Key Points
GA to guide an MDC evaluation to optimize older adult candidates for hematopoietic cellular therapy is feasible and practical.
An MDC evaluation for older adults before transplantation holds promise to mitigate transplant-related morbidity and mortality.
Abstract
Limitations found on geriatric assessment (GA) track with worse outcomes after hematopoietic cell transplantation (HCT). We report on a multidisciplinary team clinic (MDC), consisting of a cancer-specific GA and a multidisciplinary team of providers, to assess candidacy and create an individualized optimization plan for allogeneic HCT candidates aged ≥60 years and autologous HCT and adoptive T-cell therapy candidates aged ≥70 years. Among the 247 patients evaluated in the MDC, allogeneic HCT candidates comprised the majority (60%), followed by autologous HCT (37%) with occasional older cellular therapy candidates (3%). Almost all patients meeting program-required minimum ages for MDC optimization at our institution were assessed (98%). Relative to historical control subjects undergoing GA alone, allogeneic HCT patients aged ≥60 years who underwent MDC appraisal had similar frequencies of high-risk disease, reduced intensity regimens, and high comorbidity but fewer GA-graded functional impairments. The MDC cohort experienced fewer inpatient deaths, shorter length of stay, and fewer discharges to nursing facilities compared with control subjects. Improvements in early mortality were observed over time; 1-year overall survival improved from 43% in the pre-MDC era to 70% in the recent MDC era, and 1-year nonrelapse mortality decreased from 43% to 18%. The 31 autologous HCT recipients aged ≥70 years optimized by the MDC achieved 0% nonrelapse mortality and 97% overall survival at 1 year. A GA-guided MDC for older HCT candidates is feasible and seems to reduce transplant-associated morbidity and mortality. An MDC should encourage broader and safer utilization of transplantation in older patients.
Introduction
The majority of hematologic malignancies with indications for hematopoietic cell transplantation (HCT) and adoptive T-cell therapy occur in older adults.1-4 Moreover, the proportion of autologous and allogeneic HCTs performed in patients aged ≥70 years has risen almost 10-fold over the past 1 to 2 decades.5,6 This trend is likely to continue, as US life expectancy at age 65 years in 2010 was 18 years for male subjects and 20 years for female subjects.7 Although older adults may be at higher risk for nonrelapse mortality (NRM) relative to younger adults, they can still safely undergo HCT and T-cell therapies for a variety of disease-specific indications.8-13
Clinicians have traditionally used age, comorbidity, and rudimentary assessments of performance status to describe the fitness of older individuals, aiming primarily to assess eligibility for HCT rather than identifying avenues to optimize their physiologic status and social support.14 However, in addition to advanced age, factors such as disability, frailty, and geriatric syndromes (eg, falls, delirium) play a larger role in describing physiologic age.14-17
Determining an older patient’s physiologic age may be accomplished by using a geriatric assessment (GA), a multidimensional patient assessment tool developed for issues germane to older adults that include functional status, comorbidities, cognitive abilities, behavioral conditions, social and economic support, nutritional status, and polypharmacy.18,19 A GA has been shown to be more effective at predicting remaining life expectancy and tolerance of treatment than standard medical evaluations for care of the elderly,20-23 and it may aid in prognostication before autologous and allogeneic HCTs among older adults.15,24,25
The broader concept of resilience (ie, the ability to adapt and recover from stressors) better reflects the goal of both determining and maximizing physiologic age in the context of an impending stressor as in transplantation.26 Likewise, the American Society of Clinical Oncology supports this concept, recommending that GA before oncologic treatment “should be applied to develop an integrated and individualized plan that informs cancer management and to identify nononcologic problems amenable to intervention.”27
Multidisciplinary teams have long been used in geriatrics to reduce functional impairments among older adults.28 Specifically with respect to oncology, an effective in-person multidisciplinary team meeting involves tasks and evaluations that require real-time feedback and communication between providers; this requires an array of providers with an appropriate skill mix who bring complementary experiences and attributes to the team.29,30 Although not reported in the context of HCT for older adults, the high morbidity and NRM after HCT for older adults warrant consideration of a multidisciplinary team approach.
As such, we established a Transplant Optimization Program (TOP) for older adults and report on the primary intervention: a GA-guided multidisciplinary team clinic (MDC) to evaluate and enhance resilience of older adult HCT and cellular therapy candidates. The current article describes the composition, feasibility, and outcomes of the MDC.
Methods
Patients
Participation in the TOP MDC was implemented as a standard-of-care quality initiative to improve patient outcomes. Indications for the MDC included every allogeneic HCT recipient aged ≥60 years at time of transplantation beginning in March 2013. In March 2015, the TOP MDC broadened eligibility to all autologous HCT candidates aged ≥70 years and later cellular therapy candidates aged ≥70 years. In addition, patients at least 50 years of age could be referred by the transplant physician as clinically indicated. The University of Chicago Institutional Review Board granted approval to review patient data. The data were maintained in a REDCap clinical database supported by the University of Chicago.
Regimens and supportive care
The regimens and supportive care followed institutional standard of care or treatment protocols when applicable. The most common allogeneic conditioning regimens included alemtuzumab with either fludarabine/melphalan,31 clofarabine/melphalan,32 or fludarabine/busulfan.33 Busulfan dosing most often targeted an area under the curve of 4800 per day for 4 days. A CD34-selected haploidentical regimen plus a single unrelated cord unit (haplo-cord) that used conditioning with fludarabine-melphalan (140 mg/m2) and rabbit antithymocyte globulin were also administered.34,35 Low-dose total body irradiation of 200 to 400 cGy was occasionally added to the haplo-cord regimen.
GA and quality of life before the MDC
We use a modified cancer-specific GA based on the original work by Hurria et al36 and the Delphi consensus of geriatric oncology experts,37 as described in supplemental Table 1. Additional modifications to supplement the original GA of Hurria et al included 4-m walk and grip strength. In addition, the Short-Form 36 (SF-36) captured patient-reported quality of life, and several other provider-specific tools added further depth to the GA screening tools. Before the MDC, each patient fills out electronic (or less commonly, paper) versions of the Health Status Survey and the SF-36. On the day of the MDC, a clinical coordinator (who has a bachelor’s degree) or similar staff administers the cognitive screen and functional tests (ie, grip strength, 4-m walk test, timed up and go) to complete the GA. Before the creation of our MDC, a modified GA was used among allogeneic HCT patients aged ≥50 years evaluated from 2005 to 2012 as previously published15,38 ; this group served as a historical control. In addition, providers applied their own tools, complementing the screening GA.
Multidisciplinary team clinic
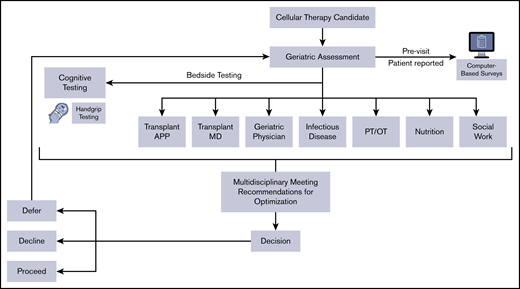
The initial referral to the MDC occurs after the transplant physician recommends consideration of hematopoietic or cellular therapy with the clinic being scheduled after pretransplant testing and usually within a 2- to 6-week window before conditioning. The patient then attends the TOP MDC, which involves ∼5 hours of evaluation per patient (supplemental Table 1). The MDC is held weekly, accommodating 1 to 3 patients per session. As shown in Figure 1, the team members include the same MDC-dedicated providers each week: an HCT advanced practice practitioner, an HCT physician, a geriatric physician (or geriatric oncologist), an infectious disease physician, a physical and/or occupational therapist, a dietitian, and a social worker. The MDC team members meet to review and discuss each patient after clinic on the same day of the visit, adhering to the following tenets: dissimilar redundancy in assessments (ie, overlap in health evaluation domains to avoid missing subtle limitations), optimize resiliency by mitigating limitations and leveraging strengths from the GA, devise recommendations to cushion the stressor (ie, morbidity and toxicities) of the proposed HCT procedure, and engage the patient and caregivers in goal-setting.
Multidisciplinary clinic evaluation, workflow, and team. APP, advanced practice practitioner; MD, physician; PT/OT, physical and/or occupational therapist.
Multidisciplinary clinic evaluation, workflow, and team. APP, advanced practice practitioner; MD, physician; PT/OT, physical and/or occupational therapist.
MDC recommendations and implementation
The multidisciplinary discussion results in a consensus recommendation on how to best optimize resilience for transplantation or cellular therapy. Table 1 describes common optimization pathways according to vulnerability. The patient and the primary referring transplant physician and team are notified of recommendations, and further discussion ensues if needed. Generally, evaluation and supportive care recommendations emanate from MDC team members coordinated by the HCT advanced practice provider under physician supervision. For example, the dietitian will educate on recommended nutritional practices, whereas the HCT advanced practice provider will submit and coordinate orders and prescriptions. Once a plan has been initiated, the primary transplant team is given responsibility to continue to observe the patients. Decisions on the transplant approach (eg, modification of conditioning regimen) ultimately reside with the transplant physician.
All patients are given recommendations to augment resiliency, even if no deficits exist, through education, review of previous toxicities and concerns, and by fortifying strengths. Finally, nonbinding recommendations about proceeding with the proposed treatment are designated into 1 of 3 categories: (1) optimize and proceed with HCT (or cellular therapy); (2) optimize and decline HCT because acceptable resilience is not likely; or (3) optimize and defer HCT until established metrics are met, usually with re-evaluation in the MDC (Figure 1). A second optimization visit if needed usually occurs after 6 weeks. When the referring physician and MDC recommendation is in conflict, the case is presented at a transplant consensus meeting.
TOP MDC: assessment of feasibility and outcomes
Heuristic approach.
The MDC was not static; modifications occurred over time with major refinements summarized here. In 2015, we began requiring attendance in those aged ≥70 years before autologous HCT and cellular therapy. We added an infectious disease physician to the team. The MDC recommendation categories were clarified to providers. To better prepare the patient for the MDC, we created a script for patients to be read before the visit over the telephone and sent patients the detailed MDC schedule of providers.
Feasibility and outcomes.
Supplemental Table 1 highlights the GA tools investigated to describe outcomes related to the MDC. Standard thresholds with validated cut points were used to define vulnerability on the GA.27,39-41
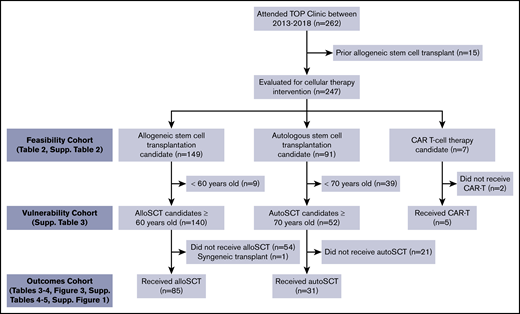
We reviewed feasibility of performing a patient-reported GA among patients who attended the TOP MDC between March 2013 and August 2018 (supplemental Table 2). To gauge patient and/or clinician adherence to the program requirements for MDC attendance, we summarized eligible allograft candidates (ie, aged ≥60 years) between March 2013 and August 2018, autograft candidates aged ≥70 years between March 2015 and August 2018, and chimeric antigen receptor T-cell therapy candidates aged ≥70 years between January 2017 and August 2018 (Figure 2). Patients with a previous allogeneic stem cell transplant were excluded.
Consolidated Standards of Reporting Trials (CONSORT) diagram of the patients studied. This includes the total number of patients seen in the TOP MDC and the subsets further studied for feasibility, prevalence of vulnerabilities, and outcomes. alloSCT, allogeneic stem cell transplant; autoSCT, autologous stem cell transplant; CAR, chimeric antigen receptor.
Consolidated Standards of Reporting Trials (CONSORT) diagram of the patients studied. This includes the total number of patients seen in the TOP MDC and the subsets further studied for feasibility, prevalence of vulnerabilities, and outcomes. alloSCT, allogeneic stem cell transplant; autoSCT, autologous stem cell transplant; CAR, chimeric antigen receptor.
Statistical analysis
Differences between groups were assessed by using the Fisher’s exact test for categorical variables and the Mann-Whitney U test for continuous variables with Stata version 15.0 (StataCorp). Inpatient length of stay was calculated as the interval from HCT infusion to the date of hospital discharge. Nursing facility admission by day 100 was defined as any overnight stay in a skilled nursing facility or acute rehabilitation center.
We restricted outcome comparison with the allogeneic transplant population aged ≥60 years because this core population participated in the MDC from inception and for whom GA data were available; outcomes studied included length of stay, nursing home admissions, readmissions, death, and 1-year overall survival (OS) and NRM. Time to readmission from discharge and OS were calculated by using the Kaplan-Meier method and compared by using the log-rank test and Cox regression analysis; there were no competing risk events to warrant using a cumulative incidence rate for time to readmission. The cumulative incidences of NRM (censored for relapse or death) and relapse at 1 year were compared by using Gray’s test. One-year outcomes were restricted to those with a full year of follow-up.
Results
Feasibility of TOP MDC attendance and recommendations
Table 2 summarizes the age ranges of the 247 patients referred to the MDC and who completed the GA. The median age of all MDC patients was 67.9 years (range, 43-83 years). Allografting represented the most frequent transplantation approach proposed (60%), with autografting constituting 37%, and chimeric antigen receptor T-cell or other adoptive T-cell therapy candidates comprising only 3% of candidates. Eleven (6.7%) allogeneic and 47 (40.7%) autologous HCT candidates were younger than the program-required age minimums of 60 and 70 years, respectively, reflecting elective referrals by the treating physician to the MDC. The primary reasons for deferring or declining transplant (n = 95) were poor health status (73%), disease status (23%), insufficient social support (3%), and patient deferral (1%).
The patient-reported tools were feasible at baseline: the Health Status Survey and SF-36 required ∼15 minutes each to complete, and the majority found the GA survey easy to understand (supplemental Table 2).
Most patients (122 of 152 [80%]) recommended by the MDC to proceed with their planned cellular therapy did receive their planned treatment. Of the 61 patients who the MDC initially recommended for deferral until additional evaluation and/or optimization, 28 (46%) eventually received cellular therapy. In contrast, only 2 patients for whom the MDC team recommended against transplant received an allogeneic HCT, both at outside institutions. Their outcomes are described later in the article.
Only 4 (3.3%) of 120 patients undergoing HCT who met our program’s age-specified standard of care requirement did not attend the MDC for logistical reasons, supporting feasibility of an institutional MDC. These three allograft recipients aged ≥60 years and 1 autograft recipient aged ≥70 years were excluded.
Supplemental Table 3 summarizes the baseline characteristics and GA impairments among patients meeting program-required age minimums for autologous and allogeneic HCT referral. Due to the older age minimum, autologous HCT candidates were older than allogeneic HCT candidates (median, 73 vs 67 years; P < .0001), although the proportion of GA-rated vulnerabilities did not differ between autologous HCT and allogeneic HCT.
Outcomes after autologous transplantation
The indications among the 31 autologous HCT recipients aged ≥70 years consisted of multiple myeloma (67.7%) and non-Hodgkin lymphoma (32.3%). Most patients with plasma cell dyscrasia (19 of 22 [86.4%]) received full-dose melphalan at 200 mg/m2, and all patients with non-Hodgkin lymphoma (n = 6) received full-dose BEAM (carmustine, etoposide, cytarabine, and melphalan) conditioning, reflecting the augmented inherent resiliency of the patients42,43 (supplemental Table 4). The median length of inpatient hospital stay was 14 days (range, 11-22 days) from time of HCT infusion; no deaths occurred during the initial hospitalization. Only 1 death occurred within day 100 (3%) of HCT in a patient with primary central nervous system lymphoma due to progressive disease. Of the 28 evaluable patients with 1 year of follow-up, 1-year OS was 97% and 1-year NRM was 0%.
Allogeneic transplantation: outcomes and improvements in the TOP MDC era
In the TOP MDC era, 85 patients aged ≥60 years underwent an allogeneic HCT between March 2013 and August 2018 (Table 3) and were compared vs 74 similar allogeneic HCT patients with a GA in the pre-TOP MDC period (2005-2012).
The cohorts exhibited similar proportions of traditional risk factors such as American Society for Blood and Marrow Transplantation high-risk disease, HCT–comorbidity index score ≥3, and use of myeloablative conditioning regimens (Table 3). Although the GA tools differed over the time period, we leveraged the similar backbones of instrumental activities of daily living (IADL) and 4-m walk speed to discern subtle differences. The pre-TOP group had a higher proportion of patients with an IADL impairment (49.2% vs 29.5%; P = .022) and frail 4-m walk (31.7% vs 5.9%; P < .001) compared with the TOP MDC cohort.
To better delineate improvements over time, the TOP MDC era was further divided into the initial phase (TOP initial of 2013-2014) and more recent era (TOP modern of 2015-2017). Early morbidity metrics showed a favorable association in the TOP era relative to the pre-TOP era in most measures: mortality during the HCT hospital stay, length of stay, discharge to nursing facility, and early death by day 100 (Table 4). Shorter length of stay and fewer inpatient deaths in the MDC era did not come at the expense of readmissions (supplemental Figure 1).
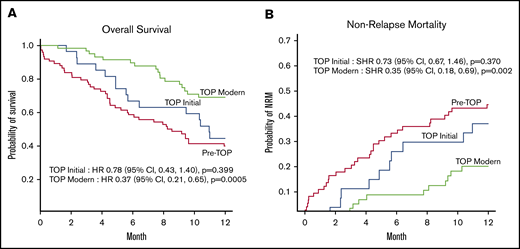
In the pre-TOP patients, 1-year OS and 1-year NRM were poor at 43%, as previously described.15,38 Steady improvements coincided with implementation of the TOP MDC (Figure 3). Relapse incidence within 1 year between the pre-TOP group and the TOP initial and TOP modern groups were similar (TOP initial subdistribution hazard ratio [SHR], 1.53; 95% CI, 0.69-3.38 [P = .30]; TOP modern SHR, 0.69; 95% CI, 0.31-1.57 [P = .38]). Similarly, there was no difference in 1-year relapse-related mortality (13.5%, 18.5%, and 12% for the pre-TOP, TOP initial, and TOP modern groups, respectively). Compared with pre-TOP patients, the SHR for NRM within 1 year for the TOP initial group was 0.73 (95% CI, 0.67-1.46; P = .37) and for the TOP modern group, it was 0.35 (95% CI, 0.18-0.69; P = .002). In parallel, the hazard ratio for death within 1 year for the TOP initial group was 0.78 (95% CI, 0.43-1.40; P = .399) and for the TOP modern group, it was 0.37 (95% CI, 0.21-0.65; P = .0005). This indicates that the improvement in OS in the TOP modern group was driven mainly by improvements in NRM. These improvements occurred despite more frequent allografting among patients aged ≥70 years during the TOP era (5% for pre-TOP, 18.5% for TOP initial, and 22% for TOP modern).
One-year outcomes before and during the TOP MDC among allogeneic transplant recipients aged ≥60 years across 3 different cohorts. Pre-TOP (2005-2012), TOP initial (2013-2014), and TOP modern (2015-2017). (A) One-year OS. (B) One-year NRM. CI, confidence interval; HR, hazard ratio.
One-year outcomes before and during the TOP MDC among allogeneic transplant recipients aged ≥60 years across 3 different cohorts. Pre-TOP (2005-2012), TOP initial (2013-2014), and TOP modern (2015-2017). (A) One-year OS. (B) One-year NRM. CI, confidence interval; HR, hazard ratio.
Excluded patients from allogeneic HCT analysis
Two patients received an allogeneic HCT at an outside institution (1 patient was <60 years of age) despite a recommendation not to proceed with transplant and were excluded from the outcomes analysis; 1 died within 1 year of HCT, and the other experienced disease relapse within 1 year of transplant although was alive beyond 1 year. Patients who did not undergo a GA before transplant in the pre-TOP period were excluded from the main analyses, and thus we could not compare baseline characteristics according to GA to evaluate patient selection. In the initial TOP MDC period of 2013 to 2014, 3 allograft recipients aged ≥60 years did not attend the TOP MDC; all 3 died within 1 year (2 of NRM and 1 of relapse). Every first allograft recipient aged ≥60 years from 2015 has been evaluated by the MDC.
Outcomes by TOP MDC recommendation for allogeneic HCT
We tracked outcomes among the patients aged ≥60 years receiving allogeneic HCT at our institution according to initial TOP MDC visit recommendation: “optimize and proceed” (n = 72), “optimize and defer” until additional evaluation and/or prehabilitation (n = 13), and “optimize and decline” (n = 0). Age and HCT–comorbidity index score did not differ between the defer and proceed patients undergoing allogeneic HCT (supplemental Table 5). Compared with those recommended to proceed, an initial deferral was associated with delayed time to HCT (median, 76 vs 35 days; P < .001). Length of inpatient stay, discharges to nursing facility by day +100, and readmissions by day +100 did not differ between these 2 cohorts. The proceed patients less often succumbed to early death by day +100 relative to patients in the defer category (4% vs 23%; P = .043). One-year outcomes did not statistically differ according to proceed or defer recommendations (1-year OS, 64% vs 46% [P = .35]; NRM, 22% vs 39% [P = .29], respectively).
Discussion
As early as 1993, transplant researchers had opined that “age alone should not be considered a contraindication to transplantation.”44 However, identifying adverse factors to ameliorate expected HCT complications in older patients has been elusive. We created an MDC to maximize resiliency to avoid biases of older age alone and more safely offer hematopoietic and cellular therapies. We describe for the first time the characteristics, feasibility, and outcomes related to an MDC attuned to older patients before HCT.
A vital component of the MDC consists of the GA, modified from the Cancer and Aging Research Group GA,36 generating a quantitative and nuanced picture of older candidates’ resiliency to aid in stratification and optimization. The novel alignment of assessment tools, providers, and process for HCT was based on a geriatrics-embedded collaborative cancer care model and heuristically modified.29,45
We required attendance as part of the standard of care for allogeneic candidates aged ≥60 years and later for autologous candidates aged ≥70 years. MDC was feasible as standard of care; 98% of patients undergoing allografting or autografting in these populations attended the MDC. Only 3 allogeneic HCT recipients were not seen, all in the first 2 years of the MDC. Furthermore, we documented frequent elective referrals of candidates at least 50 years of age not required to attend, suggesting a perceived value of the MDC.
Our data indicate that universally available and validated patient-reported tools can be routinely performed as standard of care in this setting via a computer-based survey alongside in-person clinical evaluation, to the satisfaction of patients. This finding is in keeping with previous results in older patients with solid tumors.36,46 Nevertheless, nearly one-third of patients completed surveys on paper, despite a preference among these patients to perform electronic surveys. Future efforts will be geared toward increasing electronic survey rates and attempts to reduce redundant questions to minimize patient survey effort. Consistent with previous data on pre-HCT evaluations in older adults, the GA frequently uncovered deficits in function, geriatric syndromes (eg, falls and polypharmacy), and putative cognitive impairment.25,38,47
The GA-guided recommendations to enhance resiliency before allogeneic HCT seems to mitigate early transplant-associated morbidity in older patients. Specifically, we found significant reductions in patient-centric outcomes of skilled nursing facility admissions, hospital length of stay, and early death relative to the period before our MDC. The effects, especially in the more recent time period, persisted to improve 1-year OS over time through reduced 1-year NRM. Consistent with a beneficial effect derived from the MDC, we found remarkably good survival and low NRM after autologous HCT in those aged ≥70 years despite high comorbidity and other impairments. These outcomes occurred without necessitating dose attenuation of the preparative regimen (and the attendant increased relapse rates) as 90% received the standard full doses. These data further intimate that the MDC can augment resiliency to avoid arbitrary chemotherapy dose minimization due to patient age.
Several important limitations warrant discussion. This was a single institutional observational study of heterogeneous patients and transplant types, limiting subset analysis. Prospective randomized studies will be required to ensure validity and generalizability. The MDC approach likely impeded or denied HCT for some older patients; the benefits or drawbacks of avoiding HCT for these high-risk patients cannot be ascertained. Nevertheless, among allogeneic HCT patients initially deferred by the TOP MDC but ultimately pursuing HCT after optimization, the outcomes seemed numerically (but not statistically) inferior relative to those recommended to initially proceed (eg, 1-year OS and NRM of 46% and 38.5% vs 64% and 22%, respectively) in this small subset (n = 13). Deferred patients may represent a more “at-risk” population whose comorbidities or vulnerabilities could not be fully mitigated by the MDC; in addition, they experienced a longer time from evaluation to receipt of transplant, which could have affected their outcomes. We have now instituted an MDC booster visit on day 30 after transplant for higher risk patients.
It is difficult to isolate improvements attributable to more careful patient selection vs optimization; we believe coupling selection and optimization together improved results. Historical comparisons are certainly limited by unmeasured confounding factors (eg, cytogenetic and molecular markers of disease) or supportive care changes (eg, improved molecular diagnostic techniques for cytomegalovirus and respiratory viruses) that in part account for better results in the modern era. Regimens, disease risk, and relapse-related 1-year mortality did not differ over these time periods. Patients in the MDC era were in fact older (19% who were aged ≥70 years vs 5% in the pre-TOP period), consistent with national trends.5 With the recognition that some GA components differed from the pre-TOP era, we found fewer functional impairments in IADLs or slow walk speed in the TOP cohort relative to the pre-TOP period. Therefore, better pre-HCT patient functional status may account for part of the gains, actively mediated by the GA-based MDC patient selection.
Although the MDC and optimization framework are detailed, each patient received a personalized plan in addition to standard interventions, similar to most clinical encounters. Moreover, we believe that this type of multidisciplinary clinic is generalizable to other transplant centers, as the type of specialist providers needed to engage in the MDC are typically already available at most institutions with the exception of a geriatrician. However, deploying staff in a coordinated pretransplant fashion to optimize patients requires organization and administrative effort. We believe for prospective studies, more complete characterization of the interventions and the surrogate markers for benefit will be invaluable (eg, weight and muscle gain after nutritional intervention and cognitive morbidity). Ultimately, the approach should still permit personalization and be iterative. Indeed, we have found continual improvements in 1-year NRM and OS likely through ongoing modifications in the MDC.
In conclusion, we have shown both feasibility of and favorable outcomes from an MDC for older adults before hematopoietic and cellular therapy. A focus on a GA-guided team approach, optimizing resiliency to inform eligibility, along with an institutional willingness and understanding of the need for this type of approach, were crucial to the clinic’s success. The MDC approach, if validated, may permit safer and more widespread utilization of transplantation among older adults.
Acknowledgments
The authors thank Sally Mokhtari at City of Hope National Medical Center for editorial support of the visual abstract. The authors also thank the patients and their families, investigators, study coordinators, and support staff who played a role in conducting this study.
B.A.D. is supported by National Institutes of Health Training Grant 2T32CA009566-31.
Authorship
Contribution: B.A.D. and A.S.A. designed the study; B.A.D., K.K., J.R., S.C., E.A., M.M., and A.S.A. collected and assembled data; B.A.D., K.K., W.D., S.M.L., E.A., M.M., J.P., and A.S.A. analyzed and interpreted the data; B.A.D. and A.S.A. wrote the manuscript; and all authors participated in patient care, manuscript development, and final approval.
Conflict-of-interest disclosure: K.K. receives honoraria from Kite Pharmaceuticals; served on an advisory board for Celgene; and was on the speakers bureau for Merck. J.R. is currently an employee of AbbVie. A.J.J. serves on the advisory boards of AbbVie, Amgen, Celgene, GlaxoSmithKline, Janssen, Juno, Karyopharm, and Sanofi. J.K. receives honoraria from Kite, Merck, and Seattle Genetics, and research funding from iTeos and Merck. R.A.L. serves on the advisory boards for Amgen, Novartis, Celgene, Ariad Pharmaceuticals, CVS Caremark, and Epizyme; and has received institutional research funding from Daiichi Sankyo, Celgene, Cellectis, Astellas Pharma, Rafael Pharmaceuticals, and Novartis. H.L. serves on the advisory board for Agios; receives research funding from Bristol-Myers Squibb and Karyopharm Therapeutics; and receives travel expenses from Nohla Therapeutics and Arog. M.M. serves on the advisory boards for Kite Pharma, Novartis, and Juno; and has received honorarium from Kite Pharma. O.O. serves on the advisory boards for AbbVie and Celgene; and receives research funding from Celgene, Incyte, Astex Pharmaceuticals, NS Pharma, AbbVie, Gilead Sciences, Janssen Oncology, MEI Pharma, Millennium Pharmaceuticals, Oncotherapy, Agios, AstraZeneca, and CTI/Baxalta. W.S. serves on the advisory boards for Daiichi Sankyo, Astellas, Kite Pharma, and Agios; and receives research funding from Jazz Pharmaceuticals and Pfizer, honorarium from Research To Practice, and royalties from UpToDate. A.S.A. receives research funding from Miltenyi Biotec. The remaining authors declare no competing financial interests.
Correspondence: Andrew S. Artz, Hematology/Oncology, City of Hope, 1500 E Duarte Rd, Duarte, CA 91010; e-mail: aartz@coh.org.
References
Author notes
The full-text version of this article contains a data supplement.
For original deidentified data, including deidentified individual participant data, please contact bderman@medicine.bsd.uchicago.edu.