Key Points
COVID-19 patients with blood group A or AB are at increased risk for requiring mechanical ventilation vs those with blood group O or B.
COVID-19 patients with blood group A or AB appear to exhibit a greater disease severity than patients with blood group O or B.
Abstract
Studies on severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) suggest a protective effect of anti-A antibodies against viral cell entry that may hold relevance for SARS-CoV-2 infection. Therefore, we aimed to determine whether ABO blood groups are associated with different severities of COVID-19. We conducted a multicenter retrospective analysis and nested prospective observational substudy of critically ill patients with COVID-19. We collected data pertaining to age, sex, comorbidities, dates of symptom onset, hospital admission, intensive care unit (ICU) admission, mechanical ventilation, continuous renal replacement therapy (CRRT), standard laboratory parameters, and serum inflammatory cytokines. National (N = 398 671; P = .38) and provincial (n = 62 246; P = .60) ABO blood group distributions did not differ from our cohort (n = 95). A higher proportion of COVID-19 patients with blood group A or AB required mechanical ventilation (P = .02) and CRRT (P = .004) and had a longer ICU stay (P = .03) compared with patients with blood group O or B. Blood group A or AB also had an increased probability of requiring mechanical ventilation and CRRT after adjusting for age, sex, and presence of ≥1 comorbidity. Inflammatory cytokines did not differ between patients with blood group A or AB (n = 11) vs O or B (n = 14; P > .10 for all cytokines). Collectively, our data indicate that critically ill COVID-19 patients with blood group A or AB are at increased risk for requiring mechanical ventilation, CRRT, and prolonged ICU admission compared with patients with blood group O or B. Further work is needed to understand the underlying mechanisms.
Introduction
Preliminary reports suggest a link between ABO blood groups and susceptibility to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.1-3 Specifically, individuals with blood group O have been reported to be less susceptible to SARS-CoV-2 infection. Similar associations were observed with SARS-CoV-1.4 In vitro, the anti-A antibody, found in individuals with blood group O or B, appears to antagonize the interaction between SARS-CoV-1 and the receptor for angiotensin converting enzyme 2 (ACE2), which is expressed by host target cells.5 Given that SARS-CoV-2 also binds to ACE2,6,7 it is reasonable to consider that blood groups may also be determinants of susceptibility to SARS-CoV-2 infection. Because a large proportion of individuals infected with SARS-CoV-2 remain asymptomatic, and hospital admission data are limited to patients with symptoms, population screening would be necessary to accurately assess the relationship between blood group and susceptibility to SARS-CoV-2 infection. Therefore, early preliminary data may have better reflected the relationship between ABO group and SARS-CoV-2 infection severity, and the relationship between ABO blood group and COVID-19 severity has remained unresolved, despite more recent investigations.8,9
Multiorgan tropism of SARS-CoV-2 has recently been reported.10 This is consistent with multiple reports indicating that COVID-19 is a multisystem disease that includes renal11 and hepatic12 manifestations. If ABO blood groups play a role in determining disease severity, these differences would be expected to manifest within multiple organ systems10 and hold relevance for multiple resource-intensive treatments, such as mechanical ventilation and continuous renal replacement therapy (CRRT).13 Therefore, we conducted a retrospective analysis of a multicenter case series with a nested prospective substudy of inflammatory cytokines of critically ill COVID-19 patients. In addition, we acquired nationwide population ABO blood group distribution data. Our specific aims were to determine whether ABO blood group is associated with clinical indicators of COVID-19 severity (eg, mechanical ventilation, ventilator-free days, CRRT, length of intensive care unit [ICU] stay, and mortality) and to determine whether ABO blood group is associated with differences in serum biomarkers of organ dysfunction. Further, given the role of the host immune response as a potential determinant of COVID-19 severity,11,14-21 we aimed to assess whether ABO blood type is related to the levels of serum inflammatory cytokines. We hypothesized that (1) that there would be a greater proportion of critically ill COVID-19 patients with blood group A or AB requiring mechanical ventilation and/or CRRT than those with blood group O or B, (2) clinical laboratory indices of multiorgan dysfunction would be elevated in patients with blood group A or AB compared with group O or B, and (3) serum inflammatory cytokines would be higher in critically ill COVID-19 patients with blood group A or AB compared with group O or B.
Methods
Approvals were obtained from the Fraser Health Research Ethics Board and the University of British Columbia Clinical Research Ethics Board (H20-00971; H20-01073).
