Background
Conventional cytogenetic analysis remains mandatory at the initial evaluation of a patient with acute myeloid leukemia (AML). Molecular testing by reverse transcriptase polymerase chain reaction for recurring rearrangements and NPM1, FLT3, and CEBPA mutational screening have been used in routine practice following the 2010 European LeukemiaNet recommendations.1 Diagnosis and management of AML in adults were reviewed by the European LeukemiaNET in 2017, and the recommendations from an international panel of experts include complete blood count and differential count, bone marrow aspirate, immunophenotyping, cytogenetics, screening for the gene mutations NPM1, CEBPA, RUNX1, FLT3, TP53, ASXL1, and screening for gene rearrangement, including PML-RARA, CBFB-MYH11, RUNX1-RUNX1T1, and BCR-ABL1.2 In spite of the recent accumulated evidence for molecular markers in AML risk stratification, the translation of this scenario into clinical practice in the context of developing countries remains a challenge. The lack of availability of conventional cytogenetic and molecular laboratories impairs AML risk stratification and may affect treatment decisions and patient outcomes in low- and middle-income countries. The establishment of a network of institutions in developing countries that perform AML diagnosis and risk classification is essential to overcome the barriers related to the cytogenetic and molecular evaluation of patients, including financial, structural, and human resource limitations.
Rationale and aims
We aimed to create a capacity-building initiative to provide conventional cytogenetic and molecular tests for the diagnosis and risk stratification of AML and to evaluate the feasibility of a central laboratory in performing the related tests so as to apply an integrated clinical, cytogenetic, and molecular risk stratification for Brazilian AML patients. As secondary objectives, the integrated clinical, cytogenetic, and molecular risk stratification will be correlated with clinical outcomes (disease-free survival and overall survival). The data presented here is part of the International Consortium of Acute Leukemia (ICAL) Brazilian study, “Feasibility study of the use of intermediate doses of cytarabine associated with autologous hematopoietic stem cells as consolidation treatment of adults with low- or intermediate-risk de novo acute myeloid leukemia.” In the ICAL multicentric study, all institutions perform treatment and follow-up evaluation according to a common protocol.
Study design
This study complies with the guiding principles for experimental procedures found in the Declaration of Helsinki of the World Medical Association; the study has been approved by the Institutional Ethics Committee, and informed consent has been obtained from all patients. Patients with an AML diagnosis (excluding acute promyelocytic leukemia) according to the World Health Organization classification of hematopoietic tumors and >18 years old were included. Patients were stratified into low-, intermediate-, or high-risk categories as defined by the ICAL study (Table 1), based on the European LeukemiaNet classification1 with few modifications. Total white blood cell count >50 × 109/L or the presence of BCR-ABL1 was defined as high risk, and a complex karyotype was defined as >3 chromosomal abnormalities, excluding recurrent cytogenetic abnormalities indicated by the European LeukemiaNet. All diagnostic tests were performed at the hematology laboratory of the University of São Paulo at Ribeirão Preto Medical School.
Logistics
Bone marrow samples were collected in preservative-free heparin and transported at room temperature to the Brazilian Central Laboratory. The samples from 7 centers travelled by road (distances ranging from 160 to 400 km) and from the remaining center by plane and road (>400 km).
Conventional cytogenetic and molecular analysis
At the central laboratory, all samples were processed immediately upon receipt. Bone marrow aspirates were submitted to morphology and immunophenotype analyses to confirm the diagnosis. Only patients with a confirmed diagnosis of AML were submitted to complete karyotyping and molecular evaluation. Conventional cytogenetic analysis was performed on bone marrow aspirates according to standard methods. Chromosome preparations were G-banded using trypsin and Wright eosin methylene blue, and karyotypes were described according to the International System for Human Cytogenetic Nomenclature 2013. At least 20 bone marrow metaphases were analyzed. All metaphases were photographed and karyotyped by a biologist. All karyotypes were reviewed by the hematology physician responsible for the cytogenetic laboratory and by 2 biologists currently in training. For specific cases, when necessary, karyotypes were reviewed by an independent hematology physician from another participating center. Reverse transcriptase polymerase chain reaction was used for detecting the recurring rearrangements RUNX1-RUNX1T1 and CBFB-MYH11 and NPM1 and FLT3 mutations.
Building capacity
The institutions involved had different experiences and capacities to perform cytogenetic analysis. To improve the national capacity, it was decided that samples would be processed and analyzed at the central laboratory (always) as well as at the local laboratory (whenever it was possible). Discussion of the results among members of the network was important in the training of personnel and in the identification of areas of weakness.
Preliminary results
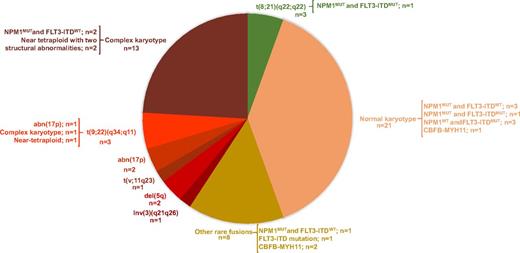
Up to the present date, 81 patients (33 males, 48 females; mean age, 49 years [range, 20-84 years; 9 patients were >66 years]) have been enrolled in the cytogenetic and molecular analysis in 8 centers in Brazil. The mean white blood cell count was 41.59 × 109/L (range, 0.7 × 109 to 288.5 × 109/L). The interval time between sample collection and sample processing was ≤24 hours in 42 cases, >24 hours in 37 cases, and not informed in 2 cases. Conventional cytogenetic analysis was informative in 64% of cases (n = 52) and was inconclusive due to the absence of metaphase suitable for analysis in 36% of cases (n = 29). The interval time between sample collection and processing significantly influenced the achievement of metaphases suitable for analysis (P < .005, Fisher’s exact test) (Table 2). Among samples with conclusive cytogenetics, normal karyotype was observed in 40% of cases (n = 21) and abnormal in 60% of cases (n = 31). Applying the proposed integrated clinical, cytogenetic, and molecular risk stratification, AML patients were classified as low risk (n = 9), intermediate risk (n = 15), high risk (n = 42), and not evaluable (n = 15) (Figure 1). The main conventional cytogenetic and molecular findings for patients with conclusive karyotyping are illustrated in Figure 2.
AML risk classification according to the proposed integrated clinical, cytogenetic, and molecular risk stratification. *Fourteen cases without cytogenetic data were stratified as high risk due to a high white blood cell count (n = 11) or FLT3-ITD mutation (n = 3). §Cases without risk classification had an absence of metaphases for analysis and a white blood cell count <50 × 109/L, and transcripts of RUNX1-RUNX1T1 or CBFB-MYH11 were not detectable by molecular biology approaches. NPM1 mutation with FLT3-ITD wild type was found in 3 patients, and cooccurrence of NPM1 and FLT3-ITD mutations were found in 2 patients.
AML risk classification according to the proposed integrated clinical, cytogenetic, and molecular risk stratification. *Fourteen cases without cytogenetic data were stratified as high risk due to a high white blood cell count (n = 11) or FLT3-ITD mutation (n = 3). §Cases without risk classification had an absence of metaphases for analysis and a white blood cell count <50 × 109/L, and transcripts of RUNX1-RUNX1T1 or CBFB-MYH11 were not detectable by molecular biology approaches. NPM1 mutation with FLT3-ITD wild type was found in 3 patients, and cooccurrence of NPM1 and FLT3-ITD mutations were found in 2 patients.
Cytogenetic and molecular findings for 52 patients with conclusive karyotyping. For each AML cytogenetic category indicated in the pie chart, cooccurring molecular findings are shown.
Cytogenetic and molecular findings for 52 patients with conclusive karyotyping. For each AML cytogenetic category indicated in the pie chart, cooccurring molecular findings are shown.
Conclusions
The results indicate the feasibility of cytogenetic and molecular tests for AML risk stratification in multicentric studies in developing countries. The results also highlight the feasibility of integrated assessment, including clinical, cytogenetic, and molecular criteria for AML risk stratification in low- and middle-income countries. The logistical planning for sending samples to the reference center remains an important challenge to be overcome, and there is a need to improve the indices of suitable metaphases for conventional cytogenetic analysis. It is important to note that the results of all the cytogenetic and molecular tests carried out by the reference center through this initiative are not available in many of the participating institutions. The present study provides the knowledge of the molecular characteristics of the Brazilian AML cohort, guides physicians’ decisions regarding patient treatment, and has great potential to directly impact survival outcomes of Brazilian AML patients.
Conflict-of-interest disclosure: K.B.B.P.: Bristol-Myers Squibb (consultancy and speakers’ bureau), Roche (speakers’ bureau), Amgen (consultancy), and Novartis (consultancy). The remaining authors declare no competing financial interests.
Correspondence: Fabiola Traina, Division of Hematology, Department of Medicine, Medical School of Ribeirão Preto, University of São Paulo, Ribeirão Preto, SP, Brazil, and Center for Cell-Based Therapy, University of São Paulo, Ribeirão Preto, SP, Brazil; e-mail: ftraina@fmrp.usp.br.