Abstract
In this study, we investigated the possibility of selective depletion of donor alloantigen-specific T cells from C57BL/6 (H-2b) mice to prevent graft-versus-host disease (GVHD). These cells were first activated with irradiated BALB/c (H-2d) host spleen cells in a 5-day mixed lymphocyte culture. Following this activation, a photoactive rhodamine derivative called 4,5-dibromorhodamine 123 (TH9402), was added. This compound is selectively retained in the mitochondria of activated host-reactive cells but not tumor- or third-party–specific resting cells. The treated cells were subsequently exposed to visible light (514 nm) to deplete the TH9402-enriched activated host-reactive cells. Treatment with photodynamic cell purging process (PDP) inhibited antihost responses measured by cytotoxic T lymphocytes (CTL) by 93%, and interferon-γ production by 66%. By contrast, anti-BCL1 (BALB/c-origin leukemia/lymphoma) and anti–third-party C3H/HeJ (H-2k) responses were preserved. PDP-treated primed C57BL/6 cells were further tested in vivo. All lethally irradiated BALB/c mice inoculated with BCL1 cells and T-cell–depleted bone marrow cells developed leukemia by day +30, with 50% mortality by 100 days. All mice died of GVHD after addition of 5 × 106untreated primed C57BL/6 cells. However, addition of same numbers of PDP-treated cells allowed 90% of the recipients to survive more than 100 days without detectable BCL1 tumor cells and free of GVHD. Moreover, PDP-treated primed C57BL/6 cells retained the ability to induce GVHD in the third-party C3H/HeJ mice. These data suggest that PDP can selectively deplete host alloantigen-specific T cells for GVHD prevention and immune and antileukemia function preserve.
Introduction
Allogeneic bone marrow transplantation (BMT) has long been recognized as a curative treatment for a variety of hematopoietic malignancy diseases.1 Unfortunately, graft-versus-host disease (GVHD) remains a major source of morbidity and mortality. Although GVHD can be prevented by removal of T cells, there is a significant increase in relapses in recipients of T-cell–depleted grafts, demonstrating the importance of donor mature T-cell–provided graft-versus-leukemia (GVL) effect.2 GVL effect may be mediated by both host histocompatibility antigen–specific T cells, which also mediate GVHD, and tumor antigen–specific T cells, which do not induce GVHD.3,4 In principle, GVL effect can be separated from GVHD by selectively depleting host histocompatibility antigen–specific T cells while sparing tumor antigen–specific T cells. Host histocompatibility antigen–specific T cells activated by the host antigens ex vivo have been successfully depleted or tolerized without impairing antileukemia or anti–third-party effect by targeting host-activated T cells.5-14 A similar strategy has also been used to prevent GVHD and graft rejection in mice in vivo.15However, it is not clear whether this strategy will preserve GVL effect and anti–third-party responses in vivo.
One potential approach is to use the photoactive rhodamine derivative called 4,5-dibromorhodamine 123 (TH9402), which is selectively retained in the mitochondria of activated cells.16 After exposure to visible light (514 nm), the cells incorporating with TH9402 are killed after oxidation of mitochondria. This procedure, termed photodynamic cell purging process (PDP), has been successfully used to purge chronic myelogenous leukemia cells,16,17 non-Hodgkin lymphoma,18 and chronic lymphocytic leukemia18in autologous bone marrow grafts. The ability of PDP to selectively eliminate activated cells could potentially allow one to separate GVHD from GVL. The photosensitizer TH9402 diffuses into both resting and activated T lymphocytes. Resting T lymphocytes pump this dye out of the cells rapidly in the incubation period in the presence of the dye because they have the active multidrug transporter (P-gp 170), while the concentration of TH9402 remains high in activated T lymphocytes due to inactivation of the multidrug transporter.19 20 Thus, when the mixture of resting and activated T lymphocytes is exposed to visible light, only activated cells are killed. In the case of the spleen cells primed by host antigens in mixed lymphocyte culture (MLC), only host antigen–specific T cells were activated and therefore killed after PDP treatment, while third-party antigen– and tumor antigen–specific resting T lymphocytes remained intact.
