Key Points
Immunotherapy for treatment of hematological malignancies is immunosuppressive, and chronic immunosuppression is a risk factor for PML.
Early diagnosis is vital for instituting prompt immune reconstitution as treatment; brain biopsy is necessary in suspicious cases.
Abstract
Progressive multifocal leukoencephalopathy (PML) is an uncommon opportunistic infection with high morbidity and mortality. This is an institutional review board–approved retrospective review of medical records identified by diagnostic coding for PML or John Cunningham virus (JCV) from 2000 to 2015. Inclusion criteria were cerebrospinal fluid (CSF) positive for JCV by polymerase chain reaction or brain biopsy–proven PML in non-HIV patients. There were 16 patients, 12 of whom were men (75%); the median age was 56 years (range, 31-71 years). All had hematologic malignancies (5 [31%] had chronic lymphocytic leukemia, 3 [19%] had acute myeloid leukemia, 3 had [19%] mantle cell lymphoma, and 1 patient each had acute lymphoblastic leukemia, Hodgkin lymphoma, myeloma, or B-cell lymphoma). One patient received no cancer-directed therapy. Of the remaining 15 patients, all received conventional chemotherapy, and 9 (60%) underwent transplant. Thirteen patients (87%) received immunomodulating therapy (predominantly rituximab). The median time from cancer diagnosis to PML diagnosis was 48.5 months. PML was diagnosed a median of 2.1 months from symptom onset; however, the median time to PML diagnosis was 5.4 months for the 4 patients presenting with a cerebellar syndrome. PML was diagnosed by CSF in 12 patients and brain biopsy in 4 following negative CSF test results. Median survival from PML diagnosis was 4.3 months for the 11 patients on treatment and 0.87 months for the 5 without treatment. PML still occurs in patients with hematologic malignancies in the absence of treatment. Twenty-five percent of our patients required brain biopsy for diagnosis, and diagnosis was delayed when the clinical presentation was unusual, such as a cerebellar syndrome.
Introduction
Progressive multifocal leukoencephalopathy (PML) is an uncommon, opportunistic infection caused by the ubiquitous human neurotropic John Cunningham polyomavirus (JCV). By early adulthood, approximately half of the general population is JCV positive, and seroprevalence continues to increase with age.1-3 Normally, cellular immunity keeps the virus quiescent where it is harbored (in the urinary tract, bone marrow, lymphoid tissue, and possibly the central nervous system).2,4,5 However, patients with abnormalities in cell-mediated immunity are vulnerable to viral reactivation, and replication occurs in glial cells (predominantly oligodendrocytes) and neurons of the cerebrum and granular cell layer of the cerebellum. This leads to demyelination and neuronal death, causing mortality within 2 to 15 months of diagnosis; however, death usually occurs within 2 months for 90% of patients who also have a hematologic malignancy.6-8
PML was first recognized in patients with chronic lymphocytic leukemia (CLL) and Hodgkin lymphoma.7,9 During the HIV/AIDS epidemic in the 1980s, 80% of PML cases were attributable to HIV, but a second surge in PML diagnoses occurred in the mid-2000s and was seen in conjunction with marketing of novel therapeutic monoclonal antibodies used in the treatment of multiple sclerosis (MS) and hematologic cancers.7 In addition to the innate immunosuppressive nature of hematologic malignancies, immune dysfunction is compounded by treatment with cytotoxic chemotherapies, stem cell transplantation, total body irradiation, and immunomodulating treatments.5 Rituximab is a monoclonal antibody targeting CD20 cells and used in the treatment of B-cell cancers. Since gaining US Food and Drug Administration approval in 1997, there have been more than 500 rituximab-associated PML cases, a large majority occurring in patients with lymphoproliferative disorders.6,10 Additionally, following approval of brentuximab, a monoclonal antibody targeting CD30-positive cells for the treatment of anaplastic large cell lymphoma and Hodgkin lymphoma, a black-box warning was added regarding the increased risk of PML.6 Cases of PML have also been reported in patients treated with adalimumab, efalizumab, and ibritumomab tiuxetan.4,11,12 The growing dependence on these immunotherapies is a concern regarding the increased risk of PML, for which there is no effective therapy and a high mortality.8 We reviewed our experience with PML in the era of widespread use of immunomodulatory agents.
Methods
This was an institutional review board–approved retrospective review at a single cancer center from 2000 to 2015. The entire institutional database of medical records was searched, and patients were identified by diagnostic coding for PML or JCV. The sole inclusion criterion in this cancer patient cohort was a cerebrospinal fluid (CSF) positive test result for the JCV by polymerase chain reaction (PCR) or a diagnostic brain biopsy for PML. Patients had to have a diagnosis of cancer, and those with HIV/AIDS were excluded. Initially, 47 patients were identified by the diagnostic code search, but PML was not confirmed in 24 patients, and they were excluded; these 24 patients had a variety of alternative diagnoses that had no particular pattern. Twenty-three patients had a proven diagnosis based on CSF testing or tissue review following a brain biopsy; 5 were HIV positive and thus excluded. Following review of each patient’s chart, 2 more patients were eliminated on the basis of false-positive CSF results. The initial positive CSF result for these patients had low JCV titers (<72 copies/mL), and results of repeat testing performed within 1 month were negative. Of note, the negative results were not influenced by reversal of the patients’ immunocompromised state or by any PML-directed therapies. Finally, neither of these patients developed neurologic deficits or had abnormalities on brain magnetic resonance imaging consistent with demyelination. In the end, the total patient cohort was 16.
Cancer diagnosis and treatments, neurologic symptom onset and disease course, method of diagnosing PML, and subsequent PML-directed treatments were documented. Additionally, the length of time from cancer diagnosis to onset of neurologic symptoms and the time to PML diagnosis and date of death were recorded.
Results
Patient characteristics
Of the 16 patients included in the study, 75% were men; the median age was 56 years (range, 31-71) years (Table 1). All had hematologic malignancies, including 5 with CLL, 3 with acute myeloid leukemia (AML), 3 with mantle cell lymphoma, and 1 patient each with acute lymphoblastic leukemia, Hodgkin lymphoma, myeloma/plasmacytoma, Waldenstrom macroglobulinemia, or B-cell lymphoma not otherwise specified.
Cancer-directed treatment
One patient with CLL never received treatment and was under surveillance only. Of the other 15 patients, all had received conventional chemotherapy (Table 1). Additionally, 9 patients had undergone a stem cell transplant (7 of which were allogeneic transplants), and 2 patients had also received total body irradiation. Finally, 81% of patients had received immunomodulating therapy. The most commonly prescribed agent was rituximab, which was used in 9 patients (8 of whom were maintained on the drug for >6 months).
Presentation and imaging findings
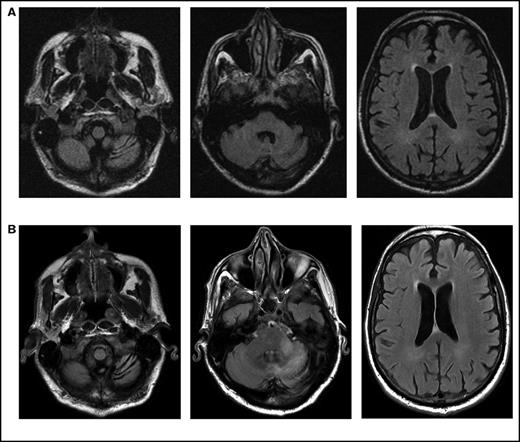
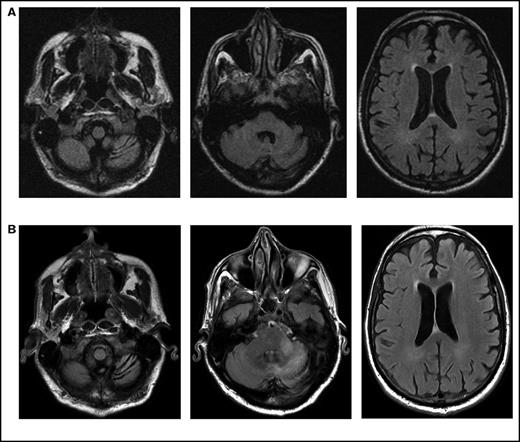
Initial symptoms included limb weakness (5 patients), visual loss (3 patients), cognitive impairment/behavior change (4 patients), and a cerebellar syndrome (4 patients). Magnetic resonance imaging abnormalities were restricted to the cerebrum in 12 patients; 2 patients had abnormalities restricted to the cerebellum and brainstem (Figure 1), and 2 patients had abnormalities in both the cerebrum and the brainstem and cerebellum.
Serial imaging of a patient diagnosed with CLL. Cancer-directed therapy included >6 months of rituximab and idelalisib. (A) Initial magnetic resonance imaging performed 2 months after the onset of headaches and balance disturbance (gait instability and repeated falls) depicting the asymmetric atrophy of the left cerebellum, which was not present in imaging performed 7 months earlier (not depicted). Fluid-attenuated inversion recovery (FLAIR) signal abnormalities are not present elsewhere. (B) Imaging repeated 3 months later (1 month prior to death) showing the continued atrophy of the left cerebellum with new confluent areas of FLAIR signal throughout the left cerebellum and left cerebellar peduncle without enhancement. Again, no FLAIR signal abnormalities are seen in the cerebrum.
Serial imaging of a patient diagnosed with CLL. Cancer-directed therapy included >6 months of rituximab and idelalisib. (A) Initial magnetic resonance imaging performed 2 months after the onset of headaches and balance disturbance (gait instability and repeated falls) depicting the asymmetric atrophy of the left cerebellum, which was not present in imaging performed 7 months earlier (not depicted). Fluid-attenuated inversion recovery (FLAIR) signal abnormalities are not present elsewhere. (B) Imaging repeated 3 months later (1 month prior to death) showing the continued atrophy of the left cerebellum with new confluent areas of FLAIR signal throughout the left cerebellum and left cerebellar peduncle without enhancement. Again, no FLAIR signal abnormalities are seen in the cerebrum.
PML diagnosis
The median time from cancer diagnosis to PML diagnosis for all patients was 48.5 months (range, 2.6-206.0 months), and the median time to diagnosis of PML was 2.1 months (range, 0.5-6.9 months) from onset of neurologic symptoms; however, the median time from symptom onset to PML diagnosis was 5.4 months (range, 1.9-5.9 months) for the 4 patients presenting with a cerebellar syndrome. The diagnosis was established by CSF testing for JCV DNA by PCR in 12 patients. In addition to the positive CSF test results, 1 patient had a confirmatory brain biopsy and another had both a confirmatory brain biopsy and an autopsy. For the 4 patients who had negative CSF test results, all had a diagnostic brain biopsy.
PML-directed treatments and outcomes
Eleven (63%) patients received PML-directed treatment (Table 2). No single regimen predominated; donor leukocyte infusions were used in 3 patients. One patient was lost to follow-up and his survival status cannot be confirmed. Median survival of all patients from PML diagnosis was 2.0 months (range, 0.4-6.7 months); median survival was 4.3 months (range, 1.6-6.7 months) for those who received treatment and 0.87 months (range, 0.4-1.1 months) for those who did not. The median Karnofsky Performance Status Scale score at PML diagnosis was 70 for the patients who received treatment and 50 for those who did not.
Discussion
The first descriptions of PML were in untreated patients with chronic hematologic malignancies. The viral etiology of this condition was not recognized for many years, at which time the inherent immune dysfunction associated with these neoplasms was appreciated as a necessary prerequisite for the full-blown syndrome. Our experience in the modern era demonstrates that PML can still occur in the untreated patient, as we observed one such patient. However, it is much more commonly associated with chronic iatrogenic immune impairment associated with vigorous and sustained treatment using immune modulating therapeutics. The only known effective therapy for PML is immune reconstitution, which is most effective in patients with minimal neurologic impairment. Plasmapheresis has been suggested in MS patients with natalizumab-related PML, but recent data demonstrate it has no benefit on clinical outcome or survival.13 In our series, we observed significant diagnostic delay in the 25% of patients who presented with a cerebellar syndrome. JCV affects the neurons in the cerebellum when there is a mutation of the VP1 gene coding for the major capsid protein, which changes the viral tropism from glial cells to cerebellar granule cells.2,4,10,14 This clinical syndrome is called granule cell neuronopathy, and the clinical manifestations include balance instability and impaired coordination with or without slurring of speech or abnormal eye movements. This atypical presentation can be diagnostically challenging, delaying testing for JCV-associated disease and the initiation of immune reconstitution. In addition to our patients, there have been documented cases of granule cell neuronopathy in patients on long-term rituximab as well as natalizumab therapy.2,10,14
A second diagnostic challenge was the 25% failure rate of CSF PCR testing among patients with JCV. Studies have demonstrated that the sensitivity of first-generation PCR detection for JCV DNA in CSF ranges from 75% to 95%.1,15,16 However, with new ultrasensitive PCR techniques, the sensitivity is >95%, and the specificity is >97%.1,3 In this series, there were 4 false-negative CSF tests, but clinical suspicion led to a confirmatory brain biopsy, which must be pursued in suspicious cases. In our series, there were also 2 false-positive results. One patient had AML and the other patient had diffuse large B cell lymphoma, both with leptomeningeal dissemination. The patient with AML presented with altered mentation in the setting of subclinical status epilepticus and returned to baseline following the initiation of antiepileptics and steroids. The patient with diffuse large B cell lymphoma was asymptomatic. Neither had imaging results suggestive of a demyelinating process. Detection of JCV DNA by PCR demonstrated only low titers, and results of repeat testing within 1 month were negative. Therefore, low-titer test results in patients without an appropriate clinical and radiographic correlate should be followed up until the situation is clarified.
Treatment duration and continued immunosuppression are risk factors for PML in any population. For patients with MS, JCV serostatus is a known risk factor for natalizumab-induced PML; it is unknown whether JCV serostatus is an important risk factor for other vulnerable populations, such as those with hematologic malignancies.17,18 When monoclonal antibodies were introduced in the treatment of MS, and as patients started to develop PML, immunosuppressive algorithms were constructed to stratify the risk potential for PML and consensus guidelines detailed the timing of periodic anti-JCV antibody testing. Biomarkers, such as the anti-JCV antibody index, are being investigated to identify MS patients at greatest risk of developing PML and to direct the use of immunomodulating therapy.19,20 These biomarkers have not been validated in other conditions, and the significance and utility of JCV testing in patients with hematologic malignancies have not been investigated but warrant study, especially in those receiving chronic immunosuppressive treatment.3,9,18
Prevention is valuable, because the condition is almost universally fatal.1 In a review of 57 HIV-negative lymphoma patients treated with rituximab who developed PML, there was a 90% fatality rate within a median survival of 2.0 months.21 So far, the aim of most PML-directed therapies is to reconstitute the immune system, which involves stopping any immunomodulating therapy.5,8 However, recovered immunity is difficult to achieve in patients with hematologic malignancies, as cancer and prior treatments have sufficiently depressed the immune system’s integrity, and recovery may not occur rapidly enough to reverse the neurologic impairment. Additionally, some pharmacological agents have been suggested based on theoretical or anecdotal evidence that they halt the exit of the virus from an infected cell, or decrease JCV replication.8,21,22 Prospective clinical studies with cidofovir, cytarabine, mefloquine, and interferon-α have not shown any benefit.5,6,23 In our patients, the median survival for the 11 patients who received PML-directed treatment was 4.3 months as opposed 0.8 months for those who were not treated; however, this is likely due to patient selection bias based on performance status and other factors, and the longer survival cannot be attributed exclusively to treatment efficacy, which is a major limitation of this retrospective review.
This study highlights the continued challenges of recognizing PML in patients with hematologic malignancies. The surveillance programs adopted in the MS community have reduced the frequency of PML associated with the chronic use of immunomodulatory therapeutics. This warrants investigation in the area of hematologic cancers, as prevention is the only approach currently used to avoid the disability and mortality associated with reactivation of JCV in the nervous system.
Acknowledgment
This research was funded by the National Institutes of Health, National Cancer Institute (cancer center support grant P30 CA008748).
Authorship
Contribution: E.C.N. cowrote the article, reviewed the institutions database to identify patients, analyzed the results, reviewed the current literature, and developed the figures; and L.M.D. reviewed the data, contributed to the analysis, cowrote the paper, and supported the research effort.
Conflict-of-interest disclosure: The authors declare no competing financial interests
The current affiliation for E.C.N. is Department of Neurology, University of Minnesota, Minneapolis, MN.
Correspondence: Lisa M. DeAngelis, Department of Neurology, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10023; e-mail: deangell@mskcc.org.