Key Points
Prednisone, as an adjunct to PEX, effectively suppresses the autoantibody inhibitor of ADAMTS13 in the treatment of iTTP.
Abstract
Although steroids are routinely used as an adjunct to plasma exchange (PEX) therapy in the treatment of immune-mediated thrombotic thrombocytopenic purpura (iTTP), limited data regarding their efficacy or effect on ADAMTS13 biomarkers are available. We report the results of a prospective, randomized study that compared the effectiveness of prednisone or cyclosporine (CSA) as adjuncts to PEX in the treatment of iTTP. A total of 26 of the planned 72 subjects were enrolled and treated from November 2007 until February 2014 before the study was halted after a planned interim analysis. Fourteen patients were randomly assigned to the prednisone arm, and 12 to the CSA arm of the study. One patient died in each arm of the study, and 2 patients in the prednisone arm of the study failed to achieve a response and crossed over to the CSA arm, leaving 11 patients in each arm of the study evaluable for the primary end point of exacerbation. One of the 11 prednisone-treated subjects (9%) suffered an exacerbation, whereas 3 of the 11 (27%) patients in the CSA arm suffered an exacerbation. Although there was no significant difference in the exacerbation rate, suppression of the anti-ADAMTS13 antibodies and improvement in ADAMTS13 activity in the first month after stopping PEX were significantly better in the prednisone-treated subjects. Side effects were manageable and comparable in both arms of the study. These data demonstrate the superiority of prednisone over CSA as an adjunct to PEX in the suppression of the anti-ADAMTS13 antibodies and improvement in ADAMTS13 activity. This trial was registered at www.clinicaltrials.gov as #NCT00713193.
Introduction
The development of plasma exchange (PEX) therapy for patients with immune-mediated thrombotic thrombocytopenic purpura (iTTP) dramatically altered the course of the disease, transforming it from a disease that was previously uniformly fatal to one where nearly all patients will recover from an acute episode. The last 20 years have also brought a greater understanding of the pathophysiology of iTTP, defining it as being mediated by autoantibodies that inhibit ADAMTS13 protease function or nonneutralizing antibodies that result in the clearance of the ADAMTS13 protease.1,2
Although the addition of immunosuppressive or modulating therapy to PEX is logically based on the pathophysiology of iTTP, very limited data exist to confirm the efficacy of steroids as an adjunct to PEX therapy and their impact on ADAMTS13 autoantibodies thought to be central to the pathophysiology of iTTP. Early reports suggested that corticosteroid therapy both alone and as an adjunct to PEX therapy may be effective in the treatment of iTTP.3 A prospective study reported by Balduini et al4 suggested that higher-dose steroids may be more efficacious than lower-dose steroids, but the relative effects of the corticosteroid therapies on ADAMTS13 activity and autoantibodies was not reported. These data, however, were before the era of more routine ADAMTS13 activity and autoantibody testing that can confirm the clinical diagnosis of iTTP and also ensure a more uniform cohort of patients for clinical study.5 In theory, this same testing could be used to judge the efficacy of immunosuppressive therapy given as an adjunct to PEX therapy.
Our group previously conducted 2 single-arm, prospective studies of adjuncts to PEX therapy in the treatment of iTTP6 : one evaluated the efficacy of corticosteroids, and one evaluated the efficacy of cyclosporine (CSA). In these 2 studies, prednisone (1mg/kg per day) or CSA (2-3 mg/kg per day) were given as an adjunct to daily PEX. These data suggested that the CSA-treated patients had a lower exacerbation rate (recurrent thrombocytopenia and the need to restart PEX in the first 30 days after the last PEX) than prednisone-treated patients and led to this prospective, randomized study (clinicaltrials.gov, identifier NCT00713193) to compare the efficacy of corticosteroids and CSA as an adjunct to PEX for the treatment of iTTP. The primary end point of the study was a comparison of the exacerbation rates between the 2 study arms, with secondary end points evaluating the effect of each immunosuppressive therapy on ADAMTS13 biomarkers in the context of clinical response criteria.
Materials and methods
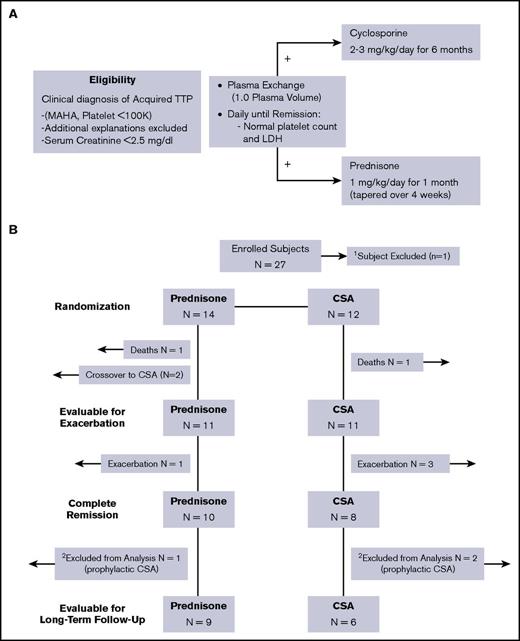
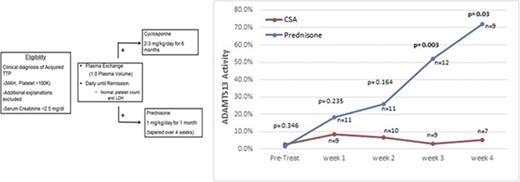
Patients with a clinical diagnosis of iTTP as defined by thrombocytopenia (<100 × 109/L) and microangiopathic hemolytic anemia, without an alternative clinical explanation, were eligible to be enrolled (Figure 1A). Both patients with newly diagnosed iTTP and a previous history of iTTP were eligible, provided that they had not been treated for an iTTP episode in the past 30 days. In light of the obvious concern to promptly initiate PEX therapy, patients were permitted to be enrolled up until 48 hours after their first PEX procedure. Given that patients could be randomly assigned to CSA, patients were also required to have a serum creatinine concentration of <2.5 mg/dL at the time of enrollment. Although the documentation of severely deficient ADAMTS13 activity was not an enrollment criterion, patients with stem cell transplant, metastatic cancer, and drug-associated forms of thrombotic microangiopathies were excluded. Institutional review board approval and oversight was obtained for this study. Patients or their legally authorized representative provided written informed consent at the time of enrollment. Patients were enrolled between November 2007 and February 2014 at 2 participating sites, The Ohio State University Hospital and Riverside Methodist Hospital, both in Columbus, Ohio. Enrolled patients were randomly assigned 1:1 in a prespecified manner to balance the number of patients with newly diagnosed TTP and relapsed TTP in each arm of the study.
Treatment plan and description of outcomes for enrolled subjects. (A) The eligibility and treatment schema for the study are shown. Eligible patients were randomly assigned to prednisone or CSA 1:1 in a prespecified manner. (B) The eligibility and treatment schema for the study are shown. A total of 11 subjects in each arm were evaluable for the primary outcome of exacerbation. Of the 18 subjects evaluable for long-term relapse rate, 3 subjects (1 in the prednisone arm, 2 in the CSA arm) elected to receive prophylactic therapy and were not evaluable for relapse.
Treatment plan and description of outcomes for enrolled subjects. (A) The eligibility and treatment schema for the study are shown. Eligible patients were randomly assigned to prednisone or CSA 1:1 in a prespecified manner. (B) The eligibility and treatment schema for the study are shown. A total of 11 subjects in each arm were evaluable for the primary outcome of exacerbation. Of the 18 subjects evaluable for long-term relapse rate, 3 subjects (1 in the prednisone arm, 2 in the CSA arm) elected to receive prophylactic therapy and were not evaluable for relapse.
Clinical definitions
For the purposes of this study, clinical response was defined as the achievement of a normal platelet count (>150 × 109/L), normal lactate dehydrogenase (LDH), and stabilization or improvement of any neurologic or renal injury present. Patients that maintained these clinical response criteria for 30 days after their last PEX procedure were defined as achieving a complete remission. An exacerbation of TTP was defined as the need to restart PEX therapy within 30 days after the last PEX procedure. A recurrence of TTP beyond 30 days after a patient’s last PEX procedure was defined as a relapse of TTP.
Treatment
All patients initiated daily PEX (1 plasma volume) using plasma as the replacement fluid. Patients randomly assigned to the corticosteroid arm received prednisone at a dose of 1 mg/kg per day rounded to the nearest 20-mg increment. Patients randomized to the CSA arm received CSA at a dose of 2 to 3 mg/kg (rounded to the nearest 50-mg increment) divided into a twice-daily dose (Figure 1A). Patients randomly assigned to the CSA arm did not receive steroids, but were permitted to receive hydrocortisone as required for any hypersensitivity reactions to the infused plasma. Daily PEX was continued until a clinical response was achieved, and then patients received PEX every other day for 2 additional procedures, with the first day that the platelet count and LDH were normal counting as the first of the 2 procedures.
Prednisone or CSA were continued at the same initial dose throughout their inpatient treatment until they were seen in the outpatient clinic. Prednisone was continued at 1 mg/kg for 4 weeks after discharge from the hospital with no dose alterations unless there were adverse effects. After completing these 4 weeks of therapy, prednisone was tapered and discontinued over the next 4 weeks, tapering the dose by 50% each week over the subsequent 4 weeks with all prednisone therapy being stopped 8 weeks after their discharge from the hospital and their last PEX procedure. CSA was continued at the same dose for 6 months after achieving a clinical response with no tapering of therapy as was done in our previous clinical trial.
Clinical follow-up
Patients were seen weekly for the first 4 weeks beginning 1 week after discharge from the hospital to monitor for exacerbations of TTP. Then, patients were seen monthly for the next 5 months, and were then seen every 3 months for 3 years. Patients who either died, suffered an exacerbation or relapse of TTP, or received any prophylactic therapy to prevent relapse while in remission were censored at the time of the event. At the time of each clinical evaluation, blood was obtained for correlative biomarkers, and subjects were assessed for any potential toxicities.
Laboratory testing
In addition to routine clinical laboratory studies to evaluate for relapse or continued remission of their TTP, blood was obtained for the purpose of studying ADAMTS13 activity and anti-ADAMTS13 antibody concentration before their first PEX procedure and at the time of each outpatient follow-up visit. For patients randomly assigned to the CSA arm of the study, trough CSA concentrations were also obtained every 3 days during inpatient treatment and then at the time of each outpatient visit. CSA concentrations were used to monitor for toxicity and decrease the dose for toxic levels, but were not used to adjust the dose to achieve any therapeutic range.
Correlative biomarker methodology
ADAMT13 activity and inhibitor titer.
The Biomarker Reference Laboratory at The Ohio State University has validated and standardized the testing for ADAMTS13 biomarkers to meet the requirements set forth by the College of American Pathologists for clinical testing. Testing is done using a surface-enhanced laser desorption/ionization time-of-flight mass spectrometer. The surface-enhanced laser desorption/ionization time-of-flight mass spectrometry methodology involves the surface chemistry of ProteinChips that allows for selective, rapid purification of protein/peptide candidates before analysis by mass spectrometry as reported previously.7
Anti-ADAMTS13 antibody concentration.
Quantification of ADAMTS13 autoantibody (immunoglobulin G [IgG]) was measured using commercially available enzyme-linked immunosorbent assay kits from Technoclone (reference #5450401) with the testing performed per the manufacturer’s instructions. To establish a range of normal for our study, 40 normal donors were studied. The range of normal established in our laboratory was 1.1 to 11.8 U/mL.
Statistical methodology
Statistical significance was concluded based on a significance level of .05. This study included an interim analysis and formal evaluation of the clinical and laboratory correlative studies, where the null hypothesis will be rejected if the calculated P value comparing the 2 observed success rates for the treatment arms is <.0002 (based on the Lan-DeMets α spending function and O’Brien-Fleming boundary calculation). The exacerbation rate was the primary end point of the study. Additionally, we evaluated several secondary clinical end points through this trial, including: (1) clinical response rate; (2) the number of PEX procedures required to achieve a normal platelet count; and (3) the safety and tolerability of each of the adjuvant treatments to PEX. The rates for each end point were calculated assuming that these end points were binomially distributed, and 95% confidence intervals were generated. For the exacerbation rate end point, we only included those patients who achieved a clinical response with their initial therapy and compared these rates between the 2 arms. Patients that crossed over to the other arm of the study were excluded for analysis of the exacerbation end point.
Results
Clinical outcomes
From November 2007 until February 2014, a total of 27 of 72 planned patients were enrolled and treated at both The Ohio State University (n = 26) and Riverside Methodist Hospital in Columbus, Ohio (n = 1). One patient who was enrolled in the CSA arm was subsequently excluded after it was determined that his clinical diagnosis was atypical hemolytic syndrome (confirmed by the finding of an MCP mutation). Four patients who were randomly assigned to the prednisone arm of this study had been previously enrolled in the single-arm CSA study when they had a previous episode of iTTP. This study has been previously reported.6 The demographic details for all enrolled patients are shown in Table 1, and the schema of enrolled patients are shown in Figure 1B. Fourteen patients were randomly assigned to the prednisone arm of the study, and 12 were randomly assigned to the CSA arm of the study. All but 1 patient had a pretreatment ADAMTS13 activity level of <10%. The 1 patient with an ADAMTS13 activity level of >10% (12.4%), however, had previously documented severely deficient ADAMTS13 activity at the time of a prior acute TTP episode. One patient died in each arm of the study during their initial hospitalization as a direct result of their TTP, and 2 patients in the prednisone arm of the study failed to achieve a response and crossed over to the CSA arm as specified in the study, leaving 11 patients in each arm of the study who achieved a response and were evaluable for the primary end point of exacerbation. The median number of PEX procedures to achieve a clinical response was 5 in both arms of the study, with a range of 4 to 8 days and 4 to 19 days for the prednisone and CSA arms, respectively (Table 2). In the prednisone arm of the study, 1of the 11 (9%) had an exacerbation, whereas 3 of the 11 (27%) patients in the CSA arm had an exacerbation of TTP. Remission rates (no initial treatment failure, no exacerbation) after their initial therapy with prednisone or CSA, respectively, were also not significantly different (10 of 14 patients [71%] vs 8 of 12 [67%] between the 2 arms of the study (Figure 1B). At the time of the planned interim analysis, it was clear that the study was unlikely to show a difference in the 2 arms of the study in terms of the primary end point. Given this in the context of the ADAMTS13 activity and inhibitor concentration data available and the slower than expected enrollment, a decision was made to close the study and report the results. Accrual to the study was also slower than expected, emphasizing the inherent difficulties in performing a prospective, randomized clinical trial for a rare disease.
Only patients that completed their planned therapy, did not suffer an exacerbation, and did not receive any additional prophylactic treatment were evaluable for the long-term relapse rate (9 patients in the prednisone arm and 6 patients in the CSA arm). The patients that received additional treatments were excluded given the additional and heterogeneous treatment they subsequently received that would confound the relapse analysis for patients in either arm of the study. A schema of enrolled patients and the number eligible for each outcome measure is shown in Figure 1B. There was no difference in the relapse rate of patients in either arm of the study. Nine subjects in the prednisone arm were evaluable for the long-term relapse rate with 4 of 9 patients (44%) relapsing at a median of 15.5 months (range, 4-24 months) from their last TTP episode, and 5 of 9 patients (56%) staying in a continuous remission for a median of 43 months (range, 12-96 months) after their last episode. Six subjects in the CSA arm were evaluable, with 3 of 6 patients (50%) relapsing after a median of 5 months (range, 2.5-9 months) from their last episode, and 3 patients remaining in remission after a median of 34 months (range, 24-70 months) of follow-up. Two of the 3 CSA patients that relapsed did so while on CSA therapy. One additional CSA-treated patient died suddenly at home after 2 years of follow-up, but the cause of death was not known.
ADAMTS13 biomarker data
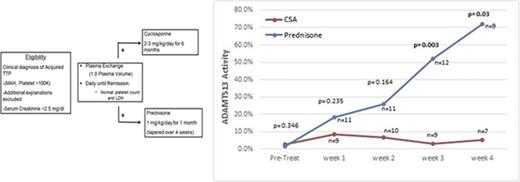
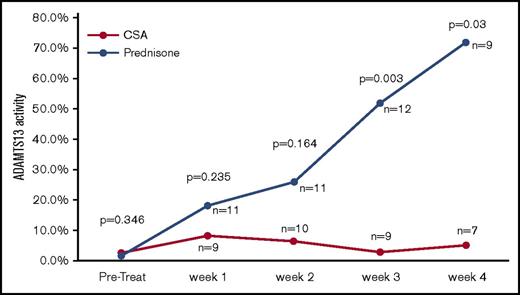
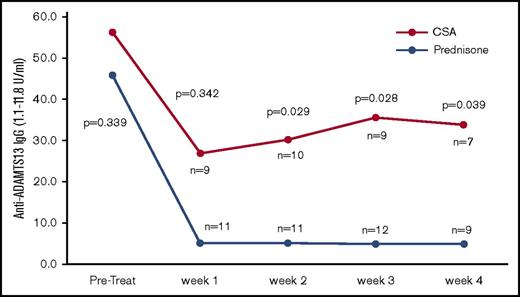
ADAMTS13 activity was measured before the first PEX procedure, weekly during the first month after stopping PEX, monthly for the next 5 months, and then every 3 months for a total of 3 years. The first measurements after being discharged from the hospital were obtained at least 5 days after their last PEX procedure to minimize the effect on ADAMTS13 biomarkers. During the first month after discharge (the time period to evaluate for exacerbation), there was no tapering of the prednisone, so the comparison of the 2 arms is with both arms continuing at the same dose that patients were treated with initially. Figure 2 shows the serial measurements of ADAMTS13 activity during the first month of follow-up. There was a clear trend toward a greater improvement in ADAMTS13 activity, with significantly higher ADAMTS13 activity from week 3 onward in the prednisone-treated patients. The inhibitor concentration data were also consistent with the ADAMTS13 activity data, with greater suppression of the anti-ADAMTS13 IgG antibodies in the prednisone-treated patients compared with the CSA-treated patients (Figure 3). Serial studies of the anti-ADAMTS13 antibodies in the first month demonstrated significantly greater suppression in the prednisone-treated subjects compared with the CSA-treated subjects beginning in week 2.
ADAMTS13 activity over the first month of follow-up after stopping PEX. The serial measurements of the median ADAMTS13 activity over the first month of follow-up after the last PEX procedure are shown. ADAMTS13 activity was significantly greater in the prednisone arm compared with the CSA arm beginning at week 3 of follow-up.
ADAMTS13 activity over the first month of follow-up after stopping PEX. The serial measurements of the median ADAMTS13 activity over the first month of follow-up after the last PEX procedure are shown. ADAMTS13 activity was significantly greater in the prednisone arm compared with the CSA arm beginning at week 3 of follow-up.
Anti-ADMATS13 antibody levels over the first month of follow-up after stopping PEX. The serial measurements of the median anti-ADAMTS13 IgG and inhibitor titer levels over the first 4 weeks after stopping PEX are shown. The anti-ADAMTS13 IgG levels are significantly lower in the prednisone-treated patients beginning in week 2, corresponding well to the improvement in ADAMTS13 activity. There was no statistically significant difference in the inhibitor titers between the 2 arms of the study.
Anti-ADMATS13 antibody levels over the first month of follow-up after stopping PEX. The serial measurements of the median anti-ADAMTS13 IgG and inhibitor titer levels over the first 4 weeks after stopping PEX are shown. The anti-ADAMTS13 IgG levels are significantly lower in the prednisone-treated patients beginning in week 2, corresponding well to the improvement in ADAMTS13 activity. There was no statistically significant difference in the inhibitor titers between the 2 arms of the study.
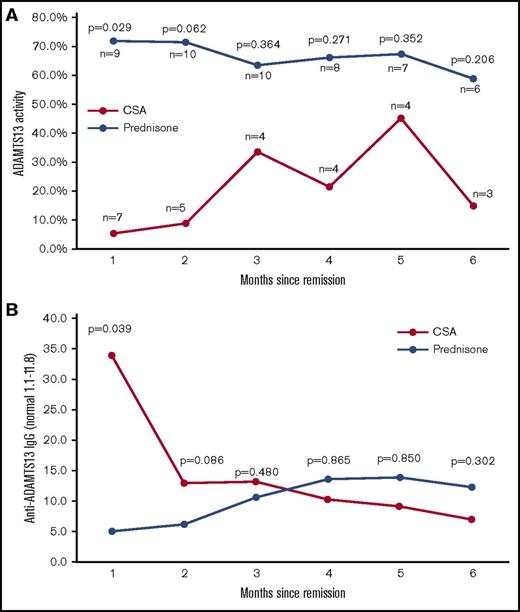
The ADAMTS13 activity and anti-ADAMTS13 IgG antibody data from the first 6 months of follow-up for both the prednisone and the CSA arms of the study are shown in Figure 4. There was a gradual improvement in ADAMTS13 activity over time during ongoing therapy with CSA, with the prednisone-treated patients having stable ADAMTS13 activity despite discontinuing all steroid treatment after month 2.
ADAMTS13 activity and anti-ADAMTS13 antibody levels over the 6 months after stopping PEX. The measurements of the median ADAMTS13 activity (A) and anti-ADAMTS13 IgG concentration (B) are shown for both the prednisone- and CSA-treated patients. Patients that suffered an exacerbation or that crossed over from another treatment arm are not included in these graphs. In the prednisone arm of the study, all prednisone was tapered and stopped at the end of month 2, whereas the CSA-treated patients continued with the full-dose treatment throughout the 6-month time period shown.
ADAMTS13 activity and anti-ADAMTS13 antibody levels over the 6 months after stopping PEX. The measurements of the median ADAMTS13 activity (A) and anti-ADAMTS13 IgG concentration (B) are shown for both the prednisone- and CSA-treated patients. Patients that suffered an exacerbation or that crossed over from another treatment arm are not included in these graphs. In the prednisone arm of the study, all prednisone was tapered and stopped at the end of month 2, whereas the CSA-treated patients continued with the full-dose treatment throughout the 6-month time period shown.
Safety and tolerability
Overall, therapy with prednisone and CSA was tolerated well with only minor side effects. Three of the 11 prednisone-treated patients that achieved remission tapered their steroids sooner than the planned 4 weeks due to side effects of the prednisone therapy (hyperglycemia, water retention, and insomnia, respectively). Two CSA-treated patients suffered side effects of therapy. One patient developed symptoms of shakiness that were associated with increased CSA concentrations and resolved with a CSA dose reduction. The other patient developed symptoms of restless legs after 3 months of CSA therapy that resolved after she stopped CSA.
Discussion
Despite their routine use in the treatment of iTTP, there are limited prospective data to support the efficacy of steroids or other forms of immunosuppressive therapy to decrease ADAMTS13 autoantibodies and improve ADAMTS13 activity in the treatment of iTTP. It has been presumed that steroids or other immunosuppressive therapies decrease the production of ADAMTS13 autoantibodies, but there have been no prospective randomized studies that demonstrate the efficacy of steroids to inhibit the production of ADAMTS13 autoantibodies in patients with iTTP. These data demonstrate for the first time, to our knowledge, the efficacy of prednisone (and CSA) in iTTP and provide evidence that their efficacy is at least in part due to the suppression of ADAMTS13 autoantibody production. Based on these results, corticosteroids should be used as an adjunct to PEX in all patients being treated for an acute episode of iTTP.
This study demonstrates the ability of both prednisone and CSA to suppress anti-ADAMTS13 autoantibody production. This will be increasingly important as we move into an era of management of iTTP, where the focus will be on therapy to directly suppress ADAMTS13 autoantibodies. Although acquired ADAMTS13 protease deficiency is known to be central to the pathogenesis of iTTP, there are also data suggesting that severely deficient ADAMTS13 activity is a risk factor for TTP relapse.8-10 Furthermore, there are also published data suggesting the prophylactic correction of ADAMTS13 deficiency can prevent relapse.11-15 A recent clinical trial of caplacizumab (a small molecule that targets the A1 domain of von Willebrand factor and prevents the interaction with platelets) demonstrated that the discontinuation of therapy before the recovery of ADAMTS13 activity to >10% was associated with exacerbations and early relapses.16 In the context of this promising therapeutic approach, there will be an increased emphasis on treatment that directly targets ADAMTS13 autoantibodies.
The primary end point of this prospective randomized study was to compare the exacerbation rates between the prednisone/PEX-treated patients and the CSA/PEX-treated patients. Although there was no significant difference in the exacerbation rates, a review of ADAMTS13 activity and autoantibody concentration data over the first 4 weeks after discharge demonstrates an advantage of prednisone over CSA in the suppression of the inhibitor and improvement in ADAMTS13 activity. Although the more effective immunosuppression with prednisone did not translate into a decreased number of PEX procedures to achieve a normal platelet count or a decreased exacerbation rate in this study, this may be a result of the relatively small numbers of subjects.
Nine patients in the prednisone arm and 6 patients in the CSA arm were evaluable for long-term outcomes and relapse (Figure 1B). Collectively from both arms, 7 of 15 (47%) patients had a relapse during follow-up. This rate of relapse is consistent with previously published reports11,17-19 and was equal in both arms of the study. The small number of subjects limits any conclusions that can be drawn regarding the relapse rates after prednisone or CSA, but the data do suggest that short-term improvement in ADAMTS13 activity and better suppression of ADAMTS13 autoantibodies do not translate into long-term clinical benefit. Given the planned every-3-months follow-up in this study, ADAMTS13 activity measurements in the visit before the relapse of these 7 subjects were available at varying time points before relapse. Two of the 7 relapsing patients had a remission ADAMTS13 activity of <10% (1.8% and <0.5%). The other 5 patients with a prerelapse sample all had ADAMTS13 activities between 10% and 20% in their last remission sample before relapse, with all 5 patients clearly declining in the months leading up to relapse. Although the small number of relapse subjects limits any conclusions that can be drawn regarding the long-term relapse rates after prednisone or CSA, these data support the role of routine ADAMTS13 monitoring in remission to identify those patients who are at an increased risk for relapse that may benefit the most from prophylactic therapy.
Although these results support the superiority of prednisone over CSA in the initial treatment of iTTP, CSA should not be interpreted as ineffective immunosuppression in iTTP. There are several reports describing the efficacy of CSA for either refractory TTP or as a prophylactic therapy to prevent relapse.8,20-22 Even though the prednisone-treated patients more quickly suppressed ADAMTS13 autoantibodies, the CSA-treated patients also showed suppression of the anti-ADAMTS13 antibodies, but took a longer time to achieve this result. The relatively longer time that was required for improvement in ADAMTS13 activity in the CSA arm may in part explain why it was less effective than the prednisone in this study, but still can be effective as a prophylactic therapy.15 Although prednisone appears to be superior to CSA as an adjuvant to PEX therapy in the acute treatment of iTTP, CSA may still have a role for refractory disease and as a prophylactic therapy.
This study provides important data regarding the ability of adjuvant immunosuppressive therapy to inhibit the production of anti-ADAMTS13 autoantibodies, but it does have limitations. Although we believe that the decision to stop the study prematurely was appropriate, this may have prevented our study from achieving the primary end point of determining which agent could more effectively prevent early recurrences or exacerbations of iTTP. In addition, the long-term effect on relapse with upfront treatment with prednisone or CSA was confounded in almost half of the subjects by the addition of other immunosuppressive medications to treat refractory disease, exacerbations, or the use of prophylactic CSA in several patients.
In conclusion, these data demonstrate the efficacy of prednisone as an adjunct to PEX therapy to efficiently suppress the production of ADAMTS13 autoantibodies. They also demonstrate that prednisone more effectively suppresses ADAMTS13 autoantibodies than CSA with a comparable side effect profile, which supports its routine use as an adjunct to PEX in the acute treatment of iTTP.
Acknowledgment
This work was supported by R01 grant 3992 from the US Food and Drug Administration Office of Orphan Products Development.
Authorship
Contribution: S.R.C. designed the study, analyzed the data, and wrote and edited the manuscript; J.N.G. and C.M. designed the study, analyzed the data, and edited the manuscript; H.M.W. designed the study and analyzed the data; P.J.K. and L.W. analyzed the data and edited the manuscript; S.Y. and H.W. performed the correlative studies, analyzed the data, and edited the manuscript; and S.G. designed the study and statistical methods, analyzed the data, and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Haifeng M. Wu died on 27 July 2015.
Correspondence: Spero R. Cataland, Department of Hematology, The Ohio State University, A361 Starling Loving Hall, 320 W. 10th Ave, Columbus, OH 43210; e-mail: spero.cataland@osumc.edu.