Key Points
A TREO-based conditioning regimen can ensure sustained donor engraftment in patients undergoing CBT.
TREO can be safely used in CBT to extend the donor pool to patients without conventional donors.
Abstract
Although the use of treosulfan (TREO) in conventional donor hematopoietic cell transplantation (HCT) has been extensively evaluated, its use in cord blood transplantation (CBT) for hematologic malignancies has not been reported. Between March 2009 and October 2019, 130 CBT recipients were enrolled in this prospective multicenter phase 2 study. The conditioning regimen consisted of TREO, fludarabine, and a single fraction of 2 Gy total-body irradiation. Cyclosporine and mycophenolate mofetil were used for graft-versus-host disease prophylaxis. The primary end point was incidence of graft failure (GF), and based on risk of GF, patients were classified as low risk (arm 1, n = 66) and high risk (arm 2, n = 64). The median age was 45 years (range, 0.6-65 years). Disease status included acute leukemias in first complete remission (CR; n = 56), in ≥2 CRs (n = 46), and myelodysplastic (n = 25) and myeloproliferative syndromes (n = 3). Thirty-five patients (27%) had received a prior HCT. One hundred twenty-three patients (95%) engrafted, with neutrophil recovery occurring at a median of 19 days for patients on arm 1 and 20 days for patients on arm 2. The 3-year overall survival, relapse-free survival (RFS), transplant-related mortality, and relapse for the combined groups were 66%, 57%, 18%, and 24%, respectively. Among patients who had a prior HCT, RFS at 3 years was 48%. No significant differences in clinical outcomes were seen between the 2 arms. Our results demonstrate that TREO-based conditioning for CBT recipients is safe and effective in promoting CB engraftment with favorable clinical outcomes. This trial was registered at www.clinicaltrials.gov as #NCT00796068.
Introduction
Umbilical cord blood (CB) is an alternative hematopoietic stem cell source with the advantages of relative tolerance of HLA disparity1-5 and rapid donor availability.6 CB transplantation (CBT) is a valuable treatment of patients with hematological malignancies, including acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), and myelodysplastic syndrome (MDS).7-9 However, optimal conditioning regimens for CBT, particularly in older and infirm patients with high-risk disease, have not yet been defined. Although aggressive myeloablative conditioning (MAC) regimens are generally associated with lower relapse risk and low risk of primary graft failure (GF), they are also associated with an increased probability of transplant-related mortality (TRM). In contrast, less-intensive conditioning regimens that rely more significantly on the graft-versus-leukemia effect to treat disease are generally associated with less toxicity but are also associated with higher risk for relapse.10 Optimizing the balance in conditioning intensity to maximize engraftment rate and disease control without increasing TRM is an ongoing challenge in hematopoietic cell transplantation (HCT) including CBT.
Treosulfan (TREO) is a busulfan (BU) analog with a distinct site of alkylation that results in a more favorable toxicity profile. It has been used increasingly in conventional donor HCT, frequently in combination with fludarabine (FLU), where it has been effective in establishing engraftment with low TRM.11 In addition, it has several attractive characteristics as compared with BU, including predictable pharmacokinetics, allowing for outpatient administration and, importantly, a decreased risk of TRM12 and potential for higher antileukemic activity that can induce sustained remissions in high-risk patients.13 These characteristics make TREO particularly appealing as an agent in less-intensive HCT-conditioning regimens. However, there is very little published experience with the use of TREO in the CBT setting,14,15 largely due to concern that use of TREO would not be sufficiently immunosuppressive to promote sustained engraftment of CB donors, given the high rates of primary GF that had been observed when BU/FLU-conditioning regimens were used in CBT.16 In this phase 2 prospective clinical trial, we sought to determine whether the combination of TREO/FLU total-body irradiation (TBI) was able to promote CB engraftment, and to estimate the curative potential of this approach in patients with hematological malignancies.
Methods
Study design
We conducted a double-arm study in patients undergoing CBT to assess the efficacy of TREO/FLU/TBI as a conditioning regimen. Incidence of GF was our primary end point. Patients were divided into the 2 arms based on their risk of GF. Arm 1 included patients at low risk for GF, including those who had received ≥2 cycles of multiagent chemotherapy and at least 1 cycle of therapy within the 3 months prior to CBT. Patients in arm 2 were high risk for GF patients, including those who had received no multiagent chemotherapy or immune-suppressive chemotherapy in the 3 months prior to transplant or had received only a single induction therapy. Primary and secondary GF were our primary end points. Other end points included platelet engraftment, acute graft-versus-host disease (GVHD; aGVHD) and chronic GVHD, chronic GVHD, overall survival (OS), relapse-free-survival (RFS), TRM, and relapse.
Patients
Eligible patients were aged 6 months to 65 years old.17 Patients had acute leukemias, myeloproliferative syndromes (MPNs), or MDS. Additional inclusion requirements included adequate performance status and organ function (see supplemental Table 1). If patients had a previous HCT, they had to be ≥3 months posttransplant. This study was conducted in accordance with the Declaration of Helsinki and was approved by the Fred Hutchinson Cancer Research Center (FHCRC) Institutional Review Board; all patients gave written informed consent.
TREO-based conditioning
TREO was supplied to investigators by Medac GmbH (Hamburg, Germany) under an institution-sponsored investigational new drug (IND) of the US Food and Drug Administration (FDA; IND 72,479, approved 22 June 2005, sponsor: FHCRC). For adult patients, TREO was administered IV at a dose of 14 g/m2 per day for a total dose of 42 g/m2. For children, TREO was given according to the same schedule, but at a daily dose (determined by body surface area) of 10 g/m2 (≤0.5 m2), 12 g/m2 (>0.5 m2 to 1 m2), or 14 g/m2 (>1 m2). Fludarabine was administered at a dose of 30 mg/m2 IV for a total dose of 150 mg/m2. If evidence suggested that the probability of GF was exceeding 5%, as defined by statistical parameters, stepwise dose escalations were set in place to first increase the dose of FLU and then to escalate TBI. For patients on arm 2, FLU was increased to 35 mg/m2 per day, for adult patients with renal impairment (defined as creatinine clearance < 70 mL/min per 24 hours), or 40 mg/m2 for a total dose of either 175 or 200 mg/m2.
Transplant procedures
CB donor selection was based on institutional guidelines and units were selected to optimize both HLA match and cell dose,7 avoiding, when possible, CB units (the patient had donor specific anti-HLA antibodies). All patients received unrelated donor CB grafts, which were 4 of 6 to 6 of 6 matched to the recipient at HLA-A, B, and DRB1 antigens. HLA typing was performed at the antigen level for HLA-A and B, and high-resolution HLA typing was performed for HLA-DRB1 alleles. The selection of 2 CB units was mandatory when a single CB unit did not meet the following criteria: HLA match 6 of 6 with a total nucleated cell count (TNC) dose of ≥2.5 × 107/kg or HLA match 5 of 6, 4 of 6 with a TNC dose of ≥4.0 (±0.5) × 107/kg. In patients receiving a double CBT, the individual CB units were at least 3 of 6 HLA-A, B, and DRB1 matched to each other, and each contained a minimum of 1.5 × 107 TNC per kilogram. Prophylaxis for GVHD consisted of cyclosporine beginning on day −3 and continuing for a minimum of 180 days, and mycophenolate mofetil beginning on day 0, administered until at least day 40, and tapered in the absence of GVHD until day 96 or potentially longer in the presence of active GVHD. Granulocyte colony-stimulating factor was given posttransplant until stable absolute neutrophil count (ANC) recovery to >2.5 × 109/L, and then as needed to maintain an ANC >1.0 × 109/L. Supportive care was standard per our institutional guidelines. All but 5 patients received an intensive strategy to prevent cytomegalovirus (CMV) in seropositive CBT recipients.18 The remaining 5 patients received letermovir for CMV prophylaxis.
Definitions for hematologic recovery, GF, and disease recurrence
The day of neutrophil (ANC) engraftment was defined as the first of 3 consecutive days of an absolute ANC of ≥0.5 × 109/L. Platelet engraftment was defined as the first of 7 consecutive days when the platelet count exceeded 20 × 109/L (untransfused). Patients were evaluated for donor cell engraftment if they were alive and in remission 28 days or more after transplantation. GF was defined either as failure to achieve an ANC ≥0.5 × 109/L combined with host CD3 peripheral blood chimerism ≥50% at day 42 or as absence of 3 consecutive days with neutrophils ≥0.5 × 109/L under any circumstances at day 55. Analysis of donor chimerism was performed on CD3+, CD56+, CD14+, and CD33+ fractions of peripheral blood sorted by flow cytometry on days 14, 28, 56, and 80 after transplantation. Chimerism analysis of unsorted bone marrow cells was performed on days +28 and +80 after transplantation.
Any detectable level of residual disease in patients with acute leukemias and MDS by flow cytometry, and cytogenetic and molecular assay below morphologically identifiable disease, was considered measurable residual disease positive (MRD+). Relapse was defined as the presence of >5% blasts by morphology in the marrow or biopsy proven extramedullary disease in patients with acute leukemia. aGVHD and chronic GVHD were defined according to published consensus criteria.19,20 GVHD-free RFS (GRFS) was defined as the absence of grade III-IV aGVHD, chronic GVHD (requiring systemic therapy or extensive stage), relapse, and death.
Statistical analysis
Cumulative incidence estimates of ANC and platelet engraftment, aGVHD, relapse, and TRM were used, with death or relapse (for GVHD), TRM (for relapse), and relapse (for TRM) included as competing risk events. Kaplan-Meier estimates were used to evaluate OS, RFS, and GRFS. OS was measured from the first day of CBT infusion until death from any cause, with censoring performed at the latest date of contact. Death of any cause other than relapse or disease progression was considered TRM. Cox regression was used to evaluate covariate associations with the hazards of overall mortality, TRM, and relapse. Accrual was completed as planned on October 2010. Data were analyzed as of 1 March 2020.
Results
Patients
From March 2009 to August 2019, 130 patients (planned accrual) received TREO/FLU/TBI conditioning followed by a single (n = 31; adults = 9 and children = 22) or double (n = 99; adults = 90 and children = 9) CBT (Table 1). The median age of patients was 45 years (range, 0.6-65 years) with the majority being nonwhite (n = 67; 51%). Median follow-up of surviving patients was 4.2 years (range, 0.46-10.9 years). Diseases included AML (n = 63), ALL (n = 37), biphenotypic leukemia (n = 2), MDS (n = 25), and MPN (n = 3). Twenty-five of the 28 patients with MDS and MPN were enrolled on arm 2. Fifty-five patients (42%) had poor cytogenetic characteristics at diagnosis, 40 (31%) were MRD+ at the time of HCT and 35 (26%) had a prior HCT (33 allogeneic, 2 autologous). Indications for second HCT were relapse of primary disease (n = 30) and onset of new hematological malignancy (n = 5). The median time from first to second HCT was 521 days (range, 38-3515 days). For patients on arm 2, we escalated the dose of FLU after the first 7 patients were enrolled. Fifty-seven patients (89%) on arm 2 received an increased FLU dose of either 35 mg/m2 (n = 5) or 40 mg/m2 (n = 52).
Hematopoietic recovery
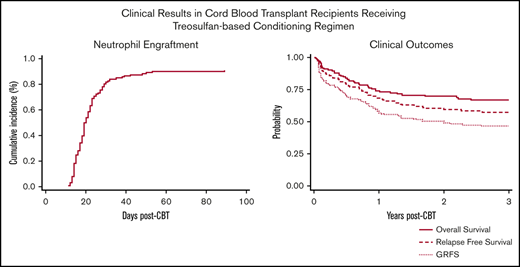
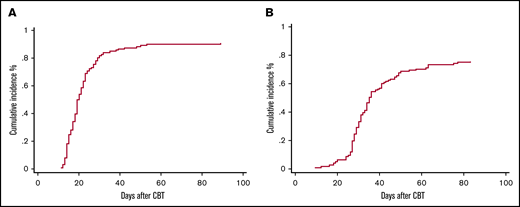
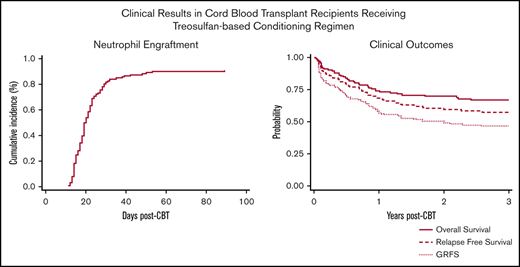
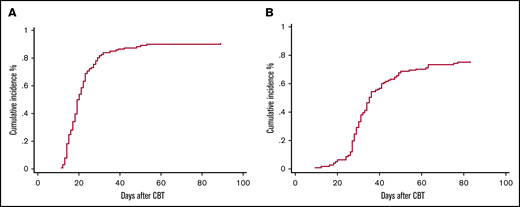
Seven patients (5%) met the definition of primary GF, 4 patients had MDS, 2 AML, and 1 ALL. Two patients ultimately recovered counts at days 66 and 89 and went on to have sustained donor engraftment. No secondary GFs were observed. Characteristics of patients with primary GF are reported in Table 2. Overall, the cumulative incidence of ANC recovery was 91% (94% confidence interval [CI], 84-94) and occurred at a median of 19 days (range, 11-89) (Figure 1A). The cumulative incidence of platelet recovery at day 100 (≥20 × 109/L) was 75% (95% CI, 67-82) at a median of 31 days (range, 12-83 days) (Figure 1B), whereas platelet count ≥50 × 109/L was reached at a median time of 37 days (range, 18-85 days). ANC and platelet recovery were similar between the 2 arms (Table 3). Chimerism was evaluable in 111 patients (85%), 89 (80%) achieved full chimerism at a median time of 28 days (range, 14-56 days), and 22 (20%) had mixed chimerism through day +100 with host persistence in 5 patients.
Cumulative incidence to day +100 in CBT recipients. (A) Neutrophil engraftment. (B) Platelet engraftment.
Cumulative incidence to day +100 in CBT recipients. (A) Neutrophil engraftment. (B) Platelet engraftment.
Transplant outcomes
Survival and relapse.
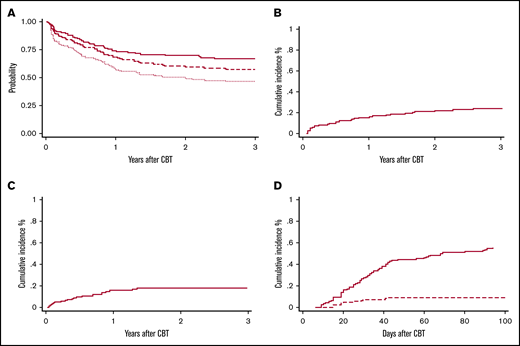
OS and disease-free survival (DFS) at 3 years were 66% (95% CI, 57-73) and 57% (95% CI, 48-65), respectively (Figure 2A), with no differences between the 2 arms. DFS for patients with AML, ALL, and MDS was 61% (95% CI, 48-72), 50% (95% CI, 33-65), and 63% (95% CI, 41-78), respectively. In patients with acute leukemias, there was no difference between patients in first complete remission (CR1) vs at least second complete remission (CR2) (61% vs 52%; P = .32). Among patients with myeloid malignancies (n = 91), OS and DFS at 3 years were 67% (95% CI, 56-76) and 61% (95% CI, 50-70), respectively. Presence of MRD at the time of HCT was associated with worse DFS (64% vs 43%; P = .02). Among patients who had a prior HCT (n = 35), DFS at 3 years was 48% (95% CI, 31-63).
Survival and other outcomes for CBT recipients. (A) OS (solid red line), RFS (dashed red line), and GRFS (dotted red line) at 3 years after a TREO-based conditioning regimen. Cumulative incidence of overall relapse (B) and TRM (C) at 3 years after a TREO-based conditioning regimen, and of grade II-IV (solid red line) and III-IV (dashed red line) aGVHD to day +100 (D).
Survival and other outcomes for CBT recipients. (A) OS (solid red line), RFS (dashed red line), and GRFS (dotted red line) at 3 years after a TREO-based conditioning regimen. Cumulative incidence of overall relapse (B) and TRM (C) at 3 years after a TREO-based conditioning regimen, and of grade II-IV (solid red line) and III-IV (dashed red line) aGVHD to day +100 (D).
The cumulative incidence of relapse at 3 years was 24% (95% CI, 17-32) (Figure 2B), with a trend of higher rate of relapse among patients in arm 1 (31% vs 17%; P = .16) and in MRD+ patients (31% vs 21%; P = .19).
TRM and cause of death.
Grade 3-4 nonhematologic toxicities included mucositis in 21 patients (16%) and hyperbilirubinemia in 28 patients (21%). In one-third of our cases, the hyperbilirubinemia was associated with either persistence of disease (n = 2) or infectious complications (n = 8; bacterial n = 4 and viral n = 4) whereas in the remaining cases, liver toxicity was transient. Only 1 patient presented with sinusoidal obstruction syndrome but recovered without need for specific treatment. The overall cumulative incidence of TRM was 7% (95% CI, 3-12) at day 100 and 18% at 3 years (95% CI, 12-26) (Figure 2C) with no difference between the 2 arms. In multivariate analysis, age (hazard ratio, 1.03; 95% CI, 1.0-1.05; P = .02) and number of HCTs (hazard ratio, 3.07; 95% CI, 1.18-8.08; P = .02) were the only variables correlated with increased risk of TRM. The Hematopoietic Cell Transplantation-specific Comorbidity Index scores had no statistically significant impact on outcome. Causes of death are summarized in Table 4.
aGVHD and chronic GVHD.
The cumulative incidence of grade II-IV and III-IV aGVHD was 55% (95% CI, 46-64) and 10% (5-15), respectively, with no difference between the 2 arms (Figure 2D). Chronic GVHD was evaluated in 109 patients who survived at least 100 days without relapse after HCT. The median day of chronic GVHD occurrence after transplantation was 176 (range, 84-798 days). Chronic GVHD occurred in 44 total cases (40%), with only 8 patients (7%) having moderate (n = 6) to severe (n = 2) chronic GVHD. At 2 years, GRFS was 50% (95% CI, 40-57) with 82% of the patients off immunosuppression (Figure 2A).
Discussion
In this phase 2 prospective study, we met our primary objective of demonstrating that a TREO-based preparative regimen allowed for sustained engraftment and disease control in patients undergoing CBT. TREO offers significant advantages over BU, specifically and especially with respect to the potential to lower the risk of regimen-related toxicities.12 Several studies have confirmed that, in the conventional donor setting, a combination of TREO and FLU has led to consistent and sustained donor engraftment with low regimen-related toxicity.17,21-24 Therefore, we investigated the use of the same combination regimen in patients undergoing CBT with the addition of a single dose of 200 cGy TBI as a means of better ensuring adequate immunosuppression to promote sustained donor engraftment and disease control.25
Previous experience with nonmyeloablative (NMA) CBT suggested that patients who have had limited prior therapy are at particularly greater risk for GF. For the purposes of determining acceptable engraftment rates following TREO/FLU/TBI, patients were classified as low risk (arm 1) or high risk for GF (arm 2).26 Based on the risk of GF, this protocol also included a dose-escalation schema, first of FLU and then TBI. A dose escalation of FLU from 30 to 40 mg/m2 was required for patients on arm 2 due to an initial excess of GF. This increase in FLU dosing in patients deemed at high risk of GF reduced the risk of GF such that no further dose escalation of TBI was required. Overall, this conditioning regimen resulted in a low rate of GF and the FLU adjustment reduced the risk of GF in patients deemed at higher risk.
The rapid conversion to full-donor chimerism observed in the majority of patients suggests that this TREO-based conditioning regimen was more intensive than protocols with attenuated (reduced)-intensity combination chemotherapy.10,16 Indeed, the median time of ANC recovery was similar to that reported with high-intensity regimens in the setting of double-cord transplantation.9,27-31
The dose of 3 × 14 g/m2 TREO in combination with FLU and TBI should be considered as a less-intense MAC regimen with reduced toxicity. We are the first to show that this combination can be successfully used in patients with a variety of high-risk hematologic malignancies undergoing CBT who may not be able to tolerate high-dose TBI, but in whom a more intensive regimen would be beneficial for better control of disease. Although NMA regimens allow older patients and those with greater comorbidities to undergo HCT, relapse rates limit the success of this strategy.
In the present study enrolling high-risk leukemia patients (26% with prior HCT), the rate of relapse was 24%, similar to that observed for CB recipients treated with other high-intensity regimens and better than that observed after NMA.9,10,27 Successful incorporation of low-dose radiation into the TREO/FLU regimen has been shown to decrease the relapse rates by increasing conditioning intensity without the toxicities seen in MAC regimens.25 The 3-year OS of 67% was higher than that reported after reduced-intensity conditioning10,16,32 and similar to that observed with high-intensity approaches.9,27,29-31,33 Furthermore, our outcomes were no different from the outcomes observed with TREO/FLU/TBI in patients receiving conventional donor HCT (no CB donors).25 Twenty-seven percent of patients in this study had MRD at the time of treatment and 26% had failed a prior transplant. The 3-year DFS of 43% and 48% in these 2 categories of patients, although not ideal, is superior to that reported in most other studies.33,34
Posttransplant toxicity was acceptable. The incidence of mucositis was similar to that reported with NMA regimens without methotrexate for GVHD prophylaxis.34 However, the incidence of hyperbilirubinemia grades III-IV was somewhat higher than the 13% reported by Casper et al in a previous pilot study where 30 patients, not eligible for standard conditioning regimens, received TREO/FLU without TBI.17 The incidence of 21% observed in the present study could be attributable to the higher dose of TREO (42 g/m2 vs 30 g/m2) or the addition of 200 cGy TBI following chemotherapy. Importantly, similar to other studies incorporating TREO, we observed only 1 case of sinusoidal obstruction syndrome that resolved with no intervention.12,17
With a combination of cyclosporine and prolonged mycophenolate mofetil administration as GVHD prophylaxis, the incidence of aGVHD was comparable to that in previous reports.24 In particular, there was no increase in the incidence of severe, grade III-IV GVHD when compared with other regimens, including those incorporating antithymocyte globulin in the conditioning regimen.16 Furthermore, the incidence of moderate or severe chronic GVHD was low with almost every patient off of immune-suppressive therapy by 2 years.
Additional clinical outcomes using this regimen have been favorable. The overall TRM in this cohort was 18%, which is similar to that observed with both high-intensity27,35,36 and reduced-intensity approaches.16,24 Kumar et al32 showed a TRM at 2 years of 19% in 121 patients of similar median age treated with NMA; however, in the same series, the 2-year cumulative incidence of relapse was 49%.32 Recently, Beelen et al reported a very low rate of TRM (10%) in patients ≥50 years who received a reduced dose of TREO of 10 g/m.2,12 In our analysis, age and prior HCTs were associated with an increase of the TRM risk. Due to these findings, it might be appropriate to consider reducing the dose of TREO from 14 g/m2 to 10 g/m2 in older and frail patients at increased mortality risk.37,38
In conclusion, the results presented from this first prospective CBT trial using a TREO-based conditioning regimen demonstrate that this approach is well tolerated and adequately immunosuppressive to promote sustained CB donor engraftment. This regimen extends donor options for patients in need of MAC HCT without conventional donors who are judged to be poor candidates for other regimens, especially those containing high-dose TBI. Notably, it would be useful to evaluate whether TBI is needed to promote engraftment and to maintain sustained remissions. Investigating whether the use of a lower dose of TREO would extend the use of CBT for patients 65 to 75 years of age would also be of great interest.
For original data, please contact fmilano@fredhutch.org.
Acknowledgments
The authors are grateful to the patients and families who consented to the use of clinical research results and biologic specimens in this trial. The authors thank Denise Ziegler, MaryJoy Lopez, and Nancy Anderson for their assistance in the preparation of this manuscript.
This work was supported by a research grant from Medac GmbH (Wedel, Germany). F.M. and L.T. were supported by the George and Fay Young Foundation.
This study is Fred Hutchinson Cancer Research Center (FHCRC) protocol 2275.
Authorship
Contribution: F.M. and C.D. participated in the study design, data analysis, and interpretation of data for the manuscript; F.M. wrote the first draft and performed the statistical analyses; and H.J.D., J.A.G., E.R.N., J.B., L.T., A.D., R.B.S., C.S., F.R.A., and C.D. provided revisions and critical review of the final manuscript.
Conflict-of-interest disclosure: J.B. is an employee of Medac GmbH, which provided support for this study. The remaining authors declare no competing financial interests.
Correspondence: Filippo Milano, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, Mailbox MDB306, Seattle, WA 98109; e-mail: fmilano@fredhutch.org.
References
Author notes
The full-text version of this article contains a data supplement.