In this study, host histocompatibility antigen–specific T cells were first activated by host spleen cells in a 5-day MLC ex vivo. The primed cells were then treated with PDP in order to eliminate the T cells activated in the MLC. These cells were then tested for the responses against host, third-party targets, and tumor antigens in vitro and in vivo to determine whether host histocompatibility antigen–specific T cells were selectively and specifically eliminated.
Materials and methods
Animals
BALB/c (H2d, Mls-2a, Mls-3a), C57BL/6 (H2b, Mls-2b, Mls-3b), and C3H/HeJ (H2k, Mls-2b, Mls-3b) mice were purchased from The Jackson Laboratories (Bar Harbor, ME). The mice were all female and were 8 to 12 weeks of age at the time of use. The mice were kept in a specific pathogen-free facility throughout the study.
BCL1 cell lines
BCL1 is a spontaneous B-cell leukemia/lymphoma cell line of BALB/c origin expressing IgM λ surface immunoglobulin, and major histocompatibility complex (MHC) class I and II molecules.21 22 There were 2 cell lines used in this study: one was for in vitro assays, the other was for in vivo assays. The in vitro line was maintained in culture, whereas the in vivo line was maintained by serial passage in normal BALB/c mice. Both cell lines were kind gifts from Dr Defu Zeng and Dr Samual Strober.
Mixed lymphocyte culture for activation of host histocompatibility antigen–specific T cells
Responder spleen cells from C57BL/6 mice were plated with irradiated (20 Gy) spleen stimulator cells from BALB/c mice at a ratio of 1:2 in T150 culture flasks. Each flask contained a total volume of 120 mL. The cultures were incubated at 37°C and 5% CO2for 112 hours. Culture medium was RPMI 1640, supplemented with 10% prescreened fetal calf serum, 2 mM l-glutamine, 50 μM 2-mercaptoethanol, 100 U/mL penicillin, and 100 μg/mL streptomycin. This medium was used for all subsequent in vitro cultures except for PDP treatment in which ex vivo–15 medium without phenol red (BioWhittaker, Walkersville, MD) was used.
Photodynamic cell purging process
The primed cells after MLC were harvested and adjusted to 1 × 106/mL in ex vivo–15 medium without phenol red containing different concentrations of 4,5-dibromorhodamine 123 (TH9402; Theratechnologies, Montreal, QC, Canada). Cells were incubated for 40 minutes at 37°C. The cells were washed once and resuspended in ex vivo–15 medium without phenol red supplemented with 10% fetal calf serum in the absence of TH9402. Cells were then transferred to a T75 flask (30 mL/flask) and incubated for 90 minutes at 37°C. Immediately following the incubation, cells were exposed to 5 J/cm2visible light energy (514 nm) delivered by a scanning lamp device (Theratechnologies). Cells were washed 3 times and counted before use for the subsequent assays.
Cytotoxic T lymphocyte assay
For cytotoxic T lymphocyte (CTL) assay, graded numbers of responder cells were plated with 2.5 × 105 irradiated (20 Gy) stimulator cells in 96-well round-bottom plates. Plates were cultured in 37°C, 5% CO2 incubator for 48 hours (peak proliferation time for 5-day primed T cells). The cells were tested in situ for lysis of 51Cr-labeled 2-day Con A blast cells or BCL1 cells. After 4 hours of culture, supernatant was removed and mixed with a scintillation fluid (PerkinElmer Wallac, Gaithersburg, MD) before being counted in a liquid scintillation and luminescence counter (PerkinElmer Wallac). In each experiment, cultures were tested in parallel for lysis of irrelevant targets to ensure the specificity of killing. Spontaneous release was obtained by incubating target cells with stimulator cells only. Maximal release was obtained after treatment with 1% Igepal CA-630 (Sigma). The percentage of specific release was calculated as follows:
Enzyme-linked immunospot assay
The assay was performed using enzyme-linked immunospot (ELISPOT) kits from R&D Systems (Minneapolis, MN) according to the manufacturer's protocol. Graded numbers of responder cells were incubated with 5 × 105/well irradiated (20 Gy) BALB/c or C3H/HeJ cells or 2.5 × 105/well irradiated (100 Gy) BCL1 cells at 37°C and 5% CO2 for 42 hours in the microplates. The wells were washed 3 times before the detection anti–cytokine antibody was added. The microplates were then incubated at 4°C overnight. At the end of incubation, the plates were washed 3 times and then 100 μL streptavidin-AP was added into each well. After 2 hours of incubation at room temperature, the microplates were washed 3 more times and the substrate solution (BCIP/NBT chromogen) was added. The plates were further incubated in the dark at room temperature for one hour. The chromogen solution was then discarded from the microplates and the microplates were rinsed with distilled water. After the microplates were dry, the ELISPOTs, which represent the number of cytokine-secreting cells, were scored using an ImmunoSpot Series 1 Analyzer (Cellular Technology, Cleveland, OH).
GVHD and GVL models
For the GVHD model, 10 million donor T-cell–depleted bone marrow cells (< 0.08% mature T cells) along with T cells were injected intravenously via the tail vein into lethally irradiated same-party BALB/c (8.5 Gy) or third-party C3H/HeJ (9.5 Gy) recipients. For the GVL model, 10 million BCL1 cells were injected intraperitoneally into BALB/c mice 24 hours before lethal radiation. All experiments were performed according to the Duke University Institutional Animal Care and Use Committee guidelines. Mortality was recorded daily. Recipients were monitored daily for clinical signs of GVHD by body weight and extent of skin changes (hair loss and erythema).23 Skin biopsies (from the ear) for histologic evidence of GVHD were routinely taken on days +30, +70, and +100 after transplantation and when mice were moribund. For evidence of tumor growth, the presence of BCL1 cells was monitored in peripheral blood using an anti–BCL1 idiotype antibody24 on days +30, +70, and +100; biopsies were taken from the spleen and liver for evidence of BCL1 cells when the animals died.
Chimerism and BCL1 cell detection by flow cytometry
Blood (50 μL) was first treated with 1× FACS lysing solution (Becton Dickinson, San Jose, CA) to lyze red blood cells, and then stained with purified anti–BCL1 idiotype antibody (clone 6A5; a generous gift from Dr Ellen S. Vitetta) and fluorescein isothiocyanate (FITC)–labeled goat anti–rat IgG. After washing and blocking with rat IgG (Jackson ImmunoResearch Laboratories, West Grove, PA), the samples were further stained with phycoerythrin (PE)–conjugated anti–H-2Db (clone CTDb), PE/Texas red–conjugated anti-B220 (cloneRA3-6B2), tricolor-conjugated anti-CD4 (clone CT-CD4), and CD8 (clone CT-CD8a). The stained cells were analyzed using a Coulter Epics XL-MCL flow cytometer equipped with System II software (Beckman-Coulter, Miami, FL). All antibodies were purchased from Caltag (San Francisco, CA) except those indicated.
Histology
Specimens were fixed in 10% formalin, embedded in paraffin, and stained with hematoxylin-eosin. The slides were studied under light microscope.
Statistical analysis
Comparison between groups was done by analysis of variance. Survival data were analyzed by log rank test. All statistical analyses were done using Statview software (SAS Institute, Cary, NC).P values less than .05 were considered significant.
Results
Titration of TH9402 for PDP treatment using CTL assay
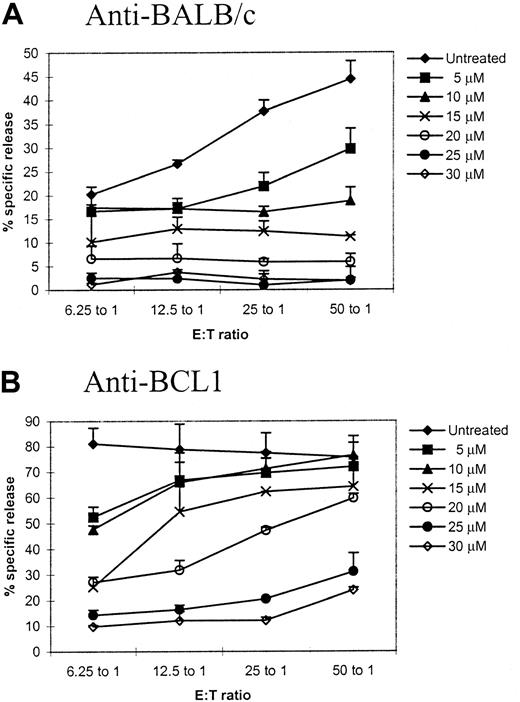
We first sought to determine the concentrations of TH9402, which could maximally eliminate host antigen–activated T cells (but minimally affect tumor antigen– and third-party–specific T cells). Primed T cells after a 5-day MLC were treated with PDP using various concentrations of TH9402 and then challenged with same-party spleen cells or BCL1 cells to test their responses against same-party or tumor antigens in a CTL assay. At an effector-to-target ratio of 50:1, anti-BALC/c response was inhibited by 87% (P = .001, compared with untreated control) and the killing against the BCL1 target remained 79% of the control (P < .05, compared with untreated control) when 20 μM of TH9402 was used (Figure1). Although the killing of BCL1 cells could be mediated by tumor antigen–specific CTLs and/or natural killer/lymphokine-activated killer cells, the concentration of 20 μM maximally eliminated host antigen–specific CTLs and in the meantime preserved the killing against the tumor cells and was therefore chosen for the subsequent assays, except where indicated.
Titration of TH9402 concentration for specific depletion of host-reactive T cells.
Spleen cells from C57BL/6 mice were first primed with BALB/c spleen cells in a 5-day mixed lymphocyte culture. The primed cells were then treated with photodynamic cell purging process using different concentrations of TH9402. After treatment, the cells were cultured with irradiated BALB/c or BCL1 stimulator. After 2 days, cytotoxicity against corresponding targets was tested in a standard 4-hour chromium release assay. Cytotoxicity against irrelevant targets was tested to ensure the specificity of the killing. The values represent mean plus SD of triplicates. This is a representative sample of 3 different experiments.
Titration of TH9402 concentration for specific depletion of host-reactive T cells.
Spleen cells from C57BL/6 mice were first primed with BALB/c spleen cells in a 5-day mixed lymphocyte culture. The primed cells were then treated with photodynamic cell purging process using different concentrations of TH9402. After treatment, the cells were cultured with irradiated BALB/c or BCL1 stimulator. After 2 days, cytotoxicity against corresponding targets was tested in a standard 4-hour chromium release assay. Cytotoxicity against irrelevant targets was tested to ensure the specificity of the killing. The values represent mean plus SD of triplicates. This is a representative sample of 3 different experiments.
Selective depletion of antigen-specific cytokine-secreting cells by PDP
Interferon-γ (IFNγ) is important in the pathogenesis of GVHD.25 We next sought to determine whether ex vivo treatment with PDP could eliminate antigen-specific IFNγ-secreting cells. The frequencies of IFNγ-secreting cells in response to same-party BALB/c and third-party C3H/HeJ were determined in PDP-treated (20 μM) primed cells using the ELISPOT assay. After PDP treatment, the frequencies of BALB/c-specific IFNγ-secreting cells were decreased 4.1-fold (P < .001), while the frequencies of third-party C3H/HeJ-specific IFNγ-secreting cells remained similar compared with untreated control (Table 1).
Effect of photodynamic cell purging process on antigen-specific interferon-γ secretion measured by enzyme-linked immunospot assay
| Groups . | Responder only . | Against BALB/c . | Against C3H/HeJ . | ||
|---|---|---|---|---|---|
| SPOTS . | SPOTS . | SI . | SPOTS . | SI . | |
| Naı̈ve C57BL/6 | 22 ± 11 | 96 ± 11 | 4.4 ± 0.5 | 63 ± 7 | 2.9 ± 0.3 |
| Untreated primed cells | 64 ± 64 | 2672 ± 68 | 36.6 ± 1.0 | 181 ± 103 | 2.5 ± 1.4 |
| PDP-treated primed cells | 73 ± 12 | 650 ± 42*,† | 10.2 ± 0.7*,† | 144 ± 11 | 2.3 ± 0.2 |
| Groups . | Responder only . | Against BALB/c . | Against C3H/HeJ . | ||
|---|---|---|---|---|---|
| SPOTS . | SPOTS . | SI . | SPOTS . | SI . | |
| Naı̈ve C57BL/6 | 22 ± 11 | 96 ± 11 | 4.4 ± 0.5 | 63 ± 7 | 2.9 ± 0.3 |
| Untreated primed cells | 64 ± 64 | 2672 ± 68 | 36.6 ± 1.0 | 181 ± 103 | 2.5 ± 1.4 |
| PDP-treated primed cells | 73 ± 12 | 650 ± 42*,† | 10.2 ± 0.7*,† | 144 ± 11 | 2.3 ± 0.2 |
Spleen cells from C57BL/6 mice were first primed with BALB/c spleen cells in a 5-day mixed lymphocyte culture. The primed cells were then treated with photodynamic cell therapy. After treatment, the cells were incubated with a variety of irradiated stimulators for 42 hours. Frequencies of antigen-specific IFNγ-secreting cells were determined by enzyme-linked immunospot (ELISPOT) assay. The values represent mean ± SD of triplicates per 106 spleen cells. The experiments were repeated twice. SI indicates stimulation index; PDP, photodynamic cell purging process.
P < .001, compared with untreated primed cells.
P < .05, compared with naı̈ve C57BL/6.
PDP treatment prevents anti–same-party GVHD but preserves anti–third-party GVHD
To further determine whether selective elimination of antigen-specific T cells by PDP treatment may be useful in controlling GVHD in vivo, PDP-treated cells were infused into lethally irradiated BALB/c (same-party) or C3H/HeJ (third-party) mice to determine whether GVHD could be induced. As illustrated in Figure2A,C, PDP-treated T cells primed with BALB/c antigens completely prevented GVHD in the BALB/c host. The recipients were healthy and survived more than 100 days after transplantation whereas all recipients of untreated primed T cells died within 30 days after transplantation (P < .0001). Full phenotypic recovery of immune cells (data not shown) further demonstrated that the recipients of PDP-treated primed T cells were free of acute or chronic GVHD.
Effect of photodynamic cell purging process on anti–same-party and anti–third-party graft-versus-host disease.
Spleen cells from C57BL/6 mice were first primed with irradiated (20 Gy) BALB/c spleen cells in a 5-day mixed lymphocyte culture. The primed cells were then treated with photodynamic cell purging process. After treatment, 5 million cells were infused into lethally irradiated BALB/c (same party, 8.5 Gy) or C3H/HeJ (third party, 9.5 Gy) mice together with 1 × 107 T-cell–depleted bone marrow cells from C57BL/6 mice. Each groups contained 4 to 10 animals. Similar experiments have been repeated twice with similar results. Panels A and C represent body weight and survival of same-party BALB/c recipients, respectively; panels B and D represent body weight and survival of third-party C3H/HeJ recipients, respectively.
Effect of photodynamic cell purging process on anti–same-party and anti–third-party graft-versus-host disease.
Spleen cells from C57BL/6 mice were first primed with irradiated (20 Gy) BALB/c spleen cells in a 5-day mixed lymphocyte culture. The primed cells were then treated with photodynamic cell purging process. After treatment, 5 million cells were infused into lethally irradiated BALB/c (same party, 8.5 Gy) or C3H/HeJ (third party, 9.5 Gy) mice together with 1 × 107 T-cell–depleted bone marrow cells from C57BL/6 mice. Each groups contained 4 to 10 animals. Similar experiments have been repeated twice with similar results. Panels A and C represent body weight and survival of same-party BALB/c recipients, respectively; panels B and D represent body weight and survival of third-party C3H/HeJ recipients, respectively.
In the third-party C3H/HeJ hosts, untreated primed cells induced severe GVHD and all mice died within 30 days after transplantation (Figure 2B,D). Similarly, PDP-treated primed cells also induced lethal GVHD, albeit with a slight delay, within 50 days after transplantation. The difference in the survival was not statistically significant between PDP-treated and untreated groups.
PDP treatment prevents GVHD while retaining GVL effect
Because anti–third-party responses of primed T cells were preserved after PDP treatment, the ability of PDP-treated cells to mediate GVL effect was further explored in a GVHD/GVL model. BALB/c mice inoculated with BCL1 leukemia/lymphoma cells were lethally irradiated and infused with T-cell–depleted bone marrow cells only or along with PDP-treated or untreated T cells primed with BALB/c antigens. All BCL1-bearing mice transplanted with only T-cell–depleted bone marrow cells recurred with BCL1 leukemia cells in peripheral blood (Table 2;P < .001, compared with normal C57BL/6 or BALB/c mice) and half of them died within 40 days after transplantation (Figure3A). Addition of 5 × 106untreated T cells primed with BALB/c antigens completely prevented the recurrence of BCL1 cells (Table 2, Figures4 and 5;P < .0001, compared to BCL1 and T-cell–depleted bone marrow only). However, lethal GVHD developed and all mice in this group died within 50 days after transplantation (Figures 3 and 5). In contrast, addition of 5 × 106 PDP-treated (20 μM TH9402) primed T cells also completely controlled the recurrence of BCL1 cells (Table 2, Figures 4 and 5; P < .0001, compared with BCL1 and T-cell–depleted bone marrow only) but without inducing GVHD (Figures 3 and 5; P < .0001, compared with untreated group). One mouse died on day +81 without evidence of GVHD or tumor.
Effect of photodynamic cell purging process on graft-versus-leukemia effect
| Groups . | n . | % BCL1 cells in peripheral blood . | Mean ± SD . |
|---|---|---|---|
| Normal C57BL/6 | 9 | 1.5, 0.6, 0.7, 0.4, 0.3, 1.0, 1.6, 1.0, 1.3 | 0.9 ± 0.5 |
| Normal BALB/c | 5 | 1.6, 0.8, 0.4, 0.4, 0.4 | 0.7 ± 0.5 |
| BCL1 + TCD BMCs only | 7 | 9.9, 8.6, 12.5, 39.5, 13.8, 6.4, 1.8 | 13.2 ± 12.3* |
| Untreated primed cells | 7 | 0.2, 0.2, 0.5, 0.5, 0.9, 1.7, 0.5 | 0.6 ± 0.5† |
| PDP-treated primed cells (20 μM) | 10 | 0.3, 0.2, 0.2, 0.2, 0.6, 0.4, 0.5, 0.7, 0.4 | 0.4 ± 0.2† |
| Groups . | n . | % BCL1 cells in peripheral blood . | Mean ± SD . |
|---|---|---|---|
| Normal C57BL/6 | 9 | 1.5, 0.6, 0.7, 0.4, 0.3, 1.0, 1.6, 1.0, 1.3 | 0.9 ± 0.5 |
| Normal BALB/c | 5 | 1.6, 0.8, 0.4, 0.4, 0.4 | 0.7 ± 0.5 |
| BCL1 + TCD BMCs only | 7 | 9.9, 8.6, 12.5, 39.5, 13.8, 6.4, 1.8 | 13.2 ± 12.3* |
| Untreated primed cells | 7 | 0.2, 0.2, 0.5, 0.5, 0.9, 1.7, 0.5 | 0.6 ± 0.5† |
| PDP-treated primed cells (20 μM) | 10 | 0.3, 0.2, 0.2, 0.2, 0.6, 0.4, 0.5, 0.7, 0.4 | 0.4 ± 0.2† |
Peripheral blood was obtained from recipients 30 days after bone marrow transplantation. Percentages of BCL1 cells were determined using anti-BCL1 idiotype monoclonal antibodies. The mice that died before day + 30 were not included. TCD indicates T-cell–depleted; BMCs, bone marrow cells; PDP, photodynamic cell purging process.
P < .001, compared with normal C57BL/6 and BALB/c.
P < .0001, compared with BCL1 + TCD BMC only.
Effect of photodynamic cell purging process on graft-versus-host and graft-versus-leukemia effect.
Spleen cells from C57BL/6 mice were first primed with BALB/c spleen cells in a 5-day mixed lymphocyte culture. The primed cells were then treated with photodynamic cell purging process. After treatment, 5 million primed cells together with 1 × 107T-cell–depleted bone marrow cells from C57BL/6 mice were infused into lethally irradiated BALB/c mice (same party, 8.5 Gy) which were previously inoculated with BCL1 cells. The data were pooled from 2 independent experiments. Each group contained 5 to 10 animals. (A) Survival. (B) Body weight.
Effect of photodynamic cell purging process on graft-versus-host and graft-versus-leukemia effect.
Spleen cells from C57BL/6 mice were first primed with BALB/c spleen cells in a 5-day mixed lymphocyte culture. The primed cells were then treated with photodynamic cell purging process. After treatment, 5 million primed cells together with 1 × 107T-cell–depleted bone marrow cells from C57BL/6 mice were infused into lethally irradiated BALB/c mice (same party, 8.5 Gy) which were previously inoculated with BCL1 cells. The data were pooled from 2 independent experiments. Each group contained 5 to 10 animals. (A) Survival. (B) Body weight.
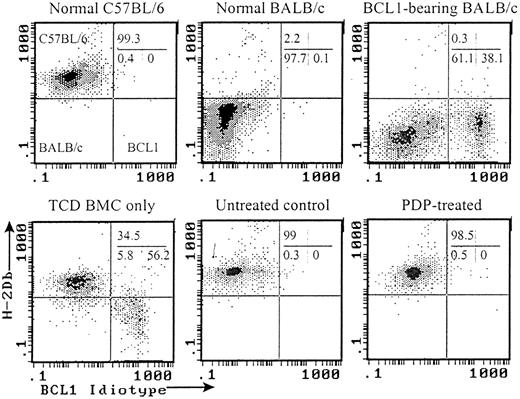
Flow cytometric analyses of chimerism and BCL1 tumor cells in the recipients of PDP-treated (20 μM) primed cells.
Peripheral blood samples taken 30 days after transplantation or treatment were analyzed by flow cytometry. All histograms were gated on white cells based on forward and side scatters. Representative histograms from each group are shown.
Flow cytometric analyses of chimerism and BCL1 tumor cells in the recipients of PDP-treated (20 μM) primed cells.
Peripheral blood samples taken 30 days after transplantation or treatment were analyzed by flow cytometry. All histograms were gated on white cells based on forward and side scatters. Representative histograms from each group are shown.
Histologic analyses.
Representative skin (A-D) and liver (E-H) sections from different groups of mice (H&E stain, ×100). Arrowhead shows BCL1 leukemia/lymphoma cells growing in the liver; arrow shows infiltrating lymphocytes; asterisk shows fibrosis of portal tracts in the liver. Analyses included 4 groups of mice: a normal BALB/c mouse inoculated with BCL1 30 days ago (A,E); a BCL1-bearing mouse transplanted with T-cell–depleted bone marrow cells only (B,F; day +30); a BCL1-bearing mouse transplanted with T-cell–depleted bone marrow cells plus untreated primed cells (C,G; day +38); a BCL1-bearing mouse transplanted with T-cell–depleted bone marrow cells plus PDP-treated (20 μM) primed cells (D,H; day +126).
Histologic analyses.
Representative skin (A-D) and liver (E-H) sections from different groups of mice (H&E stain, ×100). Arrowhead shows BCL1 leukemia/lymphoma cells growing in the liver; arrow shows infiltrating lymphocytes; asterisk shows fibrosis of portal tracts in the liver. Analyses included 4 groups of mice: a normal BALB/c mouse inoculated with BCL1 30 days ago (A,E); a BCL1-bearing mouse transplanted with T-cell–depleted bone marrow cells only (B,F; day +30); a BCL1-bearing mouse transplanted with T-cell–depleted bone marrow cells plus untreated primed cells (C,G; day +38); a BCL1-bearing mouse transplanted with T-cell–depleted bone marrow cells plus PDP-treated (20 μM) primed cells (D,H; day +126).
Discussion
In this study, we demonstrate that selective depletion of donor host histocompatibility antigen–specific T cells prevents GVHD while preserving GVL effect and anti–third-party responses. The data are in accordance with the results from other investigators demonstrating that GVHD can be prevented after ex vivo anergy or elimination of host antigen–specific T cells.5 15
Selective depletion of host-reactive T cells after PDP treatment is supported by the data presented in Figure 1 and Table 1, in which host-specific cytotoxicity T lymphocytes as well as host-specific IFNγ-secreting cells were significantly eliminated after PDP treatment, whereas third-party–specific cells were mostly retained and tumor cell–specific cells were partly preserved. Selective depletion of host antigen–specific CTLs and cytokine-producing cells explains how PDP treatment on primed T cells prevented anti–same-party GVHD (Figures 2, 3, and 5) while simultaneously preserving the GVL effect (Table 2, Figures 3, 4, and 5) and the ability to induce GVHD in a third-party host (Figure 2).
Since both GVL effect and anti–third-party effect are preserved after PDP treatment, PDP-treated primed T cells can be used to provide both GVL effect and antipathogen immunity either given together with hematopoietic stem cells or as a T-cell source for delayed lymphocyte infusion.5,13 In order to preserve GVL effect without causing GVHD, responding T cells need to be activated exclusively by host histocompatibility antigens without contamination from tumor antigens. This goal is easily achievable in inbred mice since one can use a healthy animal to obtain antigen-presenting cells. In the human setting, with hematopoietic malignancy diseases, it may not be an easy task to obtain antigen-presenting cells without tumor antigen contamination. One solution could be to use dendritic cells derived from bone marrow progenitor cells as antigen-presenting cells.26 Using dendritic cells as antigen-presenting cells may minimize the contamination of tumor antigens in the culture. However, this strategy will work only if GVL effect can be mediated by tumor antigen–specific T cells alone in human. How this strategy would be ultimately translated into clinical use would require more extensive studies.
In conclusion, ex vivo PDP treatment successfully eliminates activated T cells primed with host histocompatibility antigens but not the resting antitumor and anti–third-party T lymphocytes. A primed T-cell graft treated with PDP loses the ability to induce GVHD in the same-party hosts but retains the GVL effect and the ability to induce GVHD in the third-party recipients. This strategy may be useful to separate GVHD from GVL and provide protective immunity in clinical allogeneic bone marrow transplantation. The stem cell donor pool could be extended to haploidentical or even fully mismatched donors if this strategy can be successfully applied in the clinic.
Many thanks to Drs Defu Zeng and Samuel Strober for their generous gifts of BCL1 cells; Drs Kent J. Weinhold and Guido Ferrari for their help with the ELISPOT assay; Dr Ellen S. Vitetta for providing 6A5 and BCL1 cell lines; and Dr Yongping Li for his help with histologic studies.
Supported by a research grant from Theratechnologies, Inc, Montreal, Canada.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Nelson J. Chao, Bone Marrow Transplantation Program, Duke University Medical Center, 2400 Pratt St, Suite 1100, Durham, NC 27705; e-mail: chao0002@mc.duke.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal