Key Points
Five-year follow-up of S1106 demonstrates similar efficacy, MRD negativity, and 5-year survival with RH or RB, but RH was more toxic than RB.
RB showed excellent efficacy and survival and less toxicity compared with a cytarabine-based regimen in transplant-eligible MCL patients.
Introduction
Aggressive induction chemoimmunotherapy followed by high-dose chemotherapy and autologous stem cell transplant (ASCT) is a standard option for the initial management of transplant-eligible mantle cell lymphoma (MCL).1-3 However, the optimal induction regimen before ASCT is unknown. Cytarabine-based induction achieves excellent response rates and higher rates of minimal residual disease (MRD) negativity compared with anthracycline-based induction regimens.4-6 While effective, most cytarabine-based induction regimens require inpatient hospitalization and are associated with significant hematologic and nonhematologic toxicities.4-6 Rituximab-bendamustine (RB) is a less intensive, outpatient-based chemoimmunotherapy regimen with excellent long-term efficacy and good tolerability in transplant-ineligible MCL patients.7-9 However, its role as a pretransplant induction strategy has not been formerly evaluated.
S1106 is a randomized phase 2 multi-institutional clinical trial comparing induction R-hyper-CVAD (rituximab plus hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone) and methotrexate/cytarabine (RH) with RB followed by ASCT in newly diagnosed MCL patients. We previously reported that the 2-year progression-free survival (PFS) and overall survival (OS) were similar with either regimen. However, RH was more toxic than RB, had higher stem cell mobilization failure rates, and prompted protocol-specified early study closure.10 While premature closure limited the sample size, we demonstrated for the first time in a prospective setting that outpatient RB treatment can achieve excellent MRD negativity and could serve as an induction strategy worthy of further study. Given the increased survival of MCL patients,2 critical assessment of long-term efficacy and toxicity is needed. Here, we report the 5-year follow-up (FU) of the S1106 study.
Methods
The study design, eligibility criteria, and statistical analyses have been previously published.10 Untreated MCL patients with stage III, IV, or bulky stage II MCL were randomized to 4 cycles of RH or 6 cycles of RB followed by ASCT. Patients achieving complete response (CR) or partial response at restaging11 were eligible to proceed to ASCT. A minimum of 1.5 × 106 CD34+ cells/kg stem cells were required to proceed with ASCT. MRD was assessed at baseline and after induction on 12 paired serial samples (2 RH and 10 RB) (Adaptive Biotechnologies). The primary end point of the trial was 2-year PFS. Secondary end points were overall response rates (ORRs), CR rate, overall survival (OS), toxicity, and MRD status. To minimize selection bias, a non-preplanned 5-year landmark analysis was performed from 3 months after registration for the RH arm and from 6 months after registration for the RB arm. The 5-year landmark analysis was used for comparing survival between transplanted and nontransplanted patients.10
This randomized phase 2 study was conducted by the Southwest Oncology Group (SWOG) through the National Clinical Trials Network adult groups (SWOG/Eastern Cooperative Oncology Group/American College of Radiology Imaging Network alliance) mechanism. Patients were recruited from 4 January 2012 through 21 June 2013. The study was approved by the institutional review board at each study site, and written informed consent was obtained from all patients prior to any study-specific procedures, per the Declaration of Helsinki. The study was approved and sponsored by the Cancer Therapy Evaluation Program (https://clinicaltrials.gov/ct2/show/NCT01412879).
Results and discussion
Among 53 enrolled patients, 52 patients (17 RH and 35 RB) were evaluable, with 1 patient not starting therapy. Baseline characteristics were similar, except that more female patients were in the RH group.10 Median age was 59 and 57 years in the RH and RB arms, respectively. More than 90% patients on both arms had stage IV extranodal disease and bone marrow involvement. Approximately 20% of patients had a Ki67 index >30%, and 35% had an intermediate/high MCL international prognostic index score in each arm. A total of 9 out of 17 patients (5 patients on protocol and 4 off protocol) in the RH arm and 23 out of 35 patients (21 patients on protocol and 2 off protocol) in the RB arm underwent ASCT. The reasons for discontinuation/stopping before ASCT have been previously published. Most discontinuations in the RH arm were due to the inability to collect stem cells (n = 5) and cytopenias (n = 6), whereas in the RB arm, discontinuations were primarily due to patient choice (n = 4).10
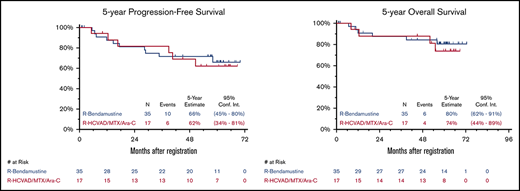
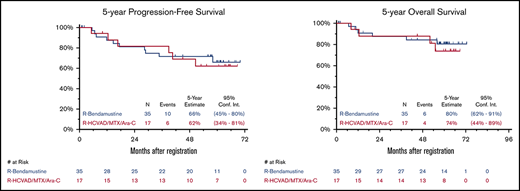
Updated efficacy results with median FU of 5 years (range, 29 days to 6 years) are detailed in Table 1. Updated ORR and CR rates were similar in both arms. As previously reported,10 the 2-year PFS was 82% and 81% and the 2-year OS was 88% and 87% in the RH and RB arms, respectively. The 5-year PFS was 62% (95% CI, 34% to 81%) and 66% (95% CI, 45% to 80%) and the 5-year OS was 74% (95% CI, 44% to 89%) and 80% (95% CI, 62% to 91%) for RH and RB, respectively (Figure 1). An updated landmark analysis at the 5-year mark with and without ASCT in each arm was also performed (Table 1). In the RH arm, the 5-year landmark PFS estimate was 50% (95% CI, 15% to 77%) vs 73% (95% CI, 28% to 93%) (P = .34) and the 5-year landmark OS was 75% (95% CI, 31% to 93%) vs 73% (95% CI: 28% to 93%) (P = .81) for patients undergoing ASCT vs those who did not. In the RB arm, the 5-year landmark PFS estimates were 70% (95% CI, 43% to 86%) vs 63% (95% CI, 23% to 86%) (P = .44) and the 5-year landmark OS was 91% (95% CI, 69% to 98%) vs 60% (95% CI, 20% to 85%) (P = .055) for patients undergoing ASCT vs those who did not.
Five-year PFS and OS. Five-year Kaplan-Meier PFS (A) and OS (B) curves. R, rituximab; R-HCVAD/MTX/Ara-C, rituximab plus hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone) and methotrexate/cytarabine.
Five-year PFS and OS. Five-year Kaplan-Meier PFS (A) and OS (B) curves. R, rituximab; R-HCVAD/MTX/Ara-C, rituximab plus hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone) and methotrexate/cytarabine.
MRD assessment was performed on 12 paired serial samples (2 RH and 10 RB). Both paired RH samples were MRD positive at baseline, and both achieved MRD negativity after induction. In the RB group, 9 out of 10 samples were MRD positive at baseline, of which 7 out of 9 (78%) achieved MRD negativity. Three patients with missing MRD assessment at baseline had negative MRD levels after induction/before ASCT. The 5-year PFS and OS was 90% (95% CI, 47% to 99%) for all patients who achieved MRD-negative status at the end of RB induction.
Of 52 evaluable patients, 10 have died (4 RH and 6 RB), mainly due to disease progression (n = 6). Other causes of death include suicide (n = 1) in the RH arm and typhlitis (n = 1), 1 lung cancer (n = 1), and an unknown cause (n = 1) in the RB arm. Two patients developed second malignancies. In the RH arm, 1 patient who did not undergo ASCT was diagnosed with acute myelogenous leukemia at 5.4 years after diagnosis and remains alive. In the RB arm, 1 patient who underwent ASCT developed lung cancer at 2.9 years after diagnosis and subsequently died.
Long-term results of this study continue to demonstrate excellent response rates, 5-year PFS, 5-year OS, and MRD negativity with either RH or RB. However, RH was more toxic and had higher stem cell mobilization failure rates. RB as induction treatment before ASCT yielded high response rates, with an ORR of 86% (95% CI, 70% to 95%) and CR of 43% (95% CI, 26% to 61%), and demonstrated excellent tolerability as well as durable remissions, with a 5-year PFS of 66% and 5-year OS of 80%. As previously published,10 the benefit of ASCT was more pronounced in the RB arm. The updated 5-year landmark OS estimates continue to suggest that the ASCT benefit is greater in patients receiving induction RB, although the limited sample size precludes definitive conclusions.
Although the optimal induction regimen is unknown, cytarabine-based induction has been favored, including the Nordic regimen, LyMa protocol and the European MCL regimens. In randomized settings, the reason for improved outcomes with cytarabine-based regimens may be the higher likelihood of achieving MRD negativity.12 However, this comes with increased cytopenias and frequent inpatient delivery of therapy. In our trial, the RH arm had significantly more grade 3/4 neutropenia, anemia, thrombocytopenia, and febrile neutropenia and is consistent with rates reported with the Nordic and European MCL Network regimens. The updated analysis of our study demonstrated that RB induction achieved a 5-year PFS of 66% and OS of 80% with MRD-negativity rates of 78%. These results are provocatively similar to intensive cytarabine-based induction therapy,4-6,12,13 albeit with decreased hematologic toxicity and outpatient treatment.
In conclusion, with 5 years of FU, S1106 shows excellent survival, with >70% patients alive and 60% patients disease-free in both arms. The comparison with RH is underpowered due to early closure of this arm. Patients receiving RB induction had excellent 5-year PFS, OS, and MRD-negativity rates. Overall, S1106 demonstrated that an outpatient-based, less intensive induction therapy of bendamustine plus rituximab is highly effective, safe, and durable in untreated transplant-eligible MCL patients. Our results have informed the design of a future Eastern Cooperative Oncology Group–led trial (EA4181) wherein a bendamustine-based induction chemotherapy backbone is being tested with cytarabine and/or a BTK-inhibitor in untreated MCL patients.
Acknowledgments
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award numbers CA180888, CA180819, CA180821, CA180820, CA180838, CA189957, CA189821, CA189972, CA189953, CA180846, CA180835, CA189808, CA46368, and CA11083 and in part by Sequenta, Inc. (Adaptive Biotechnologies).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Sequenta, Inc. (Adaptive Biotechnologies).
Authorship
Contribution: R.W.C., H.L., M.L.L., J.W.F., and S.M.S. designed research; R.W.C., J.W.F., B.S.K., T.S.F., T.C.S., T.J.P., P.M.B., L.M.R., and J.P.L. performed research; M.K. collected data; H.L. and M.L.L. performed statistical analysis; M.K., J.W.F., and S.M.S. wrote the manuscript; and all authors analyzed and interpreted data, and reviewed the manuscript.
Conflict-of-interest disclosure: M.K. is on the speakers bureau of Seattle Genetics and has received consultancy fees Genentech, AstraZeneca, and Celgene. R.W.C. has received consultancy fees from Millennium Pharmaceuticals and research funding from Affimed; consultancy, honoraria, and speakers bureau fees and research funding from Seattle Genetics; consultancy fees and research funding from Pharmacyclics and Merck; and consultancy and speakers bureau fees and research funding from Genentech. L.M.R. is an inventor on the patent for the Lymph2Cx assay (NanoString). P.M.B. has received consultancy fees from Pharmacyclics, AbbVie, Gilead, Merck, Seattle Genetics, Genentech, Celgene, Verastem, TG Therapeutics, and Janssen. T.J.P. has received consultancy fees from Genentech, Seattle Genetics, Bayer, Gilead, and Pharmacyclics and research funding from AbbVie. J.P.L. has received consultancy fees from Sutro, Bayer, Gilead, AstraZeneca, Celgene, Merck, Morphosys, Beigene, Nordic Nanovector, Roche/Genentech, ADC Therapeutics, Sandoz, Karyopharm, Miltenyi, and Akcea Therapeutics. B.S.K. has received consultancy fees from Seattle Genetics, AstraZeneca, Gilead, AbbVie, Celgene, Acerta, CTI, Juno, ADC Therapeutics, and Genentech. J.W.F. has received honoraria from Bayer. S.M.S. has received honoraria from Portola and consultancy fees from BMS. The remaining authors declare no competing financial interests.
Correspondence: Manali Kamdar, University of Colorado, 1665 Aurora Ct, MSF754, Aurora, CO 80045; e-mail: manali.kamdar@ucdenver.edu.
References
Author notes
Presented at the 60th annual meeting of the American Society of Hematology, San Diego, CA, 1 December 2018.