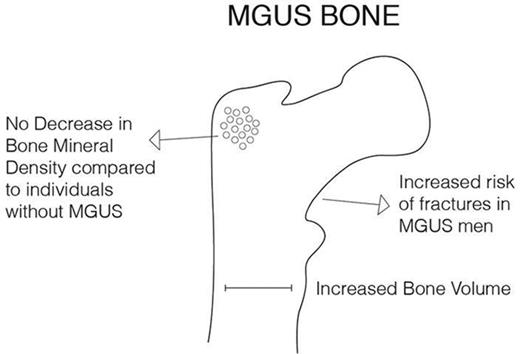
Key Points
Individuals with MGUS did not have a decreased BMD compared with others in a screened population.
Individuals with MGUS had an increased bone volume at the hip and lumbar spine compared with others.
Abstract
Previous studies have shown that individuals with monoclonal gammopathy of undetermined significance (MGUS) have an increased risk of fractures, although the underlying mechanisms remain unknown. Our aim was to analyze bone mineral density (BMD), bone volume, and risk of fractures among individuals with MGUS. We performed a screening using the Age, Gene/Environment Susceptibility (AGES)–Reykjavik Study cohort, consisting of 5764 elderly individuals, identifying 300 individuals with MGUS, and 275 with light-chain MGUS. Quantitative computerized tomography was performed in the lumbar spine and hip to evaluate BMD and bone geometry. Analysis of variance and the Tukey honest significance test were used to compare the groups. Hospital records were used to record fractures, with a mean follow-up of 6.9 years. Cox proportional hazard was used to compare fracture risk. No difference was found in BMD between subjects with MGUS and others in the spine (P = .34) or in total hip (P = .30). Individuals with MGUS had a significant increase in bone volume compared with others in the spine (P < .001) and total hip (P < .001). Overall, the risk of fractures was not significantly increased in individuals with MGUS (hazard ratio [HR], 1.19; 95% confidence interval [CI], 0.94-1.50). Men with MGUS had a significantly increased fracture risk, compared with other men (HR, 1.46; 95% CI, 1.03-2.08). Our results show that although individuals with MGUS do not have decreased BMD, bone volume is increased, and MGUS men have a 50% increased fracture risk. These results indicate that bone disease and fractures in MGUS differ from processes known from osteoporosis.
Introduction
Monoclonal gammopathy of undetermined significance (MGUS) is a precursor condition preceding multiple myeloma (MM) and other lymphoproliferative disorders. Accordingly, light-chain MGUS (LC-MGUS) is a precursor condition preceding light-chain MM.1 The prevalence of MGUS increases with age, and is higher in men than in women.2 Patients with MM have a high incidence of bone disease, including osteopenia, osteolytic lesions, and fractures, which can significantly increase morbidity and mortality in patients with MM.3 We previously performed a population-based study of 5326 MGUS patients diagnosed in Sweden, compared with 20 161 matched controls, and observed a 1.6-fold significantly increased risk of any fracture at 10 years’ follow-up in MGUS patients. Furthermore, there was a higher risk for axial (skull, vertebral/pelvis, and sternum/costae) compared with distal (arm and leg) fractures.4 Results from other published studies on fractures in MGUS patients5-8 have shown 1.4- to 6.3-fold increased risk of fractures in MGUS patients. Researchers have found a high prevalence of MGUS in osteoporotic patients,9-12 which has led some authors to suggest that protein electrophoresis should always be performed as screening for MGUS (and MM) in patients with osteoporosis and/or fractures.9,10,13 However, these studies have been limited by lack of control population, and the scientific rationale for these recommendations is scarce.
Fractures are a known result of age-related osteoporosis, and risk of fractures is increased in individuals with decreased bone mineral density (BMD).14 Traditionally, risk of fractures due to age-related bone loss is assessed from BMD, which is measured by dual-energy x-ray absorptiometry (DXA). Quantitative computerized tomography (QCT) is often used in osteoporotic research, and is a technique that has several advantages over DXA, including that cortical and trabecular bone can be separated, and trabecular volumes can be estimated. Low scores for the QCT measures vertebral trabecular BMD, femoral neck cortical thickness, and femoral neck trabecular BMD have been associated with increased risk of fractures in both men and women.15
Two studies have used skeletal imaging techniques to compare bone disease in MGUS patients to matched controls.16,17 In a study on 50 MGUS patients, compared with 100 controls, MGUS patients were found to have decreased BMD at the total femur, but no differences were found at other sites. High-resolution peripheral QCT (HRpQCT) imaging showed an overall increase in bone size, diminished cortical thickness, increased endocortical area of the distal radius, higher cortical porosity, and reduced bone strength in MGUS patients.16,17 In addition to this, bone metabolism in MGUS may be altered, although studies on bone markers have shown inconclusive results.17-21
In summary, these data indicate that MGUS patients have an increased risk of fractures and altered bone microstructure. However, it is uncertain whether the risk of fractures is a result of loss of BMD, or due to other factors affecting bone strength. MGUS is an asymptomatic condition and prior studies are mostly limited to individuals that have incidentally been diagnosed with MGUS. This limits the generalizability of previous studies due to the underlying conditions or symptoms that led to a diagnosis of MGUS. Therefore, an MGUS study using patients from a screening program is ideal to avoid this.
To better understand the underlying mechanisms of bone disease in MGUS, we conducted a screening for MGUS in a population-based cohort of 5764 elderly Icelandic men and women, in which 300 individuals had MGUS and 275 individuals had LC-MGUS, and measured BMD and bone volume in all participants. Furthermore, we collected information on fractures during a follow-up of almost 7 years. Because women have a higher risk of osteoporosis and fractures due to hormonal features,22 we assessed men and women separately.
Methods
AGES Reykjavik
The Age, Gene/Environment Susceptibility–Reykjavik Study (AGES-RS)23,24 is a continuation of the Reykjavik Study, a population-based cohort study that started in 1967. In the Reykjavik Study, men and women born in 1907 to 1935 and living in the Reykjavik area were invited to participate, and 19 381 attended (63%). The AGES-RS Study started in 2002 when 8030 randomly selected individuals of the 11 549 surviving participants from the Reykjavik Study were invited to take part again, and 5764 enrolled (72%). In 3 clinic visits, extensive data were collected and various measurements performed, including anthropometry and QCT measurements. Participants were asked to bring all medications used in the 2 weeks leading up to the first visit representing current medication, and drugs affecting bone metabolism were identified (tibolone, antiepileptics, systemic glucocorticosteroids, raloxifene, calcitonin, and bisphosphonates).
Bone measurements and fractures
The radiological measurements have been described in detail previously.22 In brief, QCT measurements were performed in the lumbar spine and the left hip with a 4-row detector CT system (Sensation; Siemens Medical Systems, Erlangen Germany). A calibration phantom was used to calibrate CT Hounsfield units to equivalent bone mineral concentrations. In the lumbar spine, a helical study of the L1 and L2 vertebrae was performed, and in the hip the helical study included the proximal femur from a point 1 cm superior to the acetabulum to a point 3 to 5 mm inferior to trochanter minor.
CT images were transferred from the CT scanner to a network of computer workstations equipped with the Linux operating system (Red Hat Version 7.2) and the AVS5 visualization program (AVS, Waltham, MA). Images were processed to extract measures of volumetric BMD and bone size from scans of the spine and hip using semiautomatic analysis techniques. This involved calibration of the images and segmentation procedures to determine trabecular, cortical, and integral regions of interest in the L1 and L2 vertebrae and the proximal femur. For each region, trabecular, cortical, and integral volumetric BMD (grams per cubed centimeters) was obtained.
Reasons for exclusion from QCT measurements were metal implants at the scanned area, weight over 150 kg, and inability to lie supine. A total of 447 spine measurements were unavailable, 31 in the MGUS group and 35 in the LC-MGUS group. Furthermore, 912 hip measurements were not available, 66 for MGUS and 60 for LC-MGUS. Individuals with data missing for both spine and hip (a total of 420, including 31 MGUS and 32 LC-MGUS) were excluded. Thus, in the final analysis, a total of 5278 spine measurements (92% of total, 269 for MGUS and 240 for LC-MGUS) and 4813 hip measurements (84% of total, 234 for MGUS and 215 for LC-MGUS) were used.
Fracture data were ascertained from the Reykjavik Study fracture registry, which is verified by medical and radiological records.25 Since the late 1960s, all patients with fractures diagnosed on an outpatient basis in Reykjavik were referred to the region’s only outpatient trauma clinic based at the Landspitali University Hospital. Hospital records for all participants from Landspitali University Hospital, and Akureyri Hospital, the largest hospital outside Reykjavik, were accessed using the participants’ personal identification numbers. All fractures were recorded from the individuals’ enrollment into the study until 31 December 2011, with a mean follow-up time of 6.9 years (median, 8.2 years). In the fracture analysis, individuals were censored after first fracture.
MGUS and LC-MGUS diagnosis
Blood was drawn from each participant at the first clinic visit in the AGES-RS study. To identify individuals with MGUS and LC-MGUS, serum samples were subjected to conventional agarose-gel serum electrophoresis (SPEP; Helena Laboratories, Beaumont, TX). In samples with M-proteins present, immunofixation electrophoresis was performed (SPIFE 3000; Helena Laboratories, Beaumont, TX). Finally, the serum free light chain (FLC) assay was performed on a SPAPLUS automated analyzer for special protein analysis using Freelite reagents (Freelite; The Binding Site Ltd, Birmingham, United Kingdom). Two individuals, blinded to details concerning the samples being tested, did all testing and interpretations. MGUS was defined as having M-protein present on SPEP and an M-protein concentration of <30 g/L. LC-MGUS was defined as having no M-protein visible on SPEP, having a pathological FLC ratio (<0.26 or >1.65) on FLC analysis as well as an increased concentration of the involved light chain (f-κ > 19.4 mg/L, f-λ > 26.3 mg/L).1 A total of 39 individuals were excluded from analysis due to previous lymphoproliferative disease at baseline (21), missing blood sample (16), missing consent form (1), or M-protein concentration above the limit for MGUS (1).
The study was approved by the Icelandic National Bioethics Committee (VSN 00-063), the Icelandic Data Protection Authority, and the US National Institute on Aging Institutional Review Board.
Statistical analysis
Mean values of QCT measurements were calculated for 3 groups: MGUS, LC-MGUS, and those without MGUS. Analysis of variance (ANOVA) was used to determine whether there was a significant difference between the means. When ANOVA showed a significant result, the Tukey honest significance difference test (Tukey HSD) was used to define between which groups there was a significant difference. Results were adjusted for age and sex, and in a separate analysis for age, sex, height, and weight.
Cox proportional hazard models were used to compare risk of fractures in individuals with MGUS, LC-MGUS, and those without MGUS. Time at risk was defined as time from enrollment in AGES-RS until first fracture, progression to MM, death, or end of follow-up. Cox proportional hazard models were also used to examine whether fractures in individuals with MGUS were a risk factor for progression to MM. Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated overall and separately for men and women, adjusted for age and sex. Results were considered significant at P < .05. All statistical analysis was performed in R version 3.2.2.26
Due to the unexpectedly high prevalence of LC-MGUS in our study cohort (4.8%) with a great κ dominance (96%), we performed further analysis. We did additional assessments using different cutoffs for LC-MGUS and defined LC-MGUS using the 97.5th percentile of the log-transformed κ and λ values (that resembled normal distribution).
Results
Of the 5725 included participants, 2419 were men and 3306 women, with a median age of 76 years. Among these, 300 individuals with MGUS (159 men and 141 women) and 275 individuals with LC-MGUS (156 men and 119 women) were identified. The median age was higher for MGUS (78 years) and LC-MGUS (80 years), than for individuals without MGUS (76 years). Furthermore, there was a male dominance in the MGUS and LC-MGUS groups, as opposed to a female dominance in the group without MGUS. Individuals with MGUS and LC-MGUS had a significantly higher mean height than the other groups. The proportion of smokers and of individuals taking osteoporosis medications or glucocorticoids was similar for the groups (Table 1).
QCT measurements
A total of 5305 individuals were included in the bone analysis, including 269 individuals with MGUS (148 men and 121 women) and 243 individuals with LC-MGUS (141 men and 102 women).
No difference was found in integral BMD between subjects with MGUS, LC-MGUS, and others at the spine (P = .34), femoral neck (P = .72), trochanter region (P = .35), or total hip (P = .30; Tables 2-4). Similarly, no difference was found in trabecular or cortical BMD (Tables 2-4).
Volumetric measurements showed that individuals with MGUS had a statistically significant increase in vertebral body cross-sectional area for both L1 (P < .001) and L2 (P < .001), as well as mean vertebral volume of L1/L2 compared with those without MGUS (P < .001; Table 2). In the femoral neck area, both cortical (P < .001) and integral volume (P < .001; Table 3) was significantly increased in MGUS compared with others. In the trochanter area, a significant difference was found in integral volume (P < .001; Table 3). In total hip, a significant increase was found in cortical (P < .001), trabecular (P < .001), and integral volume (P < .001; Table 4) in MGUS compared with those without MGUS. Furthermore, a significant difference was found in integral bone volume in total hip in MGUS men, compared with other men (P = .046).
Correspondingly, for LC-MGUS, a significant increase in bone volume was found at vertebral body cross-sectional area for L1 (P < .001) and L2 (P < .001), and mean vertebral volume of L1/L2 compared with others (P < .001; Table 2). In the femoral neck and trochanter area, integral volume was significantly increased in LC-MGUS compared with others (P = .02 and P < .001; Table 3). In total hip, a significant increase was found in cortical (P < .001), trabecular (P < .001), and integral volume (P < .001; Table 4) in LC-MGUS compared with others. Women with LC-MGUS had a significant increase in cortical volume in femoral neck (P = .015) and total hip (P = .006). Furthermore, in women with LC-MGUS, a significant decrease in integral BMD was found at the trochanter area (P = .02) and total hip (0.026) compared with other women.
Further adjustments for weight in BMD measurements, or for height in volumetric measurements, did not change the results (data not shown).
Fractures
All patients were included in the fracture analysis. A total of 1334 fractures were recorded after a median follow-up time of 6.9 years (range, 0-11.3 years; Table 5). A total of 74 fractures were in the MGUS group (34 in men and 40 in women), and 62 in the LC-MGUS group (19 in men and 43 in women). Overall, the risk of fractures was not significantly increased in individuals with MGUS (HR, 1.19; 95% CI, 0.94-1.50) or LC-MGUS (HR, 1.05; 95% CI, 0.82-1.36) as compared with others. Men with MGUS had a significantly increased risk of fractures, compared with other men (HR, 1.49; 95% CI, 1.03-2.08), whereas no increased risk of fractures was found in women with MGUS compared with women without MGUS (HR, 1.02; 95% CI, 0.74-1.40). A total of 18 MGUS patients progressed to MM in the follow-up time, of those, 4 had a fracture before progression (2 men and 2 women). MGUS patients with fractures were not at increased risk of progression to MM compared with MGUS patients without fractures (HR, 0.77; 95% CI, 0.24-2.47). Further analysis comparing individuals with MGUS with fractures to those with MGUS who did not develop fractures showed no significant difference in known risk factors for progression to MM (M-protein concentration, M-protein isotype, or FLC ratio < 0.26 or > 1.65; data not shown). Furthermore, MGUS patients who developed fractures in the follow-up time had a decreased BMD at the spine (P < .001), femoral neck (P = .005), trochanter (P < .001), and total hip (P < .001), compared with other individuals with MGUS.
Sensitivity analysis
As previously described, we performed additional analyses using different cutoffs for LC-MGUS, where we defined LC-MGUS using the 97.5th percentile of the log-transformed κ and λ values. Using 40.0 mg/L of the light chain involved as a cutoff resulted in 52 LC-MGUS cases (0.9%), 41 κ and 11 λ. Results from the sensitivity analysis confirmed previous findings, except that the significant findings of lower BMD in trochanter and total hip for LC-MGUS women were no longer significant (data not shown).
Discussion
In this screened population-based study, including >5300 individuals, we found that individuals with MGUS did not have decreased BMD, however, interestingly, bone volume was increased in lumbar spine, femoral neck, trochanter, and total hip. This increase in bone volume was more pronounced in men with MGUS, who also had a significantly increased risk of fractures. Our findings suggest an effect of MGUS on bone metabolism that does not affect BMD, but which increases our understanding of bone disease in MM.
We found no difference in BMD between MGUS, LC-MGUS, and others at any site, in fact, our results show consistently that there seems to be no difference between the groups, with comparable mean values for all groups. These results are in accordance with the findings of Ng et al, wherein DXA measurements on 50 MGUS patients, compared with 100 controls, showed no significant difference in areal BMD at lumbar spine, femur neck, or total radius, although they did find a significant difference in areal BMD at total femur.16 In a previous large screening study, a 20% increased risk for a diagnosis of osteoporosis was found in individuals with MGUS.6 This is contradictory to our results, but might be explained by the increased surveillance of individuals with MGUS, thus leading to more investigations (DXA scans) and diagnoses of osteoporosis, and not necessarily indicating a lower BMD in the group. Results from previous studies on patients with MM have shown decreased BMD compared with age- and sex-matched controls,27,28 although this decrease does not seem to correlate with the extent of osteolytic lesions,29 and might therefore not reflect the extent of the disease.
We observed an increase in bone volume at lumbar spine, femoral neck, trochanter, and total hip in MGUS and LC-MGUS, compared with those without MGUS. This is consistent with 2 studies from the Mayo Clinic using skeletal imaging, where HRpQCT measurements of the radius showed increased bone size, increased cortical porosity, and decreased cortical thickness.16,17 This increase in bone size might reflect a process similar to periosteal bone apposition, which is known to occur with increasing age, where there is a loss of trabecular bone, and in response to that a periosteal renewal of bone cells leading to an increase in cross-sectional area of bone. This is believed to contribute to maintaining bone strength in age-related bone loss.22,30 Furthermore, some studies have shown elevated levels of certain bone markers in MGUS, indicating changed metabolism in MGUS.16,18-21 Thus, there seems to be an alteration of both bone structure and metabolism in MGUS, although the association between these changes and progression to MM or bone disease in MM are still unclear. To our knowledge, no changes in bone volume have been reported in MM.
When the risk of fractures was analyzed for men and women together, no significant increase in risk of fractures was detected in individuals with MGUS. Results from previous studies, with more individuals with MGUS and longer follow-up, have shown an increased risk of fractures in MGUS, however, all were based on clinical cohorts and thus subject to bias.4,5,31 Interestingly, we found that men with MGUS had a 1.5-fold increased risk of fractures compared with other men. This is consistent with earlier findings that have shown a higher relative risk of fractures in men with MGUS than in women,4 and that men with MGUS have an unexpected high prevalence of vertebral fractures.32 In postmenopausal women, the generally enhanced rate of bone loss may mask the effect of MGUS on bone metabolism.22 Further analysis on the MGUS individuals who developed fractures showed that they had a lower BMD than individuals with MGUS who did not develop fractures, indicating that processes known from osteoporosis play a role in fractures in this group, just like in the general population.
The main strength of our study, as compared with previous studies on bone disease in MGUS, is that our study population is detected through screening and is population based, and both patients and their health care providers were blinded to their MGUS during the study. Furthermore, the AGES-RS population is well described, and includes extensive information on individual factors such as height, weight, as well as current medication affecting bone metabolism.33 Another strength of our study is the use of QCT measurements that have previously been shown to correlate well with DXA measurements as well as subsequent risk of fracture.15,34 Furthermore, QCT measurements as opposed to DXA are not confounded by skeletal size and provide measurements from trabecular and cortical bone separately.
A limitation to our study, as with other MGUS studies, is that we do not know when individuals developed MGUS, thus, some individuals might not have had the condition for a long time. Assuming that bone disease develops gradually, we might not find a difference in BMD between the groups because of the limited duration of MGUS. Another limitation is that using QCT, despite its benefits mentioned earlier in text, does not allow for comparison with other studies on bone disease in MGUS that have mostly used DXA. The bone analysis used in our study did not have sufficient resolution to study microstructural changes in bone, like HRpQCT, which has been used in other studies. Furthermore, we did not have bone marrow samples from our participants, and can therefore not rule out that the individuals that are in the MGUS group might have smoldering myeloma or MM despite the low M-protein concentration. Another limitation is that the Icelandic AGES-RS population is an ethnically homogenous population, thus, the results may not be applicable to individuals of another ethnicity than white. Moreover, the AGES population is elderly, with a median age of 76 years, therefore, results may be different for younger MGUS patients. Another consideration is that lack of power in the fracture analysis might explain why we do not find an increased risk of fractures for the whole MGUS group, however, the normal BMD speaks against increased fracture risk.
As for clinical considerations, our results do not support measuring BMD in MGUS patients to screen for osteopenia/osteoporosis, as our study shows that they do not have a lower BMD than others in the same age group. However, our results suggest that individuals with MGUS have an altered bone metabolism and the men with MGUS have an increased risk of fractures. Our findings are in line with the results of a recent meta-analysis of bone disease in MGUS, where the authors concluded that there was evidence of increased fracture risk in MGUS, although BMD did not seem to be significantly altered.35 Therefore, further studies are needed to determine how MGUS patients with increased risk of fractures can be identified and how and if they should be treated prophylactically. Treatment with bisphosphonates has been investigated to some extent in MGUS, and has been shown to increase BMD.36,37 However, the effect of bisphosphonates on future skeletal-related events and bone structure in MGUS remains uncertain.
In conclusion, our large population-based study suggests that BMD is not decreased in individuals with MGUS as compared with others. Furthermore, we found that individuals with MGUS had an increased bone volume at lumbar spine, femoral neck, trochanter, and total hip. The increased bone volume was more marked in men with MGUS, who were at increased risk of fractures. This suggests an effect of MGUS on bone metabolism in men that is not noted in women, possibly as a result of other stronger risk factors in postmenopausal women. Our results could represent compensatory bone metabolism in MGUS due to ongoing bone marrow pathology, driven by abnormal plasma cells, or other processes that are currently unclear, and warrant further studies on bone metabolism and/or structure in MGUS and MM.
Acknowledgments
This work was supported by grants from the University of Iceland Research Fund, Icelandic Centre for Research (RANNIS), Landspitali University Hospital Research Fund, Karolinska Institutet Foundations, and Marie Curie Career Integration Grants (CIG). Furthermore, this work was funded by National Institutes of Health, National Institute on Aging (NIA) contract N01-AG012100, the NIA Intramural Research Program, an Intramural Research Program Award (ZIAEY000401) from the National Eye Institute, an award from the National Institute on Deafness and Other Communication Disorders Division of Scientific Programs (IAA Y2-DC_1004-02), Hjartavernd (the Icelandic Heart Association), and the Althingi (the Icelandic Parliament). This work was also supported by National Cancer Institute Memorial Sloan Kettering Cancer Center core grant (P30 CA008748). The work was approved by the Icelandic National Bioethics Committee (VSN 00-063).
S.T. is a candidate at the University of Iceland and this work is submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Medicine.
Authorship
Contribution: S.T., S.H.L., O.L., and S.Y.K. contributed to the study concept and design; S.T. and S.Y.K. drafted the manuscript; S.T. and S.H.L. performed statistical analysis; O.L. and S.Y.K. obtained funding; E.K.L., G.S., M.T., G.E., and S.Y.K. gave administrative, technical, or material support; S.Y.K. supervised the study; and all authors contributed to the acquisition, analysis, and interpretation of data and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sigurdur Y. Kristinsson, Faculty of Medicine, University of Iceland, Sturlugata 8, 101 Reykjavik, Iceland; e-mail: sigyngvi@hi.is.