Abstract
Transforming growth factor β (TGF-β), a pleiotropic cytokine that regulates cell growth and differentiation, is secreted by many human tumors and markedly inhibits tumor-specific cellular immunity. Tumors can avoid the differentiating and apoptotic effects of TGF-β by expressing a nonfunctional TGF-β receptor. We have determined whether this immune evasion strategy can be manipulated to shield tumor-specific cytotoxic T lymphocytes (CTLs) from the inhibitory effects of tumor-derived TGF-β. As our model we used Epstein-Barr virus (EBV)–specific CTLs that are infused as treatment for EBV-positive Hodgkin disease but that are vulnerable to the TGF-β produced by this tumor. CTLs were transduced with a retrovirus vector expressing the dominant-negative TGF-β type II receptor HATGF-βRII-Δcyt. HATGF-βRII-Δcyt– but not green fluorescence protein (eGFP)–transduced CTLs was resistant to the antiproliferative and anticytotoxic effects of exogenous TGF-β. Additionally, receptor-transduced cells continued to secrete cytokines in response to antigenic stimulation. TGF-β receptor ligation results in phosphorylation of Smad2, and this pathway was disrupted in HATGF-βRII-Δcyt–transduced CTLs, confirming blockade of the signal transduction pathway. Long-term expression of TGF-βRII-Δcyt did not affect CTL function, phenotype, or growth characteristics. Tumor-specific CTLs expressing HATGF-βRII-Δcyt should have a selective functional and survival advantage over unmodified CTLs in the presence of TGF-β–secreting tumors and may be of value in treatment of these diseases.
Introduction
Immunotherapy strategies to boost cellular immune responses to tumors are increasingly applied as more tumor antigens are identified.1,2 The most successful use of adoptively transferred antigen-specific cytotoxic T cells has occurred in severely immunocompromised individuals whose tumors require few immune evasion strategies.3 By contrast, tumor immunotherapy in immunocompetent hosts has been of more limited benefit. In the presence of a normal immune system, tumors frequently develop immune evasion strategies that may influence every stage of the generation of a tumor-specific cellular immune response, from the activation of professional antigen-presenting cells (APCs) to T-cell recruitment, activation, and effector function.4
Tumor secretion of transforming growth factor β (TGF-β) is among the most widely used evasion strategies. TGF-β is a ubiquitous cytokine with pleiotropic effects on cell growth, differentiation, and matrix production. As a stimulator of mesenchymal, fibroblast, smooth muscle, and osteoblast cell growth,5,6 TGF-β induces the synthesis of extracellular matrix proteins and promotes angiogenesis.7 As a growth inhibitor, it plays a role in T-cell homeostasis by limiting immune responses to antigens and by inducing tolerance.8,9 Hence, secretion of this cytokine by malignant cells such as neuroblastoma and Hodgkin Reed-Sternberg tumor cells may diminish the effectiveness of antitumor T-cell immune responses.10-12
The binding of TGF-β to either TGF-β receptor I (TGF-βRI) or TGF-β receptor II (TGF-βRII), results in the formation of a tetramer complex, involving the dimers of the type I and II receptors, which is required for signaling.13-17 With this interaction between the 2 receptors and the ligand, phosphorylation occurs, rendering TGF-βRI active and able to phosphorylate Smad 2 and 3, resulting in their translocation to the nucleus.18,19Once in the nucleus, the Smads interact with transcription factors such as those involved in the regulation of cell growth and differentiation.20 21
TGF-β may also have adverse effects on tumor cells themselves by promoting terminal differentiation and apoptosis. Tumors may avoid this activity by mutation of their TGF-β receptors (TGF-βRI and TGF-βRII).13,22,23 Each of these receptors possesses an extracellular region, a single transmembrane domain, and a cytoplasmic signaling domain containing a serine/threonine kinase domain. Mutations in the TGF-βRII gene that correlate with loss of sensitivity to TGF-β have been identified in many tumors in which inhibition of differentiation contributes to unregulated growth.24,25Mutant forms of the TGF-βRII have also been created. HATGF-βRII-Δcyt was created with a stop codon and aBamHI site introduced after the 10th cytoplasmic codon (nt597).16 This mutant receptor lacks the entire kinase domain and most of the juxtamembrane region. HATGF-βRII-Δcyt has been shown to act in a dominant-negative fashion when transfected into the mink lung epithelial cell line, diminishing the cells' antiproliferative and transcriptional responses to TGF-β.16
We have determined whether the strategy used by tumor cells to protect themselves against the effects of TGF-β can be manipulated to shield tumor-specific cytotoxic T lymphocytes (CTLs) from the inhibitory effects of tumor-secreted TGF-β. As our model, we have taken Epstein-Barr virus (EBV) antigen-positive Hodgkin disease, a tumor that expresses virus-specific antigens and should be susceptible to specific CTLs.26 Indeed, tracking of genetically marked EBV-specific CTL infusions shows homing to Hodgkin tumor sites and CTL survival in the patient's circulation for up to 9 months after infusion.27 In addition, CTL infusions enhanced EBV-specific immune responses and reduced virus load, but only limited tumor responses of brief duration were observed. This failure may be associated with the demonstrated ability of Hodgkin tumor cells to secrete TGF-β and consequent inactivation of CTLs entering the tumor environment.12 We now show that forced expression of a dominant-negative TGF-βRII in ex vivo–expanded EBV tumor-specific CTLs from patients with relapsed Hodgkin disease renders them resistant to the inhibitory effects of TGF-β, while enabling them to retain their dependence on other growth regulatory signals.
Materials and methods
Cell lines
The ecotropic packaging cell line Phoenix28 was provided by Gary P. Nolan (Stanford, CA). PG-13 (obtained from American Type Culture Collection [ATCC], Manassas, VA) is an amphotropic retrovirus packaging cell line that produces virus pseudotyped with the gibbon-ape leukemia virus (GALV). HSB-2 (ATCC) is a T-cell lymphoma that is sensitive to lymphokine-activated killer cells and was used as a target in cytotoxicity assays. Lymphoblastoid cell lines (LCLs) were generated as described below.
pMEP5/HATGF-βRII-Δcyt plasmid
A human type II TGF-β receptor complimentary DNA (cDNA) was truncated at nt597, thereby deleting most of its cytoplasmic tail and all of its cytoplasmic kinase domain, leaving only 7 amino acids remaining in the intracellular domain.16 A sequence encoding the influenza virus hemagglutinin peptide epitope HA1 was spliced into the human TGF-βRII cDNA.14,16,29 The HA sequence was inserted after the signal sequence in the human TGF-βRII so that the HA epitope is retained near the amino terminus of the mature receptor. The presence of the HA tag does not affect ligand binding and allows the mutant construct to be distinguished from the wild type TGF-βRII receptor with an anti-HA antibody.15,16 The function and the biochemistry of pMEP5/HATGF-βRII-Δcyt have been extensively characterized.16 HATGF-βRII-Δcyt was placed into the BamHI and NcoI sites of the retroviral vector SFG30 (provided by R.C. Mulligan, Cambridge, MA).
Production of recombinant retrovirus
Cells of the ecotropic packaging cell line Phoenix-eco were transiently transfected with vector DNA by using FuGENE 6 transfection reagent (Roche, Indianapolis, IN) in Dulbecco modified Eagle medium (DMEM; Biowhittaker, Walkersville, MD) supplemented with 10% fetal calf serum (FCS; Hyclone, Logan, UT). Twenty-four hours after transfection, Iscoves modified Dulbecco medium (IMDM; Biowhittaker) supplemented with 20% FCS (20% IMDM) was added, and cells were incubated at 32°C for 24 hours. Fresh retrovirus supernatants were then collected, filtered through a 0.45-μm filter, and used to infect the packaging cell line PG13 in the presence of polybrene (8 μg/mL) for 48 hours at 32°C. The infected cells were incubated overnight at 37°C in fresh 10% DMEM and then subjected to a second round of infection under the same conditions by using freshly generated Phoenix-eco cell supernatants. Viral supernatants were harvested from the resulting bulk producer lines by adding 20% IMDM to the PG-13 cells and incubated at 32°C for 24 hours. The supernatant was harvested, filtered by using a 0.45-μm filter, and used directly to transduce the CTLs.
Generation of EBV-transformed B cell lines
Peripheral blood–derived mononuclear cells (PBMCs) (5 × 106) were incubated with 100 μL concentrated supernatant from the EBV producer cell line B95-8 in a total of 200 μL complete medium (RPMI 1640 medium [GIBCO-BRL, Gaithersburg, MD] containing 10% FCS [Hyclone], and 2 mM l-glutamine [Biowhittaker]) for 30 minutes. The cells were plated at 106 cells per well in a flat-bottomed 96-well plate (Costar; Corning, Corning, NY) containing complete medium and 1 μg/mL cyclosporin A (Sandoz Pharmaceuticals, Washington, DC). Cells were fed weekly until LCLs were established.31
Generation and transduction of EBV-specific CTL cultures
EBV-specific CTLs were prepared by stimulating PBMCs with the autologous EBV-transformed LCL.32,33 PBMCs (2 × 106) were cocultured with 5 × 104gamma-irradiated (40 Gy) autologous LCLs per well in a 24-well plate. Starting on day 10, the responder cells were restimulated weekly with irradiated (40 Gy) LCLs at a responder-to-stimulator ratio of 4:1. Two weekly doses of recombinant human interleukin 2 (rhIL-2; 50 IU/mL) were added from day 14. Twenty-four hours after LCL stimulation, CTLs ready for transduction were transferred to a 24-well plate (Costar), precoated with OKT3 (1 μg/mL; Ortho Pharmaceuticals, Raritan, NJ) and anti-CD28 antibody (1 μg/mL; Pharmingen, San Diego, CA) at 1 × 106 cells per well and incubated for 48 hours for optimal activation before transduction.34 Transductions were carried out in 24-well nontissue culture-treated plates (Becton Dickinson, Franklin Lakes, NJ), coated with recombinant fibronectin fragment (FN CH-296; Retronectin; Takara Shuzo, Otsu, Japan) at a concentration of 4 μg/cm2. The prestimulated CTL lines were resuspended at 1 × 106 cells/mL in complete medium supplemented with 45% EHAA (Clicks; GIBCO-BRL) and rhIL-2 (100 IU/mL), then incubated with equal volumes of freshly generated viral supernatant for 36 hours at 37°C and 5% CO2. Two weeks after transduction, 3 CTL lines from healthy donors were positively selected for cell surface expression of the HA-tag by using flow cytometry.
Flow cytometry
For immunophenotyping, cells were stained with fluorescein-conjugated monoclonal antibodies (Becton Dickinson, San Jose, CA) directed against CD3, CD4, CD8, CD16, CD56, and CD25 surface proteins. For each sample, 10 000 cells were analyzed by FACSCalibur with the Cell Quest Software (Becton Dickinson). Surface expression of the HA-epitope was analyzed after incubation of CTLs (1 × 106) with the HA− antibody (Sigma, St Louis, MO) at a concentration of 200 ng/5 × 105 in the presence of normal donkey serum (Jackson Immuno Research Laboratories, West Grove, PA) for 30 minutes at room temperature. This analysis was followed by incubation with fluorescein isothiocyanate (FITC)-labeled donkey antirabbit antibody (Jackson Immuno). The perforin assay was performed by fixing the CTLs in 4% paraformaldehyde (Sigma) for 20 minutes. The cells were then washed in permeabilizing buffer (1 × phosphate-buffered saline [PBS; GIBCO-BRL] + 0.1% saponin [Sigma] + 1% FCS [Hyclone]). CTLs were incubated in 3 mL permeabilizing buffer with 5% human AB-serum (C-6 Diagnostics, Germantown, WI) for 10 minutes at room temperature. CTLs were spun down and resuspended in 100 μL permeabilizing buffer. To each sample, 20 μL of either phycoerythrin (PE)-labeled antiperforin antibody (Pharmingen) or PE-labeled IgG1 isotype control (Pharmingen) was added and incubated for 30 minutes at room temperature. CTLs were washed again with permeabilizing buffer and resuspended in 1 × PBS + 1% FCS and analyzed immediately.
Analysis of transcriptional activation by Western blot
Cell pellets were resuspended in Tris sodium EDTA (TNE) buffer (100 mM Tris, 150 mM NaCl, 0.5% NP-40, 10 mM EDTA, 1 mM dithiothreitol) with phosphatase inhibitors (20 mM β-glycerol phosphate and 20 mM NaVO3) and protease inhibitors. After 5 seconds of sonication, the lysates were centrifuged at 14 000 rpm for 5 minutes. Protein concentration of the supernatants was determined by protein assay (BIO-RAD No. 500-0006, Hercules, CA). Protein (50 μg) was loaded on a 9% sodium dodecyl sulfate–polyacrylamide gel. Western blot was performed with either antiphospho-Smad 2 antibody (Upstate Biotechnology No. 06-829, Lake Placid, NY) at a final concentration of 1 μg/mL or purified anti-Smad2/3 rabbit polyclonal antisera.35
Measurement of cytokine production by enzyme-linked immunosorbent assay
To assess the effect of the truncated TGF-βRII on cytokine release in the presence of TGF-β, duplicate samples of transduced and nontransduced effector cells (5 × 104/well) were cocultured with irradiated, EBV-transformed LCLs at stimulator-to-effector ratios of 1:4 in rhIL-2 (50 U/mL) ± 5 ng/mL TGF-β1 (R&D Systems, Minneapolis, MN) in 96-well round-bottom plates (Costar). After 24 hours, the supernatants were harvested and analyzed for human granulocyte-macrophage colony-stimulating factor (GM-CSF) and/or interferon γ (IFN-γ) by using 96-well plates precoated with either antihuman GM-CSF monoclonal antibody (R&D Systems) or antihuman IFN-γ monoclonal antibody (Pharmingen) by enzyme-linked immunosorbent assay according to the manufacturer's instructions.
Cytotoxicity assays
To compare the cytotoxic specificity of transduced and nontransduced CTLs in the presence of TGF-β1, standard51Cr release assays were performed. At 72 to 96 hours before performing the cytotoxicity assay, 5 ng/mL TGF-β1 (R&D Systems) was added to 8 × 106 transduced and nontransduced CTLs. Doubling dilutions of CTLs were coincubated in triplicate for 4 hours with 5000 51Cr-labeled target cells (Amersham Pharmacia Biotech, Piscataway, NJ) in a total volume of 200 μL in a V-bottom 96-well plate (Costar) as previously described.31 The targets tested were autologous LCLs, HLA class I and II mismatched LCLs, and HSB-2. Target cells incubated in RPMI 1620 alone or in 5% Triton X-100 (Sigma) were used to determine spontaneous and maximum 51Cr release, respectively. At the end of a 4-hour incubation period at 37°C and 5% CO2, supernatants were harvested, and 51Cr release was measured on a gamma counter (Tri-CARB 4640; Packard BioScience, Downers Grove, IL). The mean percentage of specific lysis of triplicate wells was calculated as follows: [(test counts − spontaneous counts)/(maximum counts − spontaneous counts)] × 100%.
Proliferation assays
Transduced CTLs were coincubated in triplicate at 5 × 104 cells/well with irradiated autologous EBV-LCLs at a 4:1 stimulator-to-responder ratio ± titrated concentrations of TGF-β1 up to 20 ng/mL. After a 72-hour coincubation period, wells were pulsed with 0.037 MBq (1 μCi)/well of [3H]thymidine (Amersham Pharmacia Biotech) for 18 hours, and the samples were harvested onto glass fiber filter paper for β-scintillation counting (TriCarb 2500 TR; Packard BioScience).
Quantification of the transduction rate by real-time polymerase chain reaction
DNA was extracted from cytotoxic T cells by using the DNeasy Tissue Kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. For quantification of the transduction rate of CTL, real-time polymerase chain reaction (RT PCR) assays specific for the HA sequence were developed by using 5′ nuclease PCR technology and the ABI PRISM 7700 Sequence Detection System (PE Applied Biosystems, Foster City, CA).36 RT PCR amplification was performed with 2× TaqMan Universal Master Mix (PE Applied Biosystems) adjusted to 50 μL with 300 nM of each primer, 200 nM probe, template, and nuclease-free water. The forward primer (5′-GTGGACGCGTATCGCCAG-3′) binds 12 base pair (bp) upstream of the HA sequence, the reverse primer (5′-TGTCAGTGACTATCATGTCGTTATTAACC-3′) 15 bp downstream of the HA sequences, whereas the probe (5′-VIC-CCACCGTATGATGTTCCTGATTATGCTAGCC-TAMRA-3′) spans the entire HA sequence. DNA solution (250 ng) was analyzed in triplicate for each sample. As positive controls, samples were analyzed for the β-actin gene in parallel by using the TaqMan Beta-actin Detection Reagents (PE Applied Biosystems). PCR consisted of 2 minutes at 50°C (inactivation of possible carry-over contamination by uracil N′-glycosylase [UNG]), 10 minutes at 95°C (UNG inactivation and activation of DNA polymerase), and 40 2-step cycles of 15 seconds at 95°C and 60 seconds at 60°C. For quantification, serial 1:4-fold dilutions of the plasmid pMEP5/HATGF-βRII-Δcyt16 were used as the standard. A correlation coefficient of more than 0.99 was found over at least 5 orders of magnitude after amplification of the HA sequence.
Statistical analysis
The Student t test was used to test for significance in each set of values, assuming equal variance. Mean values ± SE are given unless otherwise stated.
Results
Truncated TGF-β receptor mutant was expressed by CTLs after transduction with HATGF-βRII-Δcyt
Four EBV-specific CTL lines from healthy individuals and 4 from patients with EBV-positive Hodgkin disease were transduced with HATGF-βRII-Δcyt after 21 to 105 days of culture (mean, 52 days). We used RT PCR analysis to compare the transgene copy number per cell in bulk HATGF-βRII-Δcyt–transduced CTLs with that in transduced CTLs that had been sorted by flow cytometry for HA epitope expression. Assuming that 100% of the cells sorted for the HA tag contained at least one copy of SFG:HATGF-βRII-Δcyt DNA, the transduction efficiency in unsorted CTLs ranged from 6.5% to 55% (mean = 27%) (Table 1), which is not significantly different from the transduction efficiency with SFG-eGFP (19%-51.5%; mean, 31%) (Figure 1A,B). Mutant TGF-βRII surface expression was also detected by flow cytometry (Figure 1C-E). By using the anti-HA antibody, the percentage of expression of HATGF-βRII-Δcyt on CD8+ cells ranged from 3.5% to 49.3% (mean, 17%), which is consistent with RT PCR results. Similar transduction efficiencies were also seen for CD4+cells (Figure 1F). CTLs sorted for the HA tag showed 53.15% to 62.6% HA expression on CD8+ cells 6 weeks after sorting (Figure 1G,H).
Transduction rate of HATGF-βRII-Δcyt ranges from 6.5% to 55% as determined by quantitative real-time polymerase chain reaction
| . | No. of transduced copies per 100 000 cells . | % transduced* . |
|---|---|---|
| CTL-sorted (positive selection of transduced cells by FACS) | 310 000 | 100 |
| Donor 1 (unsorted) | 67 550 | 19 |
| Donor 2 (unsorted) | 28 055 | 10 |
| Patient 1 (unsorted) | 141 000 | 45 |
| Patient 3 (unsorted) | 170 000 | 55 |
| Patient 4 (unsorted) | 22 140 | 6.5 |
| . | No. of transduced copies per 100 000 cells . | % transduced* . |
|---|---|---|
| CTL-sorted (positive selection of transduced cells by FACS) | 310 000 | 100 |
| Donor 1 (unsorted) | 67 550 | 19 |
| Donor 2 (unsorted) | 28 055 | 10 |
| Patient 1 (unsorted) | 141 000 | 45 |
| Patient 3 (unsorted) | 170 000 | 55 |
| Patient 4 (unsorted) | 22 140 | 6.5 |
To assess the transduction efficiency, DNA was extracted from transduced cytotoxic T lymphocytes (CTLs) and transduced CTLs sorted for the hemagglutinin peptide epitope tag. These sorted cells were therefore assumed to be 100% transduced. The number of copies per 100 000 cells was calculated by using a standard curve generated by serial dilutions of plasmid DNA from 256 to 131 072 copies. Sorted CTLs were compared with 5 unsorted transduced CTL lines, and the transduction rates were calculated.
Compared with 100% population of sorted CTLs.
Transduced CTLs surface express HA tag of mutant TGF-βRII receptor.
Cells of an EBV-specific CTL line 14 days after retroviral transduction with SFG:eGFP were stained with either PE-labeled anti-IgG1 (A) or anti-CD3 antibodies (B). CTLs transduced with SFG:HATGF-βRII-Δcyt (truncated TGF-βRII containing HA tag) gene were stained with FITC-labeled donkey antirabbit antibody alone (isotype control) (C). Nontransduced (D) or HATGF-βRII-Δcyt–transduced CTLs (E,F) were then stained with anti-HA monoclonal antibody followed by incubation with FITC-labeled donkey antirabbit antibody and PE-labeled CD8 or CD4 antibody. Six weeks after sorting CTLs for the HA tag, HA expression was measured on CD8+ cells (G [isotype control] and H). Surface immunofluorescence was analyzed by flow cytometry.
Transduced CTLs surface express HA tag of mutant TGF-βRII receptor.
Cells of an EBV-specific CTL line 14 days after retroviral transduction with SFG:eGFP were stained with either PE-labeled anti-IgG1 (A) or anti-CD3 antibodies (B). CTLs transduced with SFG:HATGF-βRII-Δcyt (truncated TGF-βRII containing HA tag) gene were stained with FITC-labeled donkey antirabbit antibody alone (isotype control) (C). Nontransduced (D) or HATGF-βRII-Δcyt–transduced CTLs (E,F) were then stained with anti-HA monoclonal antibody followed by incubation with FITC-labeled donkey antirabbit antibody and PE-labeled CD8 or CD4 antibody. Six weeks after sorting CTLs for the HA tag, HA expression was measured on CD8+ cells (G [isotype control] and H). Surface immunofluorescence was analyzed by flow cytometry.
HATGF-βRII-Δcyt–transduced CTLs are resistant to the antiproliferative effects of exogenous TGF-β1
To investigate whether expression of the truncated TGF-βRII by CTLs could overcome the antiproliferative effects of TGF-β on CTLs, we compared thymidine uptake by HATGF-βRII-Δcyt–transduced CTLs with eGFP-transduced and -nontransduced CTLs after addition of TGF-β1 for 72 hours (Figure 2). TGF-β1 had a dramatic antiproliferative effect on established eGFP-transduced and -nontransduced EBV-CTLs generated from both healthy donors and patients, inhibiting uptake by a mean of 59.5% (range, 44%-75%). By contrast, the mean inhibition of thymidine uptake by HATGF-βRII-Δcyt–transduced CTLs was 16% (range, 0%-30%). This resistance to the antiproliferative effects of TGF-β in HATGF-βRII-Δcyt–transduced CTLs was statistically significant when compared with the mock or nontransduced CTLs (P = .03). Importantly, when EBV-specific CTLs were maintained under normal growth conditions with the addition of TGF-β, they failed to proliferate, and most died within 12 days. HATGF-βRII-Δcyt–transduced CTLs, however, continued to proliferate and grow normally, showing that the transduced cells were resistant to the antiproliferative effects of the TGF-β1 (Figure 3A,B).
Ability of TGF-β to inhibit T-cell proliferation is significantly greater in nontransduced CTLs when compared with HATGF-βRII-Δcyt–transduced CTLs.
Nontransduced CTLs, eGFP-transduced CTLs, and HATGF-βRII-Δcyt–transduced CTLs were stimulated with irradiated autologous LCLs and IL-2 ± 5 ng/mL TGF-β1. Proliferative responses were measured after 72 hours of incubation by measurement of3[H] thymidine uptake. The mean percentage of inhibition by TGF-β was measured in nontransduced CTLs (black), eGFP-transduced CTLs (white), and HATGF-βRII-Δcyt–transduced CTLs (gray). The graph represents a pooled analysis of the mean inhibition of TGF-β on3[H] thymidine uptake in the 8 CTL lines tested.
Ability of TGF-β to inhibit T-cell proliferation is significantly greater in nontransduced CTLs when compared with HATGF-βRII-Δcyt–transduced CTLs.
Nontransduced CTLs, eGFP-transduced CTLs, and HATGF-βRII-Δcyt–transduced CTLs were stimulated with irradiated autologous LCLs and IL-2 ± 5 ng/mL TGF-β1. Proliferative responses were measured after 72 hours of incubation by measurement of3[H] thymidine uptake. The mean percentage of inhibition by TGF-β was measured in nontransduced CTLs (black), eGFP-transduced CTLs (white), and HATGF-βRII-Δcyt–transduced CTLs (gray). The graph represents a pooled analysis of the mean inhibition of TGF-β on3[H] thymidine uptake in the 8 CTL lines tested.
In the presence of TGF-β, HATGF-βRII-Δcyt–transduced CTLs continue to proliferate and grow normally in long-term cultures.
HATGF-βRII-Δcyt–transduced, nontransduced, and eGFP-transduced CTLs were harvested 2 weeks after transduction. From each group, 1 × 106 cells were stimulated weekly with LCLs and fed twice weekly with IL-2 ± 5 ng/mL TGF-β. The figures show results from one representative experiment. (A) This panel represents CTL cell numbers (× 106) recorded from weekly cell counts in the 3 CTL groups grown without TGF-β (■) and with TGF-β (▴). (B) This panel shows 3[H] thymidine uptake in nontransduced CTLs (black) and HATGF-βRII-Δcyt–transduced CTLs (gray) before addition of TGF-β then on days 4 and 12.
In the presence of TGF-β, HATGF-βRII-Δcyt–transduced CTLs continue to proliferate and grow normally in long-term cultures.
HATGF-βRII-Δcyt–transduced, nontransduced, and eGFP-transduced CTLs were harvested 2 weeks after transduction. From each group, 1 × 106 cells were stimulated weekly with LCLs and fed twice weekly with IL-2 ± 5 ng/mL TGF-β. The figures show results from one representative experiment. (A) This panel represents CTL cell numbers (× 106) recorded from weekly cell counts in the 3 CTL groups grown without TGF-β (■) and with TGF-β (▴). (B) This panel shows 3[H] thymidine uptake in nontransduced CTLs (black) and HATGF-βRII-Δcyt–transduced CTLs (gray) before addition of TGF-β then on days 4 and 12.
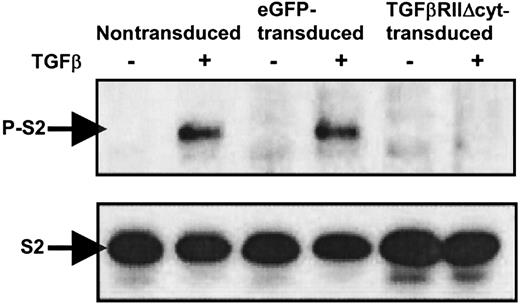
Phosphorylation of Smad2 is inhibited in TGF-βRII-Δcyt–transduced CTLs with addition of TGF-β
To confirm that downstream signaling by TGF-β is abrogated in CTLs transduced with TGF-βRII-Δcyt, TGF-β was added to nontransduced, eGFP-transduced, and TGF-βRII-Δcyt–transduced CTLs at a concentration of 5 ng/mL. After 60 minutes, the cells were harvested, and whole cell lysates were prepared. All the CTL extracts were subjected to Western immunoblotting by using anti-Smad2/3 antibody and antiphospho-Smad2 antibody (Figure4). Western blot analysis demonstrated the presence of Smad-2 (S2) in all the CTL groups in the presence and absence of TGF-β. However, phosphorylated Smad-2 (P-S2) was only detected in nontransduced and eGFP-transduced CTLs treated with TGF-β. In contrast, there was no expression of P-S2 in TGF-βRII-Δcyt–transduced CTLs with the addition of TGF-β, confirming that signal transduction was blocked by the presence of the dominant-negative TGF-βRII.
Western immunoblotting shows absence of phosphorylated Smad2 in TGF-βRII-Δcyt–transduced CTLs with addition of TGF-β.
Nontransduced, eGFP-transduced, and TGF-βRII-Δcyt–transduced CTLs were incubated for 1 hour with 5 ng/mL TGF-β, as indicated. The presence or absence of Smad2 (S2) and phosphorylated Smad2 (P-S2) was detected by Western immunoblotting by using anti-Smad2 and antiphospho-Smad2 antibodies, respectively.
Western immunoblotting shows absence of phosphorylated Smad2 in TGF-βRII-Δcyt–transduced CTLs with addition of TGF-β.
Nontransduced, eGFP-transduced, and TGF-βRII-Δcyt–transduced CTLs were incubated for 1 hour with 5 ng/mL TGF-β, as indicated. The presence or absence of Smad2 (S2) and phosphorylated Smad2 (P-S2) was detected by Western immunoblotting by using anti-Smad2 and antiphospho-Smad2 antibodies, respectively.
TGF-βRII-Δcyt–transduced CTLs continue to produce cytokines in response to antigenic stimulus in the presence of TGF-β1
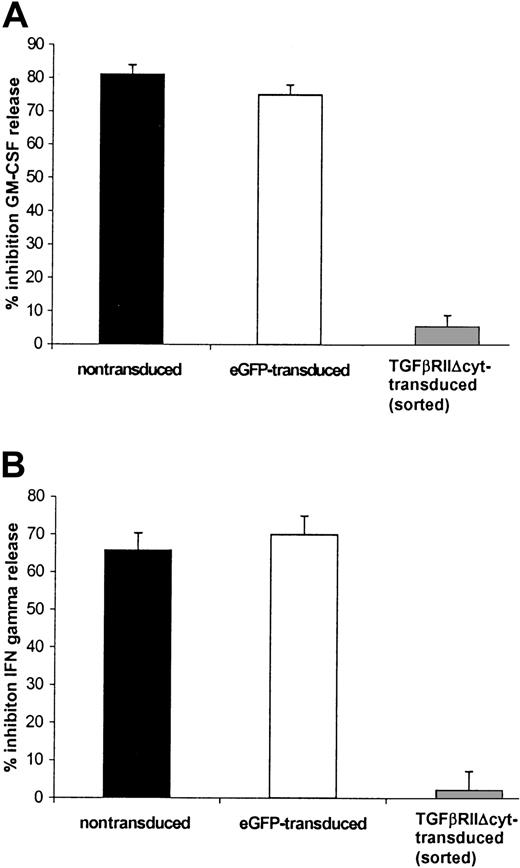
TGF-β inhibited IFN-γ and GM-CSF release from nontransduced EBV-specific CTLs after they were stimulated with irradiated LCL (effector-to-target ratio of 4:1) and 50 U/mL rhIL-2 for 24 hours. The level of inhibition was 60.5% (range, 47%-71%) for IFN-γ and 71% (range, 63%-83%) for GM-CSF. By contrast, the mean inhibition of cytokine release by HATGF-βRII-Δcyt–transduced CTLs was 43% (range, 17%-56%) for GM-CSF and 22.5% (range, 6%-39%) for IFN-γ. The effect of TGF-β on IFN-γ and GM-CSF release in HATGF-βRII-Δcyt–transduced CTLs compared with the cytokine release in nontransduced and eGFP-transduced CTLs was statistically significant (P = .05 and P = .01, respectively). This protection was even greater with HA-sorted CTLs in which there was just 4.5% (range, 0%-9%) GM-CSF inhibition (Figure5A) and 2.2% (range, 0%-9%) inhibition of IFN-γ release (P = .002) (Figure 5B).
HATGF-βRII-Δcyt–transduced CTLs sorted for the HA epitope demonstrate a significant resistance to the inhibitory effects of TGF-β on secretion of GM-GSF and IFN-γ when compared with nontransduced CTLs.
EBV-specific CTLs were transduced with the mutant TGF-βRII receptor and then positively selected for the HA tag by flow cytometry by using an anti-HA antibody. TGF-βRII-Δcyt–transduced/sorted CTLs and mock-transduced CTLs were stimulated with irradiated autologous LCLs and IL-2 ± 5 ng/mL TGF-β1. Supernatant removed after 24 hours was analyzed for GM-CSF (A) and IFN-γ (B). The graphs represent a pooled analysis of the mean percentage of inhibition of TGF-β on IFN-γ and GM-CSF release in 8 nontransduced (black) and 3 eGFP-transduced CTL (white) lines versus the 3 HATGF-βRII-Δcyt–transduced CTL (gray) lines sorted for the HA-epitope.
HATGF-βRII-Δcyt–transduced CTLs sorted for the HA epitope demonstrate a significant resistance to the inhibitory effects of TGF-β on secretion of GM-GSF and IFN-γ when compared with nontransduced CTLs.
EBV-specific CTLs were transduced with the mutant TGF-βRII receptor and then positively selected for the HA tag by flow cytometry by using an anti-HA antibody. TGF-βRII-Δcyt–transduced/sorted CTLs and mock-transduced CTLs were stimulated with irradiated autologous LCLs and IL-2 ± 5 ng/mL TGF-β1. Supernatant removed after 24 hours was analyzed for GM-CSF (A) and IFN-γ (B). The graphs represent a pooled analysis of the mean percentage of inhibition of TGF-β on IFN-γ and GM-CSF release in 8 nontransduced (black) and 3 eGFP-transduced CTL (white) lines versus the 3 HATGF-βRII-Δcyt–transduced CTL (gray) lines sorted for the HA-epitope.
CTLs transduced with retrovirus TGF-βRII-Δcyt maintain their cytolytic activity and specificity in the presence of TGF-β
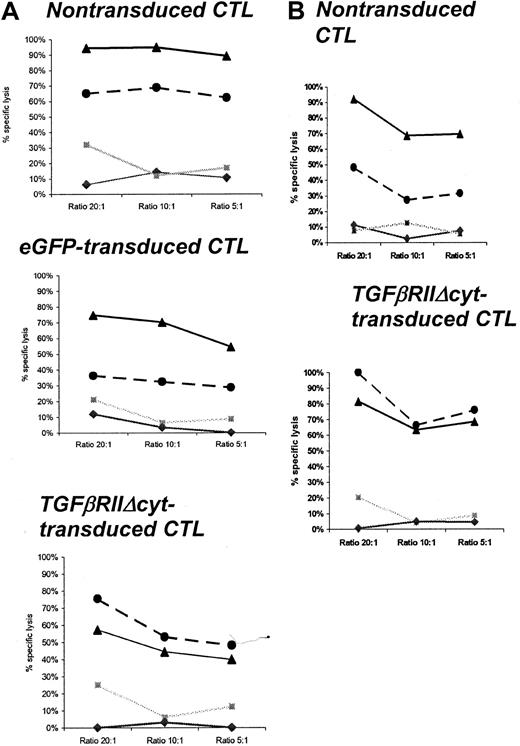
The cytotoxic activity of HATGF-βRII-Δcyt–transduced, mock-transduced, and nontransduced CTLs were compared in standard 4-hour 51Cr release assays in the presence of TGF-β1. CTL lines were tested up to 26 days after transduction in the presence of TGF-β1 (Table 2). At an effector-to-target ratio of 20:1, the percentage of autologous LCLs lysed by nontransduced CTLs was inhibited by 51% to 100% (mean, 74%) compared with a range of 27% to 57% (mean, 37.7%) after more than a 72-hour incubation with 5 ng/mL TGF-β1 (P = .02). By comparison, at the same effector-to-target ratio, the percentage of autologous LCLs lysed by transduced (unsorted) CTLs ranged from 40% to 81% (mean, 61.3%) in the absence of TGF-β1 and 41% to 100% (mean, 61.3%) in the presence of TGF-β1 (P = .7). No CTL lines had significant (> 20%) reactivity with allogeneic LCL or HSB-2 targets (Figure 6A,B).
Cytolytic characteristics of HATGF-βRII-Δcyt–transduced versus nontransduced cytotoxic T-lymphocyte lines with and without exposure to exogenous transforming growth factor β
| CTL line . | % Specific lysis (ratio 10:1) . | Day of culture* (d after transduction) . | |||
|---|---|---|---|---|---|
| Nontransduced . | TGF-βRII-Δcyt transduced . | ||||
| Auto LCL . | Auto LCL + TGF-β . | Auto LCL . | Auto LCL + TGF-β . | ||
| Patient 1 | 69 | 27 | 63 | 66 | 70 (21) |
| Patient 2 | 43 | 18 | 40 | 41 | 96 (14) |
| Patient 3 | 41 | 24 | 30 | 35 | 88 (26) |
| Patient 4 | 26 | 3 | 27 | 23 | 120 (21) |
| Donor 1 | 37 | 19 | 44 | 54 | 42 (26) |
| Donor 2 | 95 | 69 | 44 | 53 | 56 (21) |
| Donor 3 | 64 | 54 | 48 | 42 | 52 (14) |
| Donor 4 | 61 | 36 | 22 | 23 | 52 (14) |
| CTL line . | % Specific lysis (ratio 10:1) . | Day of culture* (d after transduction) . | |||
|---|---|---|---|---|---|
| Nontransduced . | TGF-βRII-Δcyt transduced . | ||||
| Auto LCL . | Auto LCL + TGF-β . | Auto LCL . | Auto LCL + TGF-β . | ||
| Patient 1 | 69 | 27 | 63 | 66 | 70 (21) |
| Patient 2 | 43 | 18 | 40 | 41 | 96 (14) |
| Patient 3 | 41 | 24 | 30 | 35 | 88 (26) |
| Patient 4 | 26 | 3 | 27 | 23 | 120 (21) |
| Donor 1 | 37 | 19 | 44 | 54 | 42 (26) |
| Donor 2 | 95 | 69 | 44 | 53 | 56 (21) |
| Donor 3 | 64 | 54 | 48 | 42 | 52 (14) |
| Donor 4 | 61 | 36 | 22 | 23 | 52 (14) |
Percentage of specific 51Cr release was determined after 4-hour coincubation with autologous lymphoblastoid cell line (LCL) in cytotoxic T-lymphocyte (CTL) lines with and without transforming growth factor β (TGF-β) added to CTL cultures 96 hours before the cytotoxicity assay. The table shows the percentage of specific lysis at an effector-to-target ratio of 10:1 in the CTL lines generated from 4 healthy donors and 4 patients with Hodgkin disease. The far right column shows the day of CTL culture/and day after transduction when the cytotoxicity assay was performed.
Cytotoxicity of transduced and nontransduced CTLs was tested on the same day of culture.
TGF-β decreases CTL-specific lysis against autologous EBV-LCL targets in mock-transduced or nontransduced CTLs but not in HATGF-βRII-Δcyt–transduced CTLs.
Percentage of specific 51Cr release was determined 4 hours after coincubation with autologous LCLs, allogeneic LCLs, and HSB-2 targets. TGF-β was added to CTL cultures 96 hours before cytotoxicity assay. The graphs show the percentage of specific lysis at effector-to-target ratios of 20:1, 10:1, and 5:1 in representative CTL lines generated from a healthy donor (A) and from a patient with Hodgkin disease (B). ▴ indicates CTLs cultured without TGF-β and autologous LCL target; ●, CTLs cultured with TGF-β and autologous LCL target; ░, CTL versus HSB-2 target; ♦, CTLs versus allogeneic (HLA class I mismatch) LCL target.
TGF-β decreases CTL-specific lysis against autologous EBV-LCL targets in mock-transduced or nontransduced CTLs but not in HATGF-βRII-Δcyt–transduced CTLs.
Percentage of specific 51Cr release was determined 4 hours after coincubation with autologous LCLs, allogeneic LCLs, and HSB-2 targets. TGF-β was added to CTL cultures 96 hours before cytotoxicity assay. The graphs show the percentage of specific lysis at effector-to-target ratios of 20:1, 10:1, and 5:1 in representative CTL lines generated from a healthy donor (A) and from a patient with Hodgkin disease (B). ▴ indicates CTLs cultured without TGF-β and autologous LCL target; ●, CTLs cultured with TGF-β and autologous LCL target; ░, CTL versus HSB-2 target; ♦, CTLs versus allogeneic (HLA class I mismatch) LCL target.
Effects of exogenous TGF-β on intracellular perforin levels in TGF-βRII-Δcyt–expressing and –nonexpressing CTLs
To identify a possible mechanism by which the cytolytic activity of CTLs is reduced by TGF-β, the intracellular perforin levels of the CTLs were measured by flow cytometry after CTLs were stained by PE-labeled antiperforin antibody (Figure7). The intracellular perforin levels in untransduced and eGFP-transduced CTLs were significantly reduced by 50% to 96% (mean, 73%, P = .002) in the presence of TGF-β (compare 7A with 7D and 7B with 7E). By comparison, CTLs transduced with HATGF-βRII-Δcyt had no significant reduction in perforin (range, 4%-11%, mean 6.8%, P = .7) (7C compared with 7F).
TGF-β significantly reduces intracellular perforin levels in nontransduced or eGFP-transduced CTLs, whereas perforin release is unaffected by TGF-β in HATGF-βRII-Δcyt–transduced CTLs.
Ninety-six hours after the addition of TGF-β (5 ng/mL), nontransduced (A,D), eGFP-transduced (B,E), and HATGF-βRII-Δcyt–transduced CTLs (C,F) were stained for intracellular perforin by using PE-labeled antiperforin antibody and were detected by flow cytometry. (A-F) These panels show representative histograms for CTLs from one donor cultured without TGF-β (A-C) versus with TGF-β (D-F). (G) This panel shows mean perforin levels from 6 CTL lines with and without TGF-β.
TGF-β significantly reduces intracellular perforin levels in nontransduced or eGFP-transduced CTLs, whereas perforin release is unaffected by TGF-β in HATGF-βRII-Δcyt–transduced CTLs.
Ninety-six hours after the addition of TGF-β (5 ng/mL), nontransduced (A,D), eGFP-transduced (B,E), and HATGF-βRII-Δcyt–transduced CTLs (C,F) were stained for intracellular perforin by using PE-labeled antiperforin antibody and were detected by flow cytometry. (A-F) These panels show representative histograms for CTLs from one donor cultured without TGF-β (A-C) versus with TGF-β (D-F). (G) This panel shows mean perforin levels from 6 CTL lines with and without TGF-β.
Expression of HATGF-βRII-Δcyt does not affect the phenotype or cytotoxic specificity of transduced CTLs
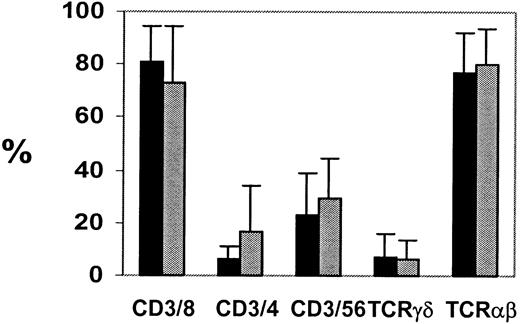
Although the dominant-negative TGF-β receptor clearly protects CTLs from the growth inhibitory effects of TGF-β, it is important to show that the transduced CTLs can continue to function normally and remain under normal growth control. The CTLs were phenotyped before and after transduction with HATGF-βRII-Δcyt. The CTLs were then maintained in culture for up to 35 days after transduction and were phenotyped weekly from day 7 after transduction. Most of the CTL lines generated had a characteristic immunophenotype with more than 90% CD3+ T cells, of which about 90% were also CD8+, whereas up to 10% of the cells had a T-cell helper phenotype (CD3+CD4+). These lines were compared with nontransduced lines for phenotypic differences. Transduction of CTLs did not result in any change in immunophenotype when compared with nontransduced cells (Figure 8). Nor was there any interference with cytolytic function. 51Cr release assays were performed between 14 and 26 days after transduction. There was no significant difference in the cytolytic specificity or activity of the transduced lines when compared with otherwise identical nontransduced lines from the same donor over several time points. As outlined in Table 2 after a range of 14 to 26 days after transduction with TGF-βRII-Δcyt, the CTL lines maintained their cytotoxic activity (Table 2) and specificity (Figure 6A).
CTL phenotype is unchanged after transduction.
To determine CTL phenotypes, the nontransduced (black) and TGF-βRII-Δcyt–transduced (gray) EBV-specific CTL cultures were stained with antibodies against T-cell surface antigens CD3, CD4, CD8, CD56, T-cell receptor αβ, and T-cell receptor γδ, and surface immunofluorescence was analyzed by flow cytometry.
CTL phenotype is unchanged after transduction.
To determine CTL phenotypes, the nontransduced (black) and TGF-βRII-Δcyt–transduced (gray) EBV-specific CTL cultures were stained with antibodies against T-cell surface antigens CD3, CD4, CD8, CD56, T-cell receptor αβ, and T-cell receptor γδ, and surface immunofluorescence was analyzed by flow cytometry.
TGF-βRII-Δcyt–transduced CTLs proliferate normally in long-term culture but die rapidly in the absence of exogenous growth factors and antigenic stimulation
Unresponsiveness to TGF-β might lead to a loss of dependence on other growth regulatory signals and, hence, to uncontrolled T lymphoproliferation. To exclude this possibility, the growth of the mature CTLs in the absence of growth stimuli was assessed. Transduced and nontransduced CTLs were cultured in the absence of antigenic stimulation (LCLs) and the growth factor rhIL-2 for 3 weeks. Both transduced and nontransduced CTLs failed to proliferate and became nonviable after 3 weeks in the absence of IL-2 and LCL stimulation (Figure 9).
HATGF-βRII-Δcyt–transduced CTLs will fail to proliferate in the absence of IL-2 or LCL stimulation.
The proliferation of long-term HATGF-βRII-Δcyt–transduced CTL (gray) and nontransduced CTL lines (black) were determined by3[H]thymidine uptake. The3[H]thymidine uptake of CTL lines left in culture for 4 weeks after transduction and stimulated weekly with LCL and IL-2 were compared with the same CTL lines grown in the absence of LCL/IL-2 stimulation for 3 weeks.
HATGF-βRII-Δcyt–transduced CTLs will fail to proliferate in the absence of IL-2 or LCL stimulation.
The proliferation of long-term HATGF-βRII-Δcyt–transduced CTL (gray) and nontransduced CTL lines (black) were determined by3[H]thymidine uptake. The3[H]thymidine uptake of CTL lines left in culture for 4 weeks after transduction and stimulated weekly with LCL and IL-2 were compared with the same CTL lines grown in the absence of LCL/IL-2 stimulation for 3 weeks.
Discussion
Secretion of TGF-β is a strategy commonly used by tumors to thwart cellular immune responses. It prevents the maturation of professional APCs and inhibits T-cell proliferation, cytokine release, and cytolytic activity.37 This effect may severely affect adoptively transferred tumor-specific CTLs used in cellular immunotherapy. Tumor cells may themselves be sensitive to TGF-β–induced differentiation and apoptosis but can avoid this fate if they also possess mutant receptors for the cytokine.24,38,39 We now show that such mutants can be adapted for the protection of tumor-specific CTLs. A dominant-negative mutant TGF-β type II receptor, in which the cytoplasmic signaling domain is deleted, protects virus-specific CTLs from the inhibitory effects of TGF-β signaling.16 Expression of the mutant receptor did not result in alterations in phenotype, cytotoxic specificity, or requirement for growth-regulatory signals. This tumor-derived defense may have clinical value when adoptively transferred T cells are used for the treatment of TGF-β–secreting tumors, including Hodgkin lymphoma.
We chose EBV-positive Hodgkin lymphoma to investigate this approach because the tumor cells express well-defined (viral) tumor antigens to which CTLs can readily be generated. Hodgkin tumors also secrete TGF-β, which may contribute to the limited clinical effectiveness of CTLs in this disease, compared with other EBV-positive malignancies.26 The experiments were performed by using polyclonal CTLs, as these cells have been successfully used in the clinical setting.3 Polyclonal CTLs are less likely to be successfully evaded by escape mutants.40 In addition, the presence of CD4+ T cells has been shown to be important for long-term CTL persistence in vivo as well as the maintenance of CD8+ T-lymphocyte–mediated antiviral or antitumor immunity.40 41 The polyclonal EBV-specific CTL lines generated from healthy individuals and patients with Hodgkin disease were readily transduced with a GALV-pseudotyped retrovirus expressing a dominant-negative TGF-βRII, and transduced CTLs were resistant to the antiproliferative effects of TGF-β. Not only was there significantly less inhibition of proliferation after 72 hours of culture with TGF-β, but also transduced CTLs continued to grow in the presence of the cytokine, whereas untransduced CTLs were nonviable after 12 days of culture. Additionally, CTLs could be transduced from day 21 to day 105 of culture, resulting in reproducible effects that were maintained for up to 120 days, thereby testifying to the robust nature of this modification.
Inhibition of the TGF-β signal transduction pathway in the TGF-βRII-Δcyt–transduced CTL was demonstrated by an absence of detectable phosphorylated Smad2 on Western blot in the presence of TGF-β. In nontransduced and transduced CTLs, TGF-β binds to TGF-βRII on the cell surface. However, in mock-transduced CTLs, the ligand-bound TGF-βRII then interacts with and phosphorylates TGF-βRI. Phosphorylation activates the intrinsic kinase activity of TGF-βRI, allowing the receptor to phosphorylate and thereby activate Smad proteins as confirmed by the detection of phosphorylated Smad2 on Western analysis in the mock-transduced CTLs. Once activated by phosphorylation, the Smad complexes migrate to the nucleus, where they recruit other transcription factors and stimulate the expression of genes, including mediators of cell growth.42 In contrast, when TGF-β is added to cells expressing the truncated dominant-negative TGF-βRII, the lack of the intracellular domain prevents phosphorylation of TGF-βRI16 and the CTL gene-modified abrogation of all downstream signaling events (including Smad phosphorylation). The complete lack of detection of phosphorylated Smad2 in the HATGF-βRII-Δcyt–transduced CTL suggests either that transduction efficiency was higher than suggested by HA detection and/or the presence of transacting protection.
TGF-β also prevented the secretion of IFN-γ and GM-CSF from EBV-CTLs in response to stimulation with autologous LCLs. In contrast, cytokine release from transduced CTLs was minimally inhibited, and protection was almost complete when the CTLs were selected for expression of the transgene by using the HA tag. Although these CTLs, which were sorted by using an anti-HA antibody, did show a near complete resistance to these TGF-β effects, the marked resistance demonstrated with the unsorted-transduced CTLs is important when we consider using such gene-modified CTLs clinically, because the approval of such a clinical protocol may be less likely with the use of antibody-selected gene-modified CTL populations. Further, selection for TGF-β–resistant tumor-specific CTLs is likely to occur in vivo and, therefore, should be unnecessary in vitro. Finally, although the cytolytic activity of EBV-specific CTLs was greatly reduced after culture in the presence of TGF-β, the cytotoxic activity of the unsorted HATGF-βRII-Δcyt–transduced CTLs was unaffected.
TGF-β has been shown to reduce the cytolytic activity of alloreactive cytotoxic CD8+ and CD4+ T cells,43and, in the presence of IL-10, TGF-β anergizes alloreactive CD4+ T cells, which remain tolerant in vivo.44In addition, TGF-β has been shown to inhibit IL-12 and IL-2–induced cell proliferation and IFN-γ production by T cells.45-47This finding is partly due to a down-regulation of the IL-12 receptor β2 chain expression by TGF-β, resulting in the inhibition of antigen-specific activation and cytokine secretion.47 The antiproliferative effects of TGF-β on cell growth appear to be secondary to the effect of TGF-β on tyrosine phosphorylation, which results in altered control of expression and activation of cell cycle regulatory molecules.44 The anticytotoxic activities of TGF-β are mediated by 2 mechanisms. First, TGF-β inhibits early signal transduction events such as Janus kinase 2, Tyk2, and STAT4 phosphorylation after the interaction of IL-12 with its receptor on activated T cells.48 This suppression of IL-12 signaling likely explains the reduction of IFN-γ release seen in our nontransduced and eGFP-transduced established EBV-CTL lines by TGF-β.46,49 Second, the regulatory effect of TGF-β on human alloreactive CTL cytotoxic activity is associated with down-regulation of perforin and granzyme B gene expression.43,50 We also found that inhibition of EBV-specific CTL killing coincides with a significant reduction in intracellular perforin expression. In addition, we found a reduction in GM-CSF release with the addition of TGF-β to CTL cultures. Although this finding has not been previously described, it may be the result of TGF-β interference of the GM-CSF signaling pathway, including an inhibitory effect on Janus kinase 2 and STAT5.51-53 All of these effects can be prevented by expression of the HATGF-βRII-Δcyt receptor.
Although the approach we describe may counteract one of the most important tumor immune evasion strategies, many others remain intact. For example, Hodgkin tumor cells down-regulate the immunodominant viral latency proteins, EBNAs 3A, 3B, and 3 that are expressed in EBV-LPD of immunosuppressed individuals and express at least one other cytokine (IL-10) that shares with TGF-β the ability to inhibit professional APC and, indirectly, CTL activation.23,48,54 Hodgkin cells also secrete the chemokine TARC, which specifically recruits IL-4–secreting Th2 cells, and a Th2 growth factor, IL-13.55-58 Both favor the creation of a noncytotoxic Th2 environment. Although this is a daunting list of evasion mechanisms, their very profligacy argues that multiple defenses are required to protect the tumor cells from attack. Abrogation of even one or two may, therefore, produce a significant change in the effectiveness of cellular immunotherapy. Adoptive immunotherapy with ex vivo–expanded tumor-specific CTLs may evade the inhibition of professional APC function, whereas transferred resistance to TGF-β may ensure that these infused CTLs can continue to proliferate and function even in a tumor environment rich in this cytokine.
One potential concern facing the clinical use of TGF-β–resistant CTLs is that lack of response to this inhibitory cytokine may undesirably impair homeostasis of the tumor-specific lymphocytes. Indeed, mice made transgenic for a human dominant-negative TGF-βRII (DNRII) expressed exclusively in T cells, develop CD8+lymphoproliferation.59 However, the T cells involved in the lymphoproliferation were naive and IL-2 independent, and they exhibited patterns of recirculation and homeostasis that were distinct from the mature “memory” T cells studied here.60,61Other mechanisms, such as Fas and tumor necrosis factor pathways as well of lack of growth stimulation by antigen and growth factors are also available to maintain mature T-cell homeostasis.23,62 63 Certainly, we found no evidence that even long-term expression of the mutant TGF-βRII had any deleterious effects on the transduced CTL lines. In particular, their phenotype and cytotoxic specificity were unmodified, and, although they continued to grow and secrete cytokines in response to stimulation and culture in IL-2, withdrawal of these stimulants led to cell death that followed identical temporal kinetics to nontransduced cells. These results were reproducible in all 4 healthy donor CTLs and in all 4 lines derived from patients with Hodgkin disease. Another possible concern is that CTLs transduced with a mutant TGF-βRII may be recognized and eliminated by the host immune response. This situation is unlikely, because the truncation used to create the mutant receptor creates no new epitopes. Hence, CTLs expressing this construct should have a selective growth and functional advantage in vivo in patients with TGF-β–secreting tumors but should not be capable of autonomous growth.
We conclude that the expression of a transdominant-negative TGF-β receptor II (HATGF-βRII-Δcyt) in tumor-specific CTLs may allow at least one human tumor evasion strategy to be overcome.
We thank the Texas Childrens' Hospital, The Methodist Hospital, and Baylor College of Medicine for their contribution.
Supported by the Department of Pediatrics, Baylor College of Medicine, Houston, TX, research grant CA61384 from the National Institutes of Health and by Royal Australasian College of Physicians Odlin Fellowship (C.M.B.) and a Distinguished Clinical Scientist Award from the Doris Duke Foundation (H.E.H.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Cliona M. Rooney, Center for Cell and Gene Therapy, Baylor College of Medicine, 6621 Fannin St, Houston, TX 77030; e-mail:cmrooney@txccc.org.

![Fig. 1. Transduced CTLs surface express HA tag of mutant TGF-βRII receptor. / Cells of an EBV-specific CTL line 14 days after retroviral transduction with SFG:eGFP were stained with either PE-labeled anti-IgG1 (A) or anti-CD3 antibodies (B). CTLs transduced with SFG:HATGF-βRII-Δcyt (truncated TGF-βRII containing HA tag) gene were stained with FITC-labeled donkey antirabbit antibody alone (isotype control) (C). Nontransduced (D) or HATGF-βRII-Δcyt–transduced CTLs (E,F) were then stained with anti-HA monoclonal antibody followed by incubation with FITC-labeled donkey antirabbit antibody and PE-labeled CD8 or CD4 antibody. Six weeks after sorting CTLs for the HA tag, HA expression was measured on CD8+ cells (G [isotype control] and H). Surface immunofluorescence was analyzed by flow cytometry.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/9/10.1182_blood.v99.9.3179/6/m_h80922475001.jpeg?Expires=1764986591&Signature=3ciWJTZvP~BjbK8j9LsIdgErjLP3CovkFPwG-yRQrm3N8TPq8cl1jDKqjiu0cTxW19gDC~ziX74SDKaqmJTDFupCwn3URngGAxn3AEbt2dbviRMULW4goocuMV92E0guZnFCYW-1gk8Uwi~NLSCkPD9yhciuc7obvAZH7B6cs9TU4LySSBtRVIug9tvN5lNFGHpOaQZfX~Pck8Ws6ewUkzBixTAq9AYRFR8~92lngt9iBM58wR5WQBe0GcBKZjlK-ybfyQI-rChzSidKlZoNpd7TrlMpyhtI-SQmGL0tL8TLJeOkiUDBpyLWhAXFFmhd-vmHlX-UY5lECMNiRym3uw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Ability of TGF-β to inhibit T-cell proliferation is significantly greater in nontransduced CTLs when compared with HATGF-βRII-Δcyt–transduced CTLs. / Nontransduced CTLs, eGFP-transduced CTLs, and HATGF-βRII-Δcyt–transduced CTLs were stimulated with irradiated autologous LCLs and IL-2 ± 5 ng/mL TGF-β1. Proliferative responses were measured after 72 hours of incubation by measurement of3[H] thymidine uptake. The mean percentage of inhibition by TGF-β was measured in nontransduced CTLs (black), eGFP-transduced CTLs (white), and HATGF-βRII-Δcyt–transduced CTLs (gray). The graph represents a pooled analysis of the mean inhibition of TGF-β on3[H] thymidine uptake in the 8 CTL lines tested.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/9/10.1182_blood.v99.9.3179/6/m_h80922475002.jpeg?Expires=1764986591&Signature=d4xeZauZiLZNepuZ0mjniPTUAVrOoBTGh81BWxLyMcYZV-7mpUQXmtccRatq~O2xphT-OcANOCS9IpBhV69Gv4OsCd~0uyAhpghcYIkhMwz2Hj2jjHJlHXEtuH8XVBuHV9XC0AnICf4bgm0iv9XhakI68kINz1HDVY1pjROe0PmYzVo03AiK5zFG4adtN2mx-oi3WQvIeKj7AyVQq1tCWjhoMHT~x-iWoKdy1S2eduB4GDcGElpRLPXYHeVMh3MjB6mFLrjOKrAGZjPqGVRQ5dvldeoMHh-IREl81KquqasvPBJa~PNw~jtbWIU1vX9g-ssSKRA4Rel9HZEtWyAzAQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. In the presence of TGF-β, HATGF-βRII-Δcyt–transduced CTLs continue to proliferate and grow normally in long-term cultures. / HATGF-βRII-Δcyt–transduced, nontransduced, and eGFP-transduced CTLs were harvested 2 weeks after transduction. From each group, 1 × 106 cells were stimulated weekly with LCLs and fed twice weekly with IL-2 ± 5 ng/mL TGF-β. The figures show results from one representative experiment. (A) This panel represents CTL cell numbers (× 106) recorded from weekly cell counts in the 3 CTL groups grown without TGF-β (■) and with TGF-β (▴). (B) This panel shows 3[H] thymidine uptake in nontransduced CTLs (black) and HATGF-βRII-Δcyt–transduced CTLs (gray) before addition of TGF-β then on days 4 and 12.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/9/10.1182_blood.v99.9.3179/6/m_h80922475003.jpeg?Expires=1764986591&Signature=Zq3mONEjEgZRAN2T3IQnA8pp8SWFTTUeavauv8HbXJLbwNLQMvzVGEh9xAR~P4u0m6-Odpg9iKZ~QojSmrjTCK9GfknGvZZY7~W2Y0T81-hczcnmF0rAbZpik6y~EC6EdJE6ZWeDGOjt3WJPA7Zu~nyoF7u5F9S34jbjSkfWl39DMz86tnsQJSS4W1qMJ0byQWwqF-undJf9L3q-OnwGG15d-Q-9OAcHGudxj0RVzkZUtCwupOwEGsd03uXflN7P-XKPlcB0EN-6wLGZaAdTW-ZJKDoatehWqr~Xihs4pO9T2yn38Dnmb2LYx33XglJxw9SZ9KeBx-syVGBzuFeJyw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 9. HATGF-βRII-Δcyt–transduced CTLs will fail to proliferate in the absence of IL-2 or LCL stimulation. / The proliferation of long-term HATGF-βRII-Δcyt–transduced CTL (gray) and nontransduced CTL lines (black) were determined by3[H]thymidine uptake. The3[H]thymidine uptake of CTL lines left in culture for 4 weeks after transduction and stimulated weekly with LCL and IL-2 were compared with the same CTL lines grown in the absence of LCL/IL-2 stimulation for 3 weeks.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/9/10.1182_blood.v99.9.3179/6/m_h80922475009.jpeg?Expires=1764986591&Signature=yCi2vl-vJX03lXvGDqBTbANBtqp~bzKQzr3rsR8aJikUTHXWSgVSuXdEusBd07TyMza-~kGTIh4kTcW~AOeOdDef~fwl7KMCtWcIA8~xF0dVwOQU5VnCqjiOXxnl2FspbgW8vKpbJTDVB4EGxfWlN7ewOfaK6kuPR9ZXMBw0yXvoMOXWGhhWqeEldPG3l-FbH6GXNwgrE8vAjt5rOJ4EZvR0Ahy2bnWIhZzPswwUOWHtXbR3Qug1ns3LrmOCukVqufwoQdntsQj5SDdAaheC~sBq3Y9Spkt151A3FwyZ0SsOGpaxM1RYWTdQxnKI9~vxmcRxdRBrA8DhMzenAAiIlg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal