Key Points
In a pilot study, the nonanticoagulant heparin derivative CX-01 was well tolerated when combined with chemotherapy for the treatment of AML.
Preliminary results show encouraging complete remission rates and rapid platelet recovery.
Abstract
Relapses in acute myelogenous leukemia (AML) are a result of quiescent leukemic stem cells (LSCs) in marrow stromal niches, where they resist chemotherapy. LSCs employ CXCL12/CXCR4 to home toward protective marrow niches. Heparin disrupts CXCL12-mediated sequestration of cells in the marrow. CX-01 is a low-anticoagulant heparin derivative. In this pilot study, we combined CX-01 with chemotherapy for the treatment of AML. Induction consisted of cytarabine and idarubicin (7 + 3) with CX-01. Twelve patients were enrolled (median age, 56 years; 3 women). Three, 5, and 4 patients had good-, intermediate-, and poor-risk disease, respectively. Day 14 bone marrows were available on 11 patients and were aplastic in all without detectable leukemia. Eleven patients (92%) had morphologic complete remission after 1 induction (CR1). Eight patients were alive at a median follow-up of 24 months (4 patients in CR1). Three patients received an allogeneic stem cell transplant in CR1. Median disease-free survival was 14.8 months. Median overall survival was not attained at the maximum follow-up time of 29.4 months. No CX-01-associated serious adverse events occurred. Median day to an untransfused platelet count of at least 20 × 109/L was 21. CX-01 is well tolerated when combined with intensive therapy for AML and appears associated with enhanced count recovery and treatment efficacy.
Introduction
Acute myelogenous leukemia (AML) afflicted 21 380 patients in the United States in 2017, with 10 590 deaths and a 5-year survival of 26.9%.1 The mainstay of therapy is variations on the combination of cytarabine and an anthracycline (“7 + 3”).2 Relapses are common because of quiescent leukemic stem cells (LSCs) in marrow stromal niches, where they are resistant to chemotherapy.3
Hematopoietic stem cells (HSCs) migrate to and are anchored by chemotactic signals produced in bone marrow stroma.4,5 Foremost among these signals is the CXC chemokine CXCL12 (stem cell-derived factor-1, or SDF-1), which ligates chemokine receptor 4 (CXCR4) on the HSC surface and activates migration toward, and attachment in, the marrow.6,7 CXCL12/CXCR4 also activates phosphatidylinositol-3-kinase and protein kinase B, enhancing survival.8 In the marrow, HSCs shuttle between an endosteal osteoblastic niche inducing quiescence and a sinusoidal endothelial vascular niche promoting proliferation and differentiation.4 Quiescence in the endosteal niche is maintained by close association with megakaryocytes.9,10 Megakaryocytes negatively regulate HSC proliferation through secretion of platelet factor-4 (PF4), a cationic chemokine that also enhances integrin-mediated endothelial adhesion during marrow homing.11
LSCs employ the same CXCL12-, CXCR4-dependent mechanisms as normal HSCs to home toward a marrow niche.3,4 CXCR4 is an unfavorable AML prognostic factor.12 Disrupting CXCR4 inhibits transmigration, survival, and chemotherapy resistance in vitro and in vivo.13,14 The CXCR4 inhibitor plerixafor has been tested in AML.15 Plerixafor administered for up to 7 days for stem cell mobilization is well tolerated,16 but carries the possibility of increased secondary myeloid malignancies.17
Heparin avidly binds to a cluster of cationic amino acids on the CXCL12 surface that mediates attachment of the chemokine to heparan sulfate on cell surfaces.18,19 Heparin can disrupt CXCL12-mediated sequestration of cells in marrow by competing with marrow heparan sulfate.20 Heparin’s competitive inhibition of CXCL12 binding to heparan sulfate is not diminished by removal of 2-O sulfates,20 but is apparent only at concentrations exceeding therapeutic plasma levels for anticoagulation.20-22 An alternative to disrupt the CXCL12/CXCR4 axis is use of a heparin analog with reduced anticoagulation. Low anticoagulant 2-O, 3-O desulfated heparin (ODSH, CX-01) is a porcine intestinal heparin derivative that retains many heparin anti-inflammatory properties.23 CX-01 binds PF4 with affinity similar to heparin, but the CX-01/PF4 complex does not bind antibodies that mediate heparin-induced thrombocytopenia.24,25 Megakaryopoiesis is negatively regulated by PF4, and anti-PF4 blocking antibodies diminish chemotherapy-induced thrombocytopenia in mice.26 By reversing the biologic activities of PF4, CX-01 is able to mitigate chemotherapy-induced thrombocytopenia in PF4-overexpressing transgenic mice.27
We conducted a pilot study combining CX-01 with chemotherapy for AML treatment. Results show no added toxicity from CX-01. Complete remission (CR) rates and count recovery were encouraging. Exploratory in vitro studies suggest CX-01 may interfere with the CXCL12/CXCR4 axis.
Methods
Clinical trial
This was an open-label pilot study conducted at 3 academic centers. The protocol was approved by the University of Utah, Augusta University, and the Medical University of South Carolina respective Institutional Review Boards (ClinicalTrials.gov identifier: NCT02056782). The study was conducted in accordance with the Declaration of Helsinki. Adult patients with previously untreated AML based on World Health Organization criteria28 with an Eastern Cooperative Oncology Group performance status of 0 to 2 and adequate organ function were enrolled. Patients with acute promyelocytic leukemia and acute megakaryoblastic leukemia were excluded. Patients needing anticoagulation were excluded.
Treatment protocol.
Induction cycles consisted of cytarabine at 100 mg/m2 as a continuous 24-hour infusion on days 1 through 7, and idarubicin at 12 mg/m2 as a slow intravenous push on days 1 through 3. CX-01 was administered as a bolus of 4 mg/kg over the course of 30 minutes immediately after the first dose of idarubicin, followed by a continuous infusion at 0.25 mg/kg per hour for 24 hours on days 1 through 7. Patients who were aged 60 years or older received further therapy off study. Patients who were younger than 60 years remained on study and received up to 4 consolidation cycles consisting of cytarabine at a dose of 3 g/m2 intravenously over the course of 3 hours every 12 hours on days 1, 3, and 5. For consolidation cycles, CX-01 was given as a bolus of 4 mg/kg over the course of 30 minutes after completion of the first dose of cytarabine, followed by a continuous infusion at 0.25 mg/kg per hour for 24 hours on days 1 through 5. Eligible patients underwent allogeneic stem cell transplant whenever ready after completion of induction therapy.
Supportive care.
Red blood cell transfusions, antibiotics, and myeloid growth factors were given per institutional guidelines. Prophylactic platelet transfusions were given to maintain a platelet count of at least 10 × 109/L for afebrile patients and of at least 20 × 109/L for febrile patients. Otherwise, platelets were given as clinically indicated.
End points.
Primary endpoints included safety and tolerability of CX-01 when combined with chemotherapy and the effect of CX-01 on platelet transfusion independence, defined as the first of 5 consecutive days with an untransfused platelet count of at least 20 × 109/L. Secondary endpoints included rate of CR, platelet nadir, number of platelet transfusions, and toxicity using Common Terminology Criteria for Adverse Events version 4.0.29 Responses were determined using International Working Group Criteria.30
Statistical analysis.
Platelet recovery and toxicity endpoints were analyzed using descriptive statistics with reporting of minimum, maximum, mean, median, and 95% confidence interval of the mean. CR rates were reported as a fraction of enrolled subjects together with an exact 95% binomial confidence interval constructed using the method of Clopper and Pearson.31 Planned enrollment was 10 evaluable patients. The target rate of unacceptable CX-01–related toxicity was 20% with the following decision rule: If 3 or more subjects experience unacceptable CX-01-related toxicity, an acceptable dose of CX-01 has been exceeded. If 0-2 patients experience unacceptable CX-01-related toxicity, an acceptable dose of CX-01 has not been exceeded. With this rule, there is a 7% chance of making an incorrect determination that an acceptable dose of CX-01 has been exceeded if the true rate of unacceptable CX-01-related toxicity is 10%. There is a 6% chance of making an incorrect determination that an acceptable dose of CX-01 has not been exceeded if the true rate of unacceptable CX-01-related toxicity is 50%.
Pharmacokinetic studies.
Single-dose and steady-state pharmacokinetic sampling at 5 points was obtained during induction cycles for 7 patients. Duplicate samples for each point were analyzed by BioCascade Incorporated (Arlington, WI), using the Sta-Chrom anti-Xa assay,23 calibrated to express CX-01 concentration in micrograms per milliliter.
In vitro studies
Surface plasmon resonance.
Surface plasmon resonance analyses on immobilized CX-01 and heparin sodium USP.
One hundred micrograms per milliliter NeutrAvidin (Thermo Scientific) in 10 mM sodium acetate at pH 4.5 was immobilized on 2 flow cells of a Series S CM5 (GE Healthcare) sensor chip, using the amine coupling method.32,33 The remaining activated groups were blocked by ethanoline injection. For direct binding studies, biotinylated CX-01 and heparin sodium were then injected at a concentration of 50 μg/mL for 2 min at 5 µL/min, resulting in approximately 174 to 243 response units CX-01 or heparin sodium immobilized. Control flow cells were used for background subtraction, having biotin captured over the immobilized neutravidin. For direct binding analysis, CXCL12α was diluted in HBS-EP (0.01 M HEPES pH 7.4, 0.15 M NaCl, 3 mM EDTA, 0.05% v/v Surfactant P20; GE Healthcare). Different dilutions of the protein were injected at a flow rate of 30 µL/min for 180 seconds. A dissociation time of 300 seconds was set up, followed by regeneration with 3 M NaCl in HBS-EP buffer.
Competition for CXCL12 binding between immobilized heparin sodium and CX-01
For the competition experiment, CXCL12α (final concentration, 100 nM) was mixed with various concentrations of CX-01 (0, 1, 5, 10, 50, 100, 1000, and 2000 nM) and then injected. The effect of CX-01 on CXCL12α binding to immobilized heparin was monitored as relative response units, used to calculate 50% inhibitory concentration values.
Surface plasmon resonance data evaluation.
Surface plasmon resonance (SPR) data were analyzed by global fitting either to a 1:1 Langmuir binding model or to a 2-state reaction binding model, using the Biacore T100 BIAevaluation software 2.0.4 (GE Healthcare). For competition studies, data were plotted and the amount of CX-01 required to inhibit 50% of the CXCL12 binding to heparin was determined.
Cell migration studies.
To determine the effect of CX-01 on the migration of AML cells toward CXCL12, we conducted in vitro migration assays using a cell migration kit from Cell Biolabs (San Diego, CA) with the U937 monocytic leukemia cell line (ATCC, Manassas, VA), after verification of CXCR4 expression by flow cytometry. U937 cells (0.2 × 106 cells/0.1 mL) were loaded in inserts with 5-μm pore membranes. Inserts were transferred to wells containing 0.5 mL OPTI-MEM media with CXCL12 (100 ng/mL), CX-01 (200 μg/mL), or both. To investigate the direct effect of CX-01 on leukemia cells in 1 variable, CXCL12 only was added to the wells, and U937 cells (2 × 106/mL) were pretreated for 30 minutes with 500 µg/mL CX-01. Cells were then washed before loading into inserts. Plates were incubated overnight at 37°C in a 5% CO2 atmosphere. Cell migration was quantitated with the CyQuant GR dye stain according to the manufacturer’s protocol.
Results
Clinical trial
Patient characteristics.
Enrollment was planned for 10 patients; 12 were enrolled (median age, 54 years; range, 22-74 years; 3 women). Two patients (patients 2001 and 1007) were taken off study because of adverse events unrelated to CX-01. They were replaced for the purposes of time to count recovery analysis and were not included in that analysis. They were included in the response and toxicity analysis. Patient 2001 was taken off study after 2 days of induction because of acute recurring angioedema-like allergic reactions that began before treatment and may have been from angiotensin-converting enzyme inhibitor therapy. Patient 1007 developed acute renal failure secondary to tumor lysis and vancomycin nephrotoxicity, and was taken off treatment after completing 5 of the 7 days of induction. All patients were evaluable for safety. Ten completed the induction cycle and were evaluable for efficacy. Table 1 summarizes patient characteristics. Three, 5, and 4 patients had good-, intermediate-, and poor-risk disease, respectively.
Response to induction.
Eleven (92%) of 12 patients obtained a morphologic CR after 1 induction cycle (CR1), including the 2 patients who did not complete induction. All patients with de novo AML (11/11) obtained a CR. Two patients in morphologic CR had minimal residual disease by molecular testing. Patient 1007 received 5 days of induction and had detectable inv(16) by polymerase chain reaction. Patient 1006 had a FLT-3/ITD mutation and an inv(3) that were detectable at the end of induction. The patient who did not obtain a CR (1003) had presented with extensive lymphadenopathy with a bone marrow showing chronic myelomonocytic leukemia-2, but was included in the study on the basis of evidence of granulocytic sarcoma on lymph node biopsy. It is worth noting that day 14 bone marrows were available on 11 patients and were aplastic in all without detectable leukemia.
Count recovery after induction.
We analyzed count recovery in the 10 patients who completed a full induction cycle. They had a median time to recovery of an untransfused platelet count of at least 20 × 109/L and of at least 50 × 109/L of 21 (mean, 21.3 ± 1.7; range, 18-22) and 23.5 (mean, 23.1 ± 2.0; range, 19-25) days, respectively. Induction cycles required a median of 6.0 platelet transfusions (mean, 6.5 ± 3.9; range, 0-10 transfusions). Patient 1010 had a platelet count nadir of 11 × 109/L and required no platelet transfusions. Patient 1002 had an induction course complicated by a rectal abscess and hematuria and received 10 platelet transfusions. The median platelet nadir count for induction cycles was 8 × 109/L (mean, 8.4 × 109/L ± 2.6 × 109/L; range, 0.5-14 × 109/L).
Patient 1010 was the only patient who received filgrastim support at the discretion of the treating physician and not because of a delay in count recovery. As we aimed to obtain preliminary results on the effect of CX-01 on bone marrow recovery, patient 1010 was not evaluated for total white blood cell (WBC) or neutrophil recovery. For patients who did not receive filgrastim support, we analyzed time to recovery to a total WBC of at least 1 × 109/L and a neutrophil count of at least 0.5 × 109/L. Eight patients were evaluable for total WBC recovery and had a median time to a WBC count of at least 1 × 109/L of 21 days (mean, 23 ± 4.8; range, 20-35 days). Patient 1009 was not included in the analysis for a total WBC recovery to a count of at least 1 × 109/L because his WBC never went below 1 × 109/L during the whole induction cycle. Five patients were evaluable for neutrophil recovery and had a median time to a neutrophil count of at least 0.5 × 109/L of 22 days (mean, 22.6 ± 2.4; range, 21-27 days). The other patients were discharged from the hospital with a rising absolute neutrophil count (ANC) before achieving the target neutrophil count, and were therefore not included in the analysis for the time to ANC recovery.
Frequency of platelet transfusions after consolidation.
Four patients received 1 or more consolidation cycles on study, with median frequencies of platelet transfusions as follows (n = evaluable patients): for the first consolidation cycle (n = 4), 2 (mean, 2.0 ± 0.7; range, 1-3) transfusions; for the second consolidation cycle (n = 3), 3 (mean, 2.3 ± 0.9; range, 1-3) transfusions; for the third consolidation cycle, (n = 2), 2 (mean, 2.0 ± 0.0; range, 2- 2) transfusions. Patient 1005 received a fourth consolidation cycle on study and had a prolonged hospitalization because of septic shock with multiorgan failure in the setting of neutropenia. He was therefore not evaluable for the number of platelet transfusions associated with that cycle. The starting platelet counts at the beginning of the first consolidation cycle were typically higher than for other cycles because most patients experienced some degree of rebound thrombocytosis on full hematologic recovery from induction. Platelet and WBC nadirs for consolidation cycles were not evaluated because subjects had blood count checks only 3 times per week on an outpatient basis on completion of chemotherapy.
Postinduction therapy and long-term follow-up.
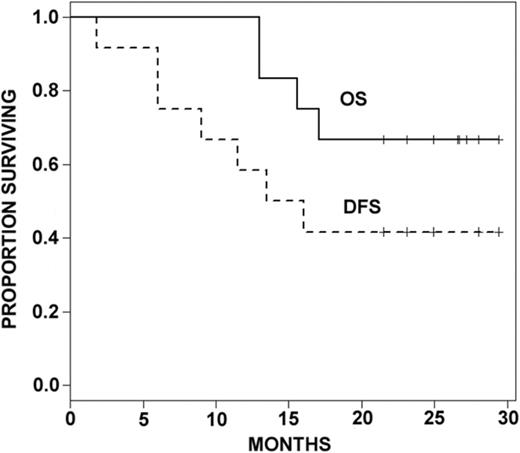
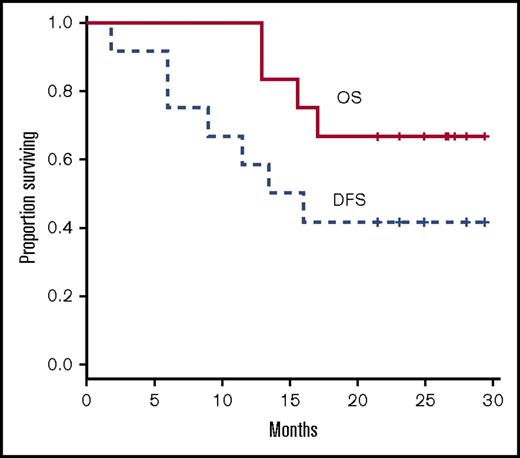
Of the 12 patients enrolled, 7 were not eligible to receive postinduction treatment on study because of age 60 years or older, induction failure, or incomplete induction cycles (Table 1). Of the remaining patients, 1 developed a line-associated deep venous thrombosis requiring anticoagulation and was taken off study before consolidation. The remaining 4 patients each received 1 or more cycles of consolidation on study as follows: patient 1005 completed all 4 cycles; patient 1009 received 3 cycles of consolidation and withdrew from study; patient 3001 completed 1 consolidation cycle, withdrew from study, and was lost to follow-up; patient 3003 received 2 consolidation cycles before relapsing. Three patients who completed induction received an allogeneic stem cell transplant in CR1 (patients 1002, 1006, 1010). Two of those patients were alive without evidence of disease at the time of final data analysis. Six of 8 patients who obtained a CR with induction therapy and did not receive a transplant in CR1 relapsed at a median time of 8 months. Among those were patient 2001, who had not completed induction and relapsed 7 weeks after diagnosis, and patient 3001, who received only 1 cycle of consolidation and relapsed 13.5 months after diagnosis. With a median follow-up of 24 months, 8 patients were alive. Median disease-free survival was 14.8 months. Median overall survival was not attained at the maximum follow-up time of 29.4 months (Figure 1).
Kaplan Meier curves of overall survival (OS) and disease-free survival (DFS).
Kaplan Meier curves of overall survival (OS) and disease-free survival (DFS).
Safety and toxicity.
CX-01 was well tolerated and did not increase the risk of bleeding. Coagulation parameters in induction and consolidation were not significantly affected by CX-01. Anti-factor Xa activity before and during administration of CX-01 was not significantly changed from baseline and remained below published therapeutic anticoagulant reference ranges. Two patients experienced grade 3 to 4 and 3 patients experienced grade 1 to 2 bleeding episodes, all deemed to be unrelated to CX-01.
A total of 5 serious adverse events occurred in 5 patients. Grade 4 sepsis occurred in 2 patients during the first and fourth consolidation. These events were deemed to be possibly related and not related to CX-01, respectively. Another patient experienced febrile neutropenia not related to CX-01 during induction. Patient 1002 had a perirectal abscess during induction requiring surgery with platelet support, an event that was unrelated to CX-01. Patients 1007 and 2001 did not complete induction because of serious adverse events that were unrelated to CX-01 (see “Patient characteristics”).
The most frequent nonserious adverse events were hematologic, followed by infectious and organ toxicity complications resulting from treatment and/or the underlying leukemia. Grade 3 and 4 hematologic toxicities were consistent with intensive AML chemotherapy. Transient asymptomatic low-grade elevations of liver transaminases were observed during chemotherapy administration in induction cycles. These were deemed by investigators to be possibly related to CX-01, irrespective of other potential causes of liver function abnormalities. Transient asymptomatic grade 3 to 4 liver transaminase elevations were observed during consolidation cycles in 2 patients and were deemed possibly related to CX-01. Serum bilirubin remained normal for all patients, except for an isolated grade 4 hyperbilirubinemia as a consequence of hepatic failure complicating septic shock in patient 1005 during the fourth consolidation.
Pharmacokinetics.
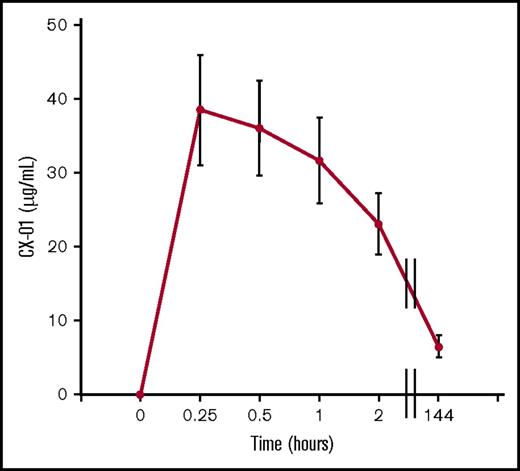
Single-dose and steady-state pharmacokinetic plasma sampling was obtained during induction at 5 points for 7 patients (Figure 2). Peak levels of 38.5 ± 7.5 μg/mL (average ± SEM) were reached 15 minutes after CX-01 bolus injection. For unknown reasons, patients 3001 and 3002 had substantially lower peak levels of CX-01 than the remaining patients (13.3 ± 1.85 vs 48.6 ± 5.25 μg/mL). Steady state levels at day 6 were 6.5 ± 1.5 μg/mL.
Peak and steady-state CX-01 plasma concentrations vs time curves in 7 patients with AML during induction. Shown are averages and standard errors of the mean for each time point.
Peak and steady-state CX-01 plasma concentrations vs time curves in 7 patients with AML during induction. Shown are averages and standard errors of the mean for each time point.
In vitro studies
To explore possible mechanisms of action of CX-01, we conducted in vitro studies to evaluate the effect of CX-01 on the CXCR4/CXCL12 axis.
SPR.
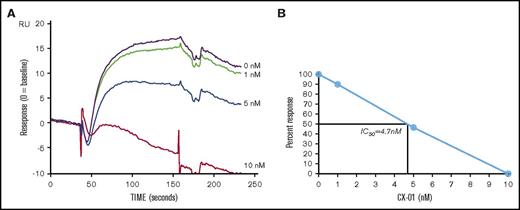
The interaction properties of CX-01 or heparin sodium with CXCL12α were compared. CXCL12α bound to immobilized CX-01 or heparin sodium with comparable association and dissociation constants, yielding apparent KD values from 26 to 40 nM and 5 to 30 nM, respectively. To determine whether CX-01 inhibits binding of CXCL12α to immobilized heparin, a constant amount of CXCL12α was incubated with varying concentrations of CX-01 and injected over the immobilized heparin. The amount of residual binding was determined at each concentration of CX-01, and the corresponding amount of free CXCL12α was determined. Competition experiments demonstrated that binding of 100 nM CXCL12α to immobilized heparin was inhibited by progressively higher concentrations of CX-01 (Figure 3A), with 5 nM CX-01 inhibiting 46.8% of the interaction between CXCL12α and heparin, for a 50% inhibitory concentration of 4.7 nM (0.055 µg/mL) (Figure 3B).
Surface plasmon resonance assay of the binding of CX-01 to CXCL12α. (A) BIAcore sensorgrams for the binding of 100 nM CXCL12α after preincubation with different concentrations of CX-01 (0, 1, 5, 10, 50, 100, 1000, and 2000 nM). (B) Competition assay shows the CX-01 concentration (50% inhibitory concentration = 4.7 nM or 0.055 μg/mL) that inhibits 50% of the maximum CXCL12α attachment response to immobilized heparin.
Surface plasmon resonance assay of the binding of CX-01 to CXCL12α. (A) BIAcore sensorgrams for the binding of 100 nM CXCL12α after preincubation with different concentrations of CX-01 (0, 1, 5, 10, 50, 100, 1000, and 2000 nM). (B) Competition assay shows the CX-01 concentration (50% inhibitory concentration = 4.7 nM or 0.055 μg/mL) that inhibits 50% of the maximum CXCL12α attachment response to immobilized heparin.
Cell migration studies.
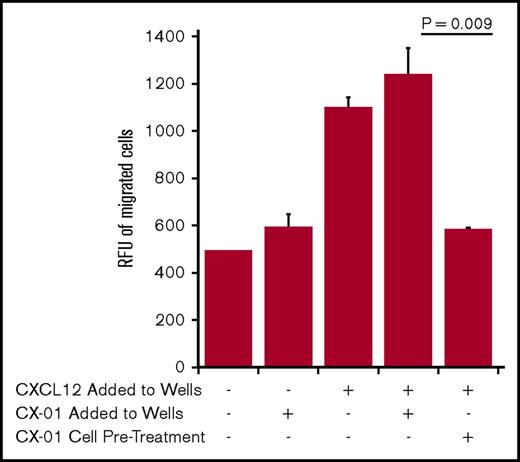
To test the effects of CX-01 on chemotaxis, we performed in vitro migration studies using U937 AML cells. CXCL12 at a concentration of 100 ng/mL induced chemotaxis of U937 AML cells. Addition of CX-01 (200 μg/mL) to CXCL12 in the wells did not inhibit migration. However, pretreating U937 cells with CX-01 (500 μg/mL) nullified the chemotactic effect of CXCL12, suggesting a direct effect of CX-01 on leukemic cells (Figure 4).
Effect of CX-01 on U937 cell migration toward CXCL12. The assay was conducted using a cell migration assay kit from Cell Biolabs (San Diego, CA), as detailed in the Methods section. CXCL12 was added at a concentration of 100 ng/mL in the wells. CX-01 was added at a concentration of 200 µg/mL in the wells. To investigate the direct effect of CX-01 on leukemia cells in 1 variable, CXCL12 only was added to the wells and U937 cells were pretreated for 30 minutes with 500 µg/mL CX-01 before loading them into inserts. The graph shows averages and standard errors of the mean of 2 to 3 replicate wells in each group (the media blank variable had 1 well). The legend indicates CXCL12 and/or CX-01 added to the wells or pretreatment of U937 cells with CX-01. The graph is representative of 2 separate experiments. RFU, relative fluorescence unit.
Effect of CX-01 on U937 cell migration toward CXCL12. The assay was conducted using a cell migration assay kit from Cell Biolabs (San Diego, CA), as detailed in the Methods section. CXCL12 was added at a concentration of 100 ng/mL in the wells. CX-01 was added at a concentration of 200 µg/mL in the wells. To investigate the direct effect of CX-01 on leukemia cells in 1 variable, CXCL12 only was added to the wells and U937 cells were pretreated for 30 minutes with 500 µg/mL CX-01 before loading them into inserts. The graph shows averages and standard errors of the mean of 2 to 3 replicate wells in each group (the media blank variable had 1 well). The legend indicates CXCL12 and/or CX-01 added to the wells or pretreatment of U937 cells with CX-01. The graph is representative of 2 separate experiments. RFU, relative fluorescence unit.
Discussion
In this pilot exploratory study, CX-01 in combination with standard intensive AML therapy was safe and well tolerated, with no unexpected serious adverse events, suggesting that the approach is feasible. The combination was associated with an encouraging CR rate of 92%. Hematologic recovery was also encouraging, with a median of 22 days to reach an ANC larger than 0.5 × 109/L, and 21 days to reach a platelet count higher than 20 × 109/L in induction cycles.
From a toxicity point of view, we observed transient asymptomatic low-grade elevations in liver transaminases during induction cycles. Elevation of liver transaminases is a class effect for heparins.34 It has also been described for idarubicin and cytarabine induction regimens in the absence of heparin.35 Transient asymptomatic grade 3 to 4 liver transaminase elevations were also noted during consolidation cycles in 2 patients. Simultaneous coadministration of CX-01 with cytarabine in this study may confound attribution of causality for this effect, as hepatotoxicity has also been reported for cytarabine.36 Although the CX-01 dose and schedule were identical for induction and consolidation cycles (except for the duration of the CX-01 continuous infusion), consolidation cycles employed a much higher dose of cytarabine than induction cycles, raising the possibility of multifactorial causation of elevated liver transaminase levels.
Pharmacokinetic studies showed a similar pattern of CX-01 clearance as in normal volunteers.23 It should be noted that the doses we used were lower than doses use in the latter study.23 These results suggest that CX-01 can be administered with cytarabine and idarubicin without significant drug-drug interaction. Hematologic recovery was rapid compared with published data, with a median of 22 days to reach an ANC higher than 0.5 × 109/L, and 21 days to reach a platelet count higher than 20 × 109/L in induction cycles. A randomized comparison will be required to confirm these findings.
The combination was associated with an encouraging CR rate of 92%, higher than the 71% CR rate reported by Vogler et al using a similar regimen of cytarabine and idarubicin.37 None of our patients needed a second induction cycle to achieve CR, compared with 23% in the Vogler study.37 Although our study is small, it is conceivable that the improvement over the expected response is the result of disrupting the CXCL12/CXCR4-mediated homing of LSCs to the protective marrow niche.3,4 Intravenous boluses of sulfated polysaccharides mobilize normal stem cells from the niche into the circulation, suggesting that sulfated polysaccharides also mobilize LSCs.38 Promoting cell division and permissive migration closer to the vascular compartment may expose leukemic cells to chemotherapy in a vulnerable state. Our hypothesis is supported by the similar binding affinity of CX-01 and unfractionated heparin for CXCL12 and the ability of CX-01 to inhibit binding of CXCL12 to immobilized heparin at concentrations below those achieved in the circulation of treated patients. This suggests similar competition in vivo of CX-01 with marrow glycosaminoglycans. Interestingly, when CX-01 was added to CXCL12 in U937 cell migration studies, it did not inhibit migration of the leukemic cells toward CXCL12. The discrepancy between the SPR data and the cell migration data may be a result of differences between a cell-free assay (SPR) and a cell-based assay (migration). However, pretreatment of U937 cells with CX-01 abrogated migration toward CXCL12, suggesting a direct effect on the leukemic cells to block the CXCL12/CXCR4 axis. As the CX-01 concentration in these experiments was higher than the measured drug concentrations in patients, further work will be required to determine whether that observation can be reproduced at lower CX-01 concentrations.
The mean days of recovery to a platelet count above 50 × 109/L and to a WBC count above 1 × 109/L were 23.1 and 23.0 days, respectively; a more rapid return of platelets (mean of 35.1 days for a platelet count higher than 50 × 109/L) and WBC (mean of 31.2 days for WBC count higher than 1 × 109/L) than previously reported with this regimen.37 Furthermore, the median time to recovery of neutrophils for induction cycles in CX-01-treated patients is essentially identical (22 days) to that of patients supported with myeloid growth factors during induction.39 Although the number of patients in our study is small, and while acknowledging the inherent limitations of such comparisons, our results are encouraging with respect to WBC and neutrophil recovery.
PF4 levels inversely correlate with recovery of platelet counts after chemotherapy in children with acute lymphoblastic leukemia,40 and PF4 suppresses the proliferation of normal HSCs.9,10 Because CX-01 inhibits the biologic action of PF4, our observations suggest that CX-01 might be abrogating the suppressive effect of PF4 on marrow recovery. Furthermore, the effects on platelet and neutrophil counts in our study are compatible with the recently appreciated role of megakaryocytes and PF4 in promoting quiescence of normal HSCs.9,10 Thus, PF4 might conceivably play a role in maintaining LSC quiescence, similar to normal HSCs. In these conditions, CX-01 (a polyanion that charge-inhibits critical cationic structural sequences of PF4) could remove any PF4 constraint on LSC activation, while simultaneously promoting megakaryocytic and myeloid recovery through neutralization of the inhibitory effect of PF4 on HSCs.
Results of this pilot study are preliminary and exploratory in nature. They require confirmation in large studies, as well as more extensive evaluation of our mechanistic hypotheses. However, if confirmed, CX-01 might offer a valuable adjunctive therapy for one of hematology’s most challenging malignancies.
Acknowledgment
This study was supported with funding from Cantex Pharmaceuticals.
Authorship
Contribution: T.J.K. participated in study design, patient care, data and analysis; A.M. participated in study design and patient care; M.E.S. participated in pathologic review of bone marrows; J.P. participated in study design and patient care; N.R. participated in laboratory studies; K.M.K. participated in laboratory studies; P.A. participated in laboratory studies; M.J.G. participated in patient care; M.W.N.D. participated in patient care; K.M.B. participated in statistical design and analysis; L.M.B. participated in data analysis; G.G.-S. participated in laboratory studies; T.P.K. participated in data analysis; S.G.M. participated in study design and data analysis; P.J.S. participated in study design, patient care, and data analysis; and all authors participated in manuscript preparation.
Conflict-of-interest disclosure: T.P.K. is a stockholder in Cantex Pharmaceuticals. S.G.M. is employed by and is a stock holder in Cantex Pharmaceuticals. P.J.S. received research support from Cantex Pharmaceuticals. The remaining authors declare no competing financial interests.
The current affiliation for A.M. is Division of Hematology, Ohio State University, Columbus, OH.
Correspondence: Paul J. Shami, Huntsman Cancer Institute, Suite 2100, 2000 Circle of Hope, Salt Lake City, UT 84112; e-mail: paul.shami@utah.edu.