Data were collected from consecutively admitted ICU patients in 6 metropolitan Vancouver hospitals between 21 February 2020 and 28 April 2020. All hospitals are affiliated with the University of British Columbia, and the associated ICUs function in a mixed medical-surgical closed ICU model with subspecialty fellowship-trained intensivists in attendance. We collected the following data: age, sex, comorbidities, dates of symptom onset, hospital admission, ICU admission, mechanical ventilation, CRRT, and laboratory parameters, including hematology, chemistry, coagulation, inflammatory markers (white cell count, lymphocyte count, platelet count, fibrin D-dimer, aspartate aminotransferase [AST], alanine aminotransferase [ALT], creatinine, bilirubin, lactate, ferritin, and C-reactive protein). Fibrin D-dimer results were reported up to a maximum of 4000 μg/L. Laboratory results, including serum inflammatory cytokines, were reported as a baseline (day 1) or peak value within the first 3 days of ICU admission (see Table 1 for specific baseline/peak values). ABO blood type was determined with a standard group and screen (NEO Blood Bank Analyzer; Immucor, Norcross, GA).
Patients and management
All ICU patients with real-time reverse transcription polymerase chain reaction confirmed SARS-CoV-2 infection22 on nasopharyngeal or tracheal samples were included. British Columbia provincial management guidelines for critically ill COVID-19 patients are in accordance with the Surviving Sepsis Campaign.23 Admission to the ICU and endotracheal intubation were at the discretion of the attending intensivist for COVID-19 patients who required mechanical ventilation once noninvasively administered oxygen requirement >6 L/min was necessary to maintain a peripheral oximetry saturation >94%. The primary sedative of use across sites is IV propofol, with benzodiazepine or narcotics as second-line infusion agents. The decision to extubate was at the discretion of the attending intensivist with considerations of level of consciousness, fraction of inspired oxygen <40%, positive end expiratory pressure ≤8 cm H2O, pressure support ≤8 cm H2O, and minimal pre-extubation tracheal secretions or moderate to strong cough. For routine thromboprophylaxis, enoxaparin (30-40 mg) was administered subcutaneously every 12 hours. CRRT was initiated as medically indicated for acute kidney injury with refractory metabolic acidemia, hyperkalemia, uremic encephalopathy, or positive fluid balance. Clinical decisions for ICU discharge were made at the discretion of the attending intensivist.
National and provincial blood group distributions
The distribution of national and provincial ABO blood groups for Canada and British Columbia, respectively, were acquired from Canadian Blood Services. These data encompass all unique Canadian and British Columbian blood donors over the period from 1 May 2019 to 30 April 2020.
Inflammatory cytokine analysis
In a subcohort of patients, which was a consecutive sample of patients enrolled at Vancouver General Hospital, we prospectively collected serum samples for research purposes. These samples were analyzed for serum concentrations of interleukin-1β (IL-1β), IL-6, IL-10, and tumor necrosis factor-α (TNF-α) using the Quanterix Single Molecule Array (Simoa) HD-1 analytical platform.24
Outcomes
Our primary outcome was the proportion of patients requiring mechanical ventilation. Our secondary outcome was the probability of requiring mechanical ventilation during hospital stay. Tertiary outcomes included other clinical indices of disease severity (ie, proportion of patients requiring CRRT, probability of requiring CRRT, ventilator-free days, ICU length of stay, probability of extubation, probability of ICU discharge, all-cause hospital mortality, and overall hospital survival), clinical laboratory serum biomarkers of multiorgan dysfunction (eg, AST, ALT, creatinine), and, within our subcohort, peak serum inflammatory cytokines. The primary outcome of mechanical ventilation was chosen because it is a clinically relevant and reproducible end point associated with disease severity and an event that would occur frequently enough to ensure reasonable power given a smaller sample size. Ventilator-free days were defined as the number of days alive and successfully weaned from mechanical ventilation within the first 28 days following ICU admission.
Statistical analyses
Descriptive statistics were used to summarize baseline demographics, clinical characteristics, and laboratory values. Continuous variables are presented as median and interquartile range (IQR), and categorical variables are presented as total number (proportions; %). Differences in the blood group distribution between Canadian data and British Columbia data and our cohort were assessed by a 2-tailed χ2 goodness-of-fit test. To assess whether ABO blood group influences clinical indices of disease severity (see "Outcomes"), patients were split into 2 groups based on previous data demonstrating that the anti-A antibody may impact SARS-CoV-2 interaction with its cell entry receptor ACE25,6 (ie, those with the anti-A antibody [groups O and B] or without the anti-A antibody [groups A and AB]).
