM-CSF, the administration of rIL-4 as a single agent did not increase spleen cellularity or DC number (data not shown). This corresponds to in vitro studies in which IL-4 alone failed to induce the proliferation or differentiation of myeloid progenitors.7,37,38 When rIL-4 was administered in combination with rGM-CSF, spleen cellularity increased significantly, but not to the degree observed with rGM-CSF alone. This finding is consistent with prior studies in which IL-4 partially suppressed hematopoietic stem cell proliferation in response to GM-CSF.28 39 However, though limiting the expansion of T cells and B cells, GM-CSF/IL-4 still allowed DCs to increase by approximately 6-fold compared with control spleens. Taken together, these facts suggest a more targeted effect on DC proliferation and differentiation than occurs with GM-CSF alone.
In addition to increasing DC number, rGM-CSF/rIL-4 promoted a more balanced expansion of myeloid and lymphoid DCs than observed with rGM-CSF alone. Similar to other reports,20 we found that myeloid DCs predominate de novo in the spleen at a ratio of approximately 1.5:1 compared with lymphoid DCs and that this ratio increased to roughly 7:1 in response to the effects of GM-CSF on the myeloid subset. By comparison, exposure to rGM-CSF/rIL-4 increased the number of myeloid DCs in spleen tissue by approximately 6-fold and the number of lymphoid DCs by approximately 3.7-fold, resulting in a more balanced myeloid-to-lymphoid ratio. A similar pattern was observed in lymph nodes, in which the combination of rGM-CSF/rIL-4 resulted in a greater expansion of the lymphoid DC subset than occurred with rGM-CSF alone. Although treatment with flt3-ligand also increases both DC populations, it favors the expansion of lymphoid DCs in spleen by approximately 2:1 over myeloid DCs.5,6,20 25
In untreated mice, myeloid and lymphoid DCs are present in scant numbers and occupy distinct microenvironments within the spleen.6,40,41 Lymphoid DCs primarily reside in T-cell areas of the white pulp, whereas myeloid DCs are distributed throughout the marginal zones with some extension into the red pulp. In response to rGM-CSF in vivo, a striking splenic enlargement develops with a diffuse increase in CD11c+ DCs throughout all regions, including a significant increase in DEC-205+ cells infiltrating into the T-cell–enriched PALS. These effects are similar to those described for flt3-ligand6 and IL-1242and suggest a general response pattern to dendropoietic factors. However, in response to rGM-CSF/rIL-4, DCs expressing CD11c and CD11b and those expressing DEC-205 became highly concentrated within enlarged PALS. This localization within T-cell zones and the distinct increase in staining intensity observed in response to rGM-CSF/rIL-4 are similar to the changes that occur in mice treated with lipopolysaccharide in vivo.40 These changes are also reminiscent of the homing that occurs when activated DCs migrate from the periphery to lymphoid tissue.43 In contrast to other dendropoietic factors, these immunohistology results suggest that combined treatment with rGM-CSF/rIL-4 produces the proliferation and mobilization of DCs and some degree of activation and localization to T-cell–enriched areas.
Considerable controversy exists regarding the relative origins and roles of myeloid versus lymphoid DCs. Although it has been reported that CD11c+/CD8α+ DCs closely resemble thymic DCs and are derived from lymphoid-committed precursors,5,44-46 others have reported that myeloid-committed precursors give rise to both DC subsets in vivo and that myeloid DCs acquire the expression of CD8α when trafficking from the periphery to regional lymph nodes.43,47 As such, it is difficult to conclude anything from our experiments about the types of progenitors that respond to GM-CSF/IL-4 in vivo. Further studies are warranted to investigate this. Similar controversy exists about the functional impact of the up-regulation of myeloid versus lymphoid DCs. In the thymus, CD8α+ DCs play a role in clonal deletion, yet when CD11c/CD8α+ DCs are purified from flt3-ligand–treated mice, they produce high quantities of IL-12 and stimulate strong Th1 responses.25,48 In contrast, myeloid DCs produce less IL-12 and stimulate a mixed Th1–Th2 T-cell response.6,25 When directly compared for their ability to stimulate antitumor immunity in vivo, tumor cells secreting rGM-CSF were more potent than tumor cells secreting recombinant flt3-ligand.49 This difference correlated with the induction of exclusively myeloid DCs and a mixed Th1–Th2 response in GM-CSF–exposed animals versus a lymphoid-predominant DC response that stimulated exclusively Th1 cytokines in mice exposed to flt3-ligand. However, the co-administration of tumor cells secreting GM-CSF and IL-4 was found to produce more potent antitumor responses than mice inoculated with GM-CSF–secreting tumors alone.50 In addition, when a combination of GM-CSF/IL-4 was administered to human subjects at 2-week intervals, antitumor responses were observed in patients with metastatic prostate cancer.13
We also examined GM-CSF/IL-4 for evidence that it altered the differentiation or activation state of resultant DC. Two clear effects were observed. GM-CSF/IL-4 increased the expression of MHC class I and MHC class II on myeloid and lymphoid DCs and significantly increased their capacity for endocytosis and macropinocytosis. These changes closely recapitulate the effects when GM-CSF and IL-4 are used together in vitro7,8 22 and suggest that combined GM-CSF/IL-4 results in greater maturation along a DC pathway. Despite these changes, freshly isolated DCs from both treatment groups were still functionally immature when tested immediately in an MLR assay. Their full T-cell stimulatory activity was not observed until after a short-term in vitro culture. We conclude from these experiments that though IL-4 promotes greater differentiation along a DC pathway, it does not promote the terminal activation or maturation required for them to function as potent T-cell activators. Additional stimulatory activity might be needed in the form of antigen or pathogen (virus, bacteria, parasite) for the induction of a second signal to induce full maturation of these DCs for vaccination strategy.
Finally, we examined the in vivo administration of rGM-CSF and rGM-CSF/rIL-4 for their ability to enhance the response to a vaccine and to stimulate antigen-specific antitumor immunity. Neither rGM-CSF alone nor the combination of rGM-CSF/rIL-4 produced antitumor effects in a model of established tumor growth (data not shown). We hypothesized that DCs generated in response to these cytokines lacked access to the appropriate tumor antigens required for initiating an antitumor response. To test this hypothesis, mice that had been inoculated with tumor were pretreated with rGM-CSF or with combination rGM-CSF/rIL-4 and were vaccinated with an adenoviral vector expressing a model tumor-specific transgene (β-gal). In this setting, combined therapy with rGM-CSF/rIL-4 followed by antigen-specific vaccination resulted in a significant and reproducible attenuation of tumor growth. The same regimen, but using rGM-CSF alone or an empty adenoviral-vector lacking the tumor antigen transgene (AdV/RR5), was ineffective at generating any antitumor response. These results indicate that rGM-CSF/rIL-4 is more efficient than rGM-CSF alone for stimulating an antitumor response and that the administration of a tumor-specific antigen is required to direct the effects of rGM-CSF/rIL-4 against the tumor. These results also lead us to speculate that DCs generated in vivo by rGM-CSF/rIL-4, which express higher levels of MHC and more avidly sample the environment for antigens and which relocate to T-cell–enriched areas of spleen, are more effective than their rGM-CSF–generated counterparts in responding to an antigen challenge and stimulating T-cell responses. These findings agree with earlier studies in which a mixture of tumor cell line(s) secreting rGM-CSF and rIL-4 generated more effective antitumor responses than did the same number of tumor cells secreting only rGM-CSF.22,23 It was also recently reported that tumors secreting rGM-CSF and rIL-4, but not rGM-CSF alone, induce bone marrow mononuclear cells capable of transferring immunity to syngenic mice.50 This response was associated with a higher expression of higher DEC-205+and CD11c+ cells than observed after treatment with rGM-CSF–secreting tumors, suggesting that a more balanced expansion of lymphoid and myeloid DCs might play a role in this difference.
In summary, our studies demonstrate a synergism between rIL-4 and rGM-CSF, when administered in vivo, similar to that previously reported for these 2 cytokines when used to activate DC precursors in vitro. In contrast to GM-CSF alone, which promoted a broad-spectrum increase in spleen cells of all types, the combination of GM-CSF/IL-4 more selectively expanded the number of DCs, including myeloid and lymphoid subsets. DCs generated in response to GM-CSF/IL-4 expressed higher levels of MHC and were capable of greater antigen uptake and T-cell stimulatory activity. However, as experienced in vitro, additional activation is required for these cells to express their full potency. Finally, we demonstrate that the differences generated in response to treatment with rGM-CSF/rIL-4, compared with rGM-CSF alone, enhanced the animal's response to an adenoviral-based vaccination and induced antitumor immunity. Further studies are indicated to determine the types of precursors that respond to rGM-CSF/rIL-4 and the impact of this therapy on immune responsiveness mediated by different DC subpopulations in vivo. The striking similarity between the effects we observed in mice and those previously reported in humans13suggest that the infusion of rGM-CSF/rIL-4 by miniature osmotic pumps may provide an important model for developing and testing DC-based immunization strategies.
Flow cytometry was performed in the UCLA Jonsson Comprehensive Cancer Center and Center for AIDS Research Flow Cytometry Core Facility, which is supported by National Institute of Health awards CA-16042 and AI-28697.
Supported by grants from the Tobacco-Related Disease Research Program of California (#7RT-0040), the Parker B. Francis Foundation (S.K.B.), the Jonsson Comprehensive Cancer Center/UCLA, and the National Institutes of Health/National Cancer Institute (CA 85686, IP50 CA90388) and by Merit Review Research Funds from the Department of Veteran Affairs.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Saroj K. Basak, Division of Pulmonary and Critical Care, Department of Medicine, UCLA School of Medicine, Los Angeles, CA 90095-1690; e-mail: sbasak@mednet.ucla.edu.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal