Key Points
Children with primary MDS should be tested for GATA2 mutations, regardless of karyotype, family history, or features of GATA2 deficiency.
Screening children with GATA2-MDS for somatic mutations may reveal mutations predictive of clinical outcomes.
Abstract
Approximately 10% of children with primary myelodysplastic syndrome (MDS) have germ line GATA2 mutations, leading to the proposal that all children with primary MDS and certain cytogenetic findings, including monosomy 7, be tested for germ line GATA2 mutations regardless of family history or other clinical features associated with GATA2 deficiency. In adults with familial GATA2-MDS, those with somatic mutations in ASXL1 experience rapid disease progression to acute myeloid leukemia (AML) and poor prognosis after stem cell transplantation; however, the prevalence of somatic mutations in primary pediatric GATA2-MDS is unclear. Here, we studied a cohort of 8 pediatric patients with MDS and lacking additional GATA2-associated clinical features or significant family history and identified heterozygous germ line GATA2 mutations in 5 patients, including 1 with a normal karyotype. For those with GATA2-MDS, we screened for somatic mutations in genes with prognostic relevance in AML/MDS, using a targeted next-generation sequencing panel. Although no somatic mutations in ASXL1 were observed, somatic mutations were found in RUNX1, SETBP1, IKZF1, and CRLF2. One subject with deleterious mutations in RUNX1, SETBP1, and IKZF1 rapidly progressed to AML with disease that was refractory to treatment. Our findings confirm the importance of GATA2 testing in primary pediatric MDS, even in the absence of other clinical features of GATA2 deficiency. Further, similar to what has been observed in adults with GATA2-MDS, somatic mutations with potential prognostic effect occur in children with MDS associated with mutations in GATA2.
Introduction
Myelodysplastic syndrome (MDS) is a heterogeneous disorder manifesting as ineffective hematopoiesis with risk for infection, hemorrhage, and evolution to acute myeloid leukemia (AML). MDS is rare in childhood, but risk is increased in the setting of certain inherited bone marrow failure syndromes (IBMFS)1-5 or after cancer treatment that includes radiation or specific chemotherapy.6 Recent genomic analyses of children and young adults with idiopathic MDS or IBMFS revealed a proportion with germ line mutations in GATA2, a gene that encodes a zinc finger transcription factor involved in hematopoiesis and lymphatic development.7,8 In primary pediatric MDS, heterozygous GATA2 mutations are the most common germ line defect, occurring in 7% of cases and frequently associated with monosomy 7.9
A spectrum of conditions falls under the umbrella of GATA2 deficiency-associated phenotypes, all the result of germ line mutations in GATA2. Among these are monocytopenia and mycobacterial infection (MonoMAC syndrome),10-12 dendritic cell, monocyte, B and NK cell lymphoid deficiency,13 primary lymphedema with myelodysplasia progressing to AML (Emberger syndrome),12,14 and a subset of familial MDS.15 The extent to which children with GATA2-MDS may present without other clinical features suggestive of GATA2-deficiency is unknown.
Somatic mutations that affect disease prognosis are relatively common in adults with MDS, most often involving TET2, SF3B1, ASXL1, SRSF2, DNMT3A, and RUNX1.16,17 Somatic mutations in ASXL1 are also described in familial GATA2-MDS,18,19 correlating with an increased risk for myeloid transformation.20 In a small cohort of children with GATA2-MDS and a family history of MonoMac or Emberger syndromes, mutations in additional driver genes were noted, including ASXL1, RUNX1, SETBP1, and NRAS.21
The primary objective of this study was to determine whether GATA2 mutations would be readily identified in children diagnosed with MDS, but without features suggestive of GATA2 deficiency, such as those described by Spinner et al.22 We screened 8 children with primary MDS lacking past medical or family history suggestive of GATA2 deficiency for GATA2 mutations, and found 5 to have germ line mutations, emphasizing that MDS may be the presenting feature of GATA2 deficiency in children. In addition, we show that among children with GATA2-MDS, next-generation sequencing (NGS) may detect somatic mutations in genes related to AML/MDS disease progression.
Methods
Subject population
We queried 2 institutional review board–approved Baylor College of Medicine studies (H-3342 and H-17698) for subjects diagnosed with MDS. The objective of these protocols is to enroll subjects (primarily children at our clinical site, Texas Children’s Hospital) who are undergoing bone marrow aspiration for suspected or known malignancy (H-3342) or bone marrow failure disorder (H-17698). All subjects enrolled have provided written informed consent to participate in this research. Among the MDS cases identified, we excluded those who lacked tissue samples; were treatment-related; were previously diagnosed with an IBMFS or severe aplastic anemia; had a history of other manifestations of GATA2-deficiency, such as certain viral infections or Mycobacterium avium intracellular infections (history of limited, localized warts allowed); or had a known or suspected family history of a GATA2-associated disorder. Additional clinical and laboratory information was obtained from medical records. The MDS diagnosis of refractory cytopenia of childhood (RCC), based on World Health Organization criteria,23 was confirmed by independent pathology review.
GATA2 analysis
Initial sequence analysis of GATA2 consisted of bidirectional sequencing of DNA from peripheral blood cells, bone marrow, or buccal swab, and included upstream and intronic regulatory regions using previously described primers.10 GATA2 single nucleotide variants were interrogated for germ line status using targeted sequencing on DNA isolated from mouthwash samples or buccal swabs obtained after hematopoietic stem cell transplantation (HSCT). For cases with mouthwash samples post-HSCT, PCR products were Topo TA cloned (Thermo Fisher Scientific) and sequenced to detect the germ line mutant allele among the wild-type allele present in contaminating donor-derived lymphocytes. Array comparative genomic hybridization was applied to peripheral blood mononuclear cell at diagnosis and fibroblast DNA for 1 subject as a clinical evaluation (Baylor Medical Genetics Laboratories).
Testing for somatic mutations
A custom-designed, targeted Agilent SureSelect QXT NGS panel was used to sequence promoter, exonic, and intronic gene regions relevant to pediatric MDS/AML. The sequencing panel design is provided in supplemental Table 1. For subjects with GATA2 mutations and available diagnostic (4 patients) or relapse (1 patient) bone marrow samples, NGS libraries were prepared from extracted DNA. All samples were multiplexed and sequenced on a MiSeq flow cell in paired-end mode. Read alignment to the reference human genome (GRCh37), variant calling, and annotation were performed using NextGENe and Oncotator software. Variants passing defined depth of coverage (>100×) and variant allele fraction (>0.05) filters were queried against publicly available databases and reviewed for pathogenic significance.
Results
Among subjects enrolled on the above-described study protocols, 15 were identified with MDS. Eight subjects remained after exclusion of cases lacking tissue samples (2 patients), cases with treatment-related MDS (1 patient), or a previous diagnosis of an IBMFS (3 patients) or severe aplastic anemia (1 patient), and cases characterized by infections associated with GATA2 deficiency (0 patients) or known or suspected family history of a GATA2-associated disorder (0 patients).
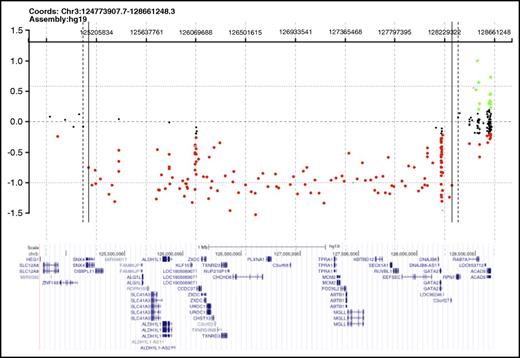
Germ line GATA2 mutations were detected in 5 of these 8 presumed primary MDS cases (Table 1). In 2 subjects (BMF41 and BMF67), sequencing identified previously described GATA2 splice site mutations that abolish the splice acceptor for intron 4 (mRNA transcript NM_032638.4): 1 with c.1018-1G>A10 and 1 with c.1018-2A>C, albeit with a unique nucleotide substitution.9,21 A third subject (BMF109) had a novel GATA2 splice site mutation, c.1144-1G>C, which alters the intron 5 splice acceptor (in the same isoform noted earlier). A fourth subject (BMF129) harbored a nucleotide deletion in exon 3 (isoform as noted earlier), c.599delG, leading to a frameshift p.G200VfsX189 (see supplemental Figure 1 for sequencing chromatograms). In all 4 of these cases, the GATA2 mutation identified was determined to be germ line by targeted sequencing of either mouthwash or buccal samples obtained post-HSCT, when the subjects were in clinical remission. In the fifth subject (BMF52), a large deletion was suspected, given that the GATA2 sequence analysis of pre-BMT peripheral blood mononuclear cell DNA showed absence of heterozygosity at each of 6 polymorphic sites within the GATA2 sequence with minor allele frequencies ≥0.20. Clinical array comparative genomic hybridization testing revealed a 3.1- to 3.3-Mb heterozygous deletion encompassing the GATA2 locus and contiguous genes, which was determined germ line by analysis of DNA from cultured skin fibroblasts (Figure 1). Familial testing for GATA2 mutations revealed no parental mutations in 2 cases for which both parents were available for testing (BMF52 and BMF129), nor in the remaining 3 cases for which only 1 parent was available for testing.
Heterozygous deletion encompassing GATA2 in a subject with primary MDS and lacking additional features of GATA2 deficiency. Array comparative genomic hybridization of fibroblast DNA (courtesy of Baylor Medical Genetics Laboratories), demonstrating the 3.1- to 3.3-Mb deletion encompassing GATA2 identified in BMF52. The GRCh37/hg19 genomic location of GATA2 is chromosome 3, nucleotides 128198265 through 128212030. Red dots represent copy number losses, green dots represent copy number gains, and orange dots represent known polymorphisms. A subset of additional genes within the deleted segment is shown.
Heterozygous deletion encompassing GATA2 in a subject with primary MDS and lacking additional features of GATA2 deficiency. Array comparative genomic hybridization of fibroblast DNA (courtesy of Baylor Medical Genetics Laboratories), demonstrating the 3.1- to 3.3-Mb deletion encompassing GATA2 identified in BMF52. The GRCh37/hg19 genomic location of GATA2 is chromosome 3, nucleotides 128198265 through 128212030. Red dots represent copy number losses, green dots represent copy number gains, and orange dots represent known polymorphisms. A subset of additional genes within the deleted segment is shown.
All 5 GATA2-MDS subjects had bone marrow pathology consistent with RCC. Four patients demonstrated monosomy 7 on routine karyotyping, and 1 subject had a normal karyotype and a negative MDS FISH panel for 5q-, 7-, 7q-, trisomy 8, and 20q-. Other common morphologic and laboratory features of the GATA2-MDS cohort included megakaryocytic and erythroid dysplasia (n = 5), hypocellular bone marrow aspirates for age (n = 4), monocytopenia (n = 3), decreased granulocytes (n = 3), and absence of reticulin fibrosis in all cases.
Next-generation sequencing of detected mutations in genes associated with poor AML/MDS prognosis was noted in 3 of 5 GATA2-MDS subjects (Table 1). These included 5 unique mutations in RUNX1, SETBP1, IKZF1, and CRLF2. Mutations were present in the diagnostic bone marrow sample for all subjects save 1, BMF109, who presented to our institution in relapse after initial diagnosis and treatment at an outside institution. One of the RUNX1 variants, p.L56P, which was detected in BMF109, was determined to be germ line after detection of the mutation by targeted sequencing of DNA from a mouthwash sample post-HSCT (supplemental Figure 2), consistent with its reported minor allele frequency of 1.6% in the Exome Aggregation Consortium database (accessed November 2016). In contrast, the RUNX1 p.D198G mutation detected in BMF41 was absent in an LCL-derived sample obtained from this patient, and was therefore somatic (supplemental Figure 2). Of the 5 GATA2-MDS subjects, then, 2 were found to have somatic mutations in AML-driver genes.
Discussion
We detected germ line GATA2 mutations in 5 of 8 pediatric primary MDS cases, despite the absence of other clinical features suggestive of GATA2 deficiency. Although limited by the constraints of a retrospective case review, our observations are consistent with recent studies suggesting GATA2 analysis is critical to the diagnostic workup of pediatric MDS.9 As expected, monosomy 7, prevalent in GATA2-MDS,12,15 was noted in 4 of 5 cases. The GATA2 mutations detected included 3 splice site mutations, a frameshift mutation, and a large heterozygous deletion, which was initially suspected by the absence of heterozygosity across the several polymorphic sites within the GATA2 locus. These classes are in line with the frameshift, nonsense, and splice site GATA2 mutations that account for approximately half of reported germ line alterations in GATA2-MDS.9 Heterozygous GATA2 gene deletions, however, are rare in primary GATA2-MDS.9 Limited reports of germ line large, de novo deletions spanning GATA2 and neighboring genes have been noted in association with MDS, but in patients with developmental and neurological deficits, as well as dysmorphic features.12 In contrast, our case lacked any discernable phenotype and had normal neurocognitive function. The absence of relevant family history in our cohort, as well as the failure to detect parental mutations, suggests the mutations we observed arose de novo, which has been shown to be the case in approximately two-thirds of GATA2-MDS.24 However, only 1 parent was available for testing in 3 of 5 cases, so we cannot exclude the possibility that the nontested parent was a carrier.
The routine assessment of morphologic, flow cytometric, and cytogenetic findings in bone marrow aspirates can be suggestive of GATA2 deficiency in a subset of patients with MDS. The constellation of bone marrow hypocellularity for age and atypical megakaryocytes, particularly the presence of separate nuclear lobes, abnormal granulocytic maturation, and reticulin fibrosis, along with peripheral blood B-cell and NK-cell lymphopenia and monocytopenia, is indicative of the diagnosis.22,25 In our GATA2-MDS cohort, dysplastic megakaryocytes and bone marrow hypocellularity were common to all diagnostic marrows. However, peripheral blood monocytopenia and abnormal granulocytic maturation were not consistent features, and there was no appreciable fibrosis in any of the bone marrows examined. Notably, for 1 patient (BMF129), the pathologist recommended germ line GATA2 sequencing at the time of diagnosis because of the combination of trilineage dysplasia, characteristic megakaryocytic dysplasia, and profound monocytopenia and neutropenia. Our findings highlight the evolving spectrum of morphology and laboratory findings in GATA2-MDS, particularly for those who lack GATA2-associated clinical features or significant family history.
We observed somatic mutations in several genes associated with AML disease progression (Table 1). Somatic mutations in SETBP1, including p.G870S, are independent and poor prognostic indicators in adult AML and MDS.26 Somatic mutations in RUNX1 are also common in adult MDS/AML,27 and the p.D198G substitution observed in our cohort is described in cases of MDS progressing to AML.28 Large IKZF1 focal deletions are rare, but recurrent, events in pediatric AML, in conjunction with monosomy 7.29 Although CRLF2 mutations are well-characterized in relapsed B-acute lymphoblastic leukemia,30 their clinical significance in AML/MDS is unclear. Our study was not powered to detect the prognostic effect of somatic mutations on disease course, although the 2 cases with somatic mutations experienced relapse or disease progression. The subject with the CRLF2 mutation developed AML after a second HSCT, but remains in remission after a third HSCT. The subject harboring concomitant mutations in SETPB1, RUNX1, and IKFZ1 experienced an aggressive clinical course marked by multiple disease relapses and progression to AML despite receiving 3 HSCTs, ultimately dying of refractory disease.
Our results confirm the importance of GATA2 sequencing in the evaluation of primary pediatric MDS, as the identification of a germ line mutation has crucial implications for related HSCT donor selection and genetic counseling. Moreover, GATA2 sequencing reported as “no mutation identified” should prompt deletion analysis, as absence of neurodevelopmental findings does not preclude a GATA2 gene deletion. Last, similar to observations in adult and familial GATA2-MDS, we provide evidence that the acquisition of potential driver mutations in genes predictive of AML/MDS disease progression occur in children with primary GATA2-MDS. Therefore, testing for these mutations using a disease-specific NGS panel may detect prognostic mutations predictive of disease clinical course.
The full-text version of this article contains a data supplement.
Acknowledgments
GATA2 sequence analysis was performed by GeneDx for 1 patient.
This work was supported, in part, by a Cancer Prevention Research Institute of Texas grant (RP129976) (A.A.B.).
Authorship
Contribution: K.E.F. drafted the manuscript and analyzed the NG sequencing data for somatic mutations; A.P.H. performed GATA2 sequence analysis and edited the manuscript; C.L.W. prepared samples, performed GATA2 sequencing, and drafted the methods; H.S. performed NG sequencing for somatic mutations and drafted the methods; B.Y.M. provided pathological review and edited the manuscript; M.T.E. provided pathological review and edited the manuscript; S.M.H. assisted in study design and data analysis and edited the manuscript; A.A.B. assisted in study design and data analysis and revised/edited the manuscript; and M.M.G. assisted with study design, extracted and analyzed medical data, and revised/edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Maria Monica Gramatges, Texas Children’s Hospital, Feigin Center, 1102 Bates, Suite 1200, Houston, TX 77030; e-mail: gramatge@bcm.edu.