Group differences in the proportion of patients requiring mechanical ventilation, our primary outcome, were assessed using Pearson’s χ2 test. Our secondary outcome, the probability of requiring mechanical ventilation between groups, was determined using a competing-risks Fine & Gray regression model (STATA command stcrreg), adjusting for age (years), sex (male vs female), and comorbidity status (yes/no). Comorbidity status was defined as having ≥1 of the following comorbidities: hypertension, diabetes, dyslipidemia, asthma, chronic kidney disease, cardiovascular disease, chronic obstructive pulmonary disease, or being a current or former tobacco smoker. Death was specified as a competing risk. A subdistribution hazard ratio (sHR) and corresponding 95% confidence intervals (CIs) are presented. Differences in tertiary outcomes between groups were assessed using Pearson’s χ2 test for dichotomous or categorical variables (eg, proportion requiring CRRT, 28-day all-cause hospital mortality), or a Mann-Whitney U test for continuous variables (eg, ICU length of stay) where appropriate. Probability of requiring CRRT during hospital stay, probability of extubation, and probability of ICU discharge were assessed with competing-risks Fine & Gray regression models adjusting for age, sex, and comorbidity status and displayed as sHR (95% CI), in accordance with the methods described above. Overall survival was assessed using a Cox-proportional hazards regression model (STATA command stcox), adjusting for age, sex, and comorbidity status (yes/no). This was presented as a hazard ratio (HR) with 95% CIs. A Wald test of significance for differences between groups was used for all competing risk and Cox-proportional hazard regression models. Within our subcohort, differences in serum inflammatory biomarkers between groups was assessed using a Mann-Whitney U test.
All statistical tests were 2-tailed, and we considered P < .05 to be statistically significant. No imputation was made for missing data. All analyses were performed using STATA 15.2 (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC).
Results
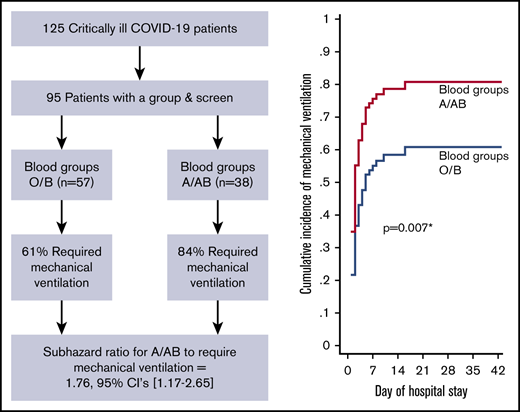
A total of 125 critically ill COVID-19 patients were admitted to the ICU from 1 March 2020 to 28 April 2020. Of these 125 patients, 95 had ABO blood group data available from an ICU admission group and screen and were included in the analyses. Baseline characteristics and outcomes from 117/125 patients (87/95 with blood group data) have been summarized previously25 ; however, this investigation addressed novel hypotheses that did not share any overlap with previous reporting.
Demographics and clinical laboratory results are presented in Table 1. There were no differences in age or sex between groups or overall comorbidities. ABO blood group data from all unique blood donors nationally (N = 398 671) and provincially (n = 62 246) from 1 May 2019 to 30 April 2020 were acquired from Canadian Blood Services. Our ICU cohort’s blood group distribution was not different from the national blood group distribution (P = .38) or the provincial blood group distribution (P = .60; Table 2); this reflects the regional demographics from which our patient population is drawn. Indeed, ∼85% of provincial patients with COVID-19 were admitted to 1 of the 6 hospitals included in this investigation.25
Clinical indicators of disease severity
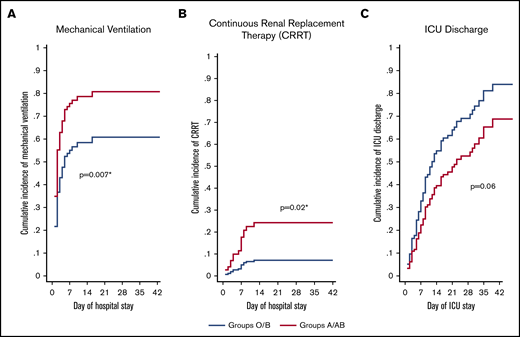
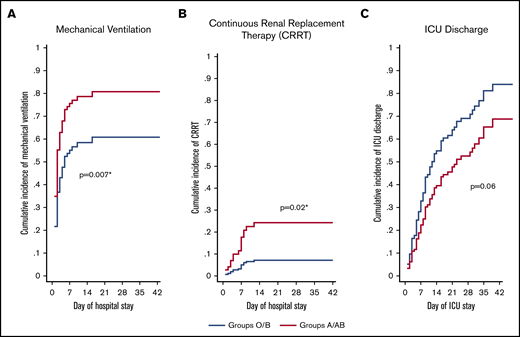
Of the 95 ICU patients included in the analyses, 57 were blood group O or B, and 38 were blood group A or AB. A greater proportion of A or AB patients (32; 84%) required mechanical ventilation compared with O or B patients (35; 61%; P = .02) and had a greater probability of requiring mechanical ventilation after adjusting for sex, age, comorbidity status (yes/no), and treating death as a competing risk (adjusted sHR, 1.76; 95% CI, 1.17-2.65; P = .007; Figure 1A;Table 3). A greater proportion of A or AB patients (12; 32%) required CRRT compared with O or B patients (5; 9%; P = .004) and had a greater probability of requiring CRRT after adjusting for age, sex, comorbidity status (yes/no), and treating death as a competing risk (adjusted sHR, 3.75; 95% CI, 1.28-10.9; P = .02; Figure 1B; Table 3). Median ICU length of stay was longer in A or AB patients (13.5 days; IQR, 7-26) than in O or B patients (9 days; IQR, 5-18; P = .03), but there were no differences in the probability of ICU discharge after adjustment for age, sex, comorbidity status (yes/no), and death as a competing risk (adjusted sHR, 0.63; 95% CI, 0.39-1.03; P = .06; Figure 1C; Table 3). The median number of ventilator-free days was not different between A or AB patients (7 days; IQR, 0-19) and O or B patients (13 days; IQR, 0-21; P = .50). Further, there were no differences in the probability of extubation between groups after adjustment for age, sex, comorbidity status (yes/no), and death as a competing risk (adjusted sHR, 0.92; 95% CI, 0.52-1.62; P = .78; Table 3). Overall hospital length of stay did not differ between groups (P = .13; Table 3). A total of 67 (71%) patients had been discharged from the ICU at the time of study completion, with no statistically significant differences between the 2 groups (43 [75%] of O or B vs 24 [63%] of A or AB; P = .20; Table 3). For discharged patients, ICU length of stay was longer in A or AB patients (P = .03), whereas there was no difference in hospital length of stay (P = .08; Table 3). There was no difference in the proportion of O or B (8; 14%) and A or AB (9; 24%) patients who died during their hospital stay (P = .23).
Adjusted cumulative incidence plots for mechanical ventilation, CRRT, and ICU discharge. Adjusted cumulative incidence of requiring mechanical ventilation (A) or CRRT (B) during hospital stay by group. (C) Cumulative incidence of being discharged from the ICU by group. Cumulative incidence models were created with death as a competing risk and are adjusted for age, sex, and the presence of ≥1 comorbidity status (binary, yes/no). sHR ratio >1 indicates an increased probability of an event occurring during the study period, whereas a ratio <1 indicates a decreased probability. *Statistically significant P value for a difference between groups.
Adjusted cumulative incidence plots for mechanical ventilation, CRRT, and ICU discharge. Adjusted cumulative incidence of requiring mechanical ventilation (A) or CRRT (B) during hospital stay by group. (C) Cumulative incidence of being discharged from the ICU by group. Cumulative incidence models were created with death as a competing risk and are adjusted for age, sex, and the presence of ≥1 comorbidity status (binary, yes/no). sHR ratio >1 indicates an increased probability of an event occurring during the study period, whereas a ratio <1 indicates a decreased probability. *Statistically significant P value for a difference between groups.
Clinical laboratory results
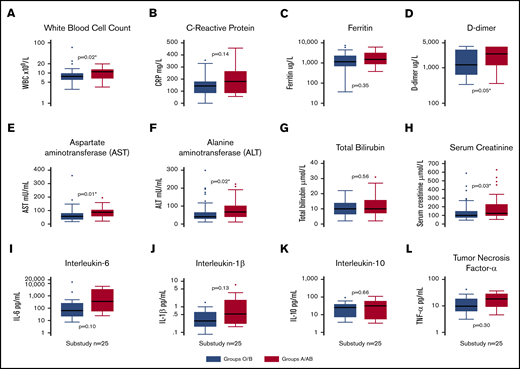
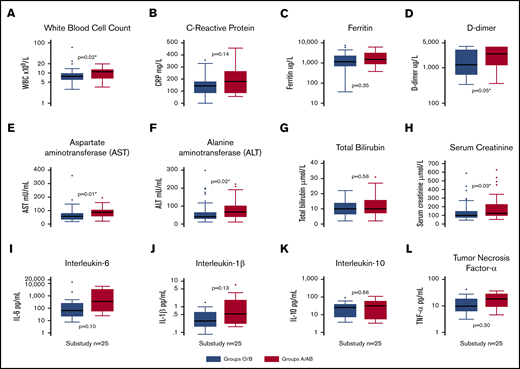
Clinical laboratory results for both groups are shown in Table 1. For all 95 ICU patients, ICU admission white blood cell count (P = .02; Figure 2A), highest recorded value for fibrin D-dimer (P = .05; Figure 2D), AST (P = .02; Figure 2E), ALT (P = .01; Figure 2F), and highest recorded value for serum creatinine (P = .03; Figure 2H) were lower in O or B patients than in A or AB patients.
Box plots of clinical laboratory results and serum inflammatory biomarkers. White blood cell count (A), C-reactive protein (B), ferritin (C), and fibrin D-dimers (D) in the entire cohort (n = 95), split into blood groups O/B and A/AB. Markers of liver and kidney dysfunction, including AST (E), ALT (F), bilirubin (G), and peak creatinine (H) in the entire cohort (n = 95), split into blood groups O/B and A/AB. Inflammatory biomarker levels, including IL-6 (I), IL-10 (J), IL-1β (K), and TNF-α (L), in a substudy (n = 25) of patients. *Statistically significant P value for a difference between groups.
Box plots of clinical laboratory results and serum inflammatory biomarkers. White blood cell count (A), C-reactive protein (B), ferritin (C), and fibrin D-dimers (D) in the entire cohort (n = 95), split into blood groups O/B and A/AB. Markers of liver and kidney dysfunction, including AST (E), ALT (F), bilirubin (G), and peak creatinine (H) in the entire cohort (n = 95), split into blood groups O/B and A/AB. Inflammatory biomarker levels, including IL-6 (I), IL-10 (J), IL-1β (K), and TNF-α (L), in a substudy (n = 25) of patients. *Statistically significant P value for a difference between groups.
Serum inflammatory cytokines
There were no differences between blood group O or B compared with A or AB for peak IL-1β (P = .13), IL-6 (P = .10), IL-10 (P = .66), or TNF-α (P = .30) (Figure 2I-L).
Discussion
We present a comprehensive assessment of the effect of ABO blood group on clinical indices of COVID-19 severity in critically ill patients. Although the distribution of ABO blood groups in our sample of ICU patients did not differ from the national and provincial ABO blood group distribution, a greater proportion of blood group A or AB patients required mechanical ventilation and CRRT compared with blood group O or B patients. Similarly, biomarkers of renal and hepatic dysfunction were higher in blood group A or AB patients. In our subcohort there were no differences in serum inflammatory cytokines. Collectively, our data indicate that critically ill COVID-19 patients with blood group A or AB are associated with an increased risk for requiring mechanical ventilation, CRRT, and prolonged ICU length of stay compared with patients with blood groups O or B.
As of 28 July 2020, >16 000 000 people worldwide have been infected with SARS-CoV-2, resulting in >650 000 deaths.26 Yet, apart from age and sex,13,27 and more recently blood group,9 there is a striking lack of knowledge of the clinical or demographic risk factors that influence susceptibility to SARS-CoV-2 infection and the severity of subsequent COVID-19. Recently, Li and colleagues observed that blood groups A and O are present at higher and lower proportions, respectively, in hospital-admitted patients with COVID-19 (n = 265) compared with the general population.3 This finding is consistent with SARS-CoV-1 data.4 The similarities between the SARS-CoV-1 and SARS-CoV-2 receptor binding domains,6 coupled with the observation that anti-A antibody inhibits the interaction between SARS-CoV-1 and the ACE2 receptor,5 suggest that ABO blood groups could influence SARS-CoV-2 infection and resultant COVID-19 severity.1-3 However, the relationship between ABO blood group and COVID-19 severity may be more complicated and involve such factors as the specific anti-A titers,28 the immunoglobulin isotype of anti-A antibodies,29 and ABO group differences in von Willebrand factor.30
Importantly, the proportion of asymptomatic infection ranges from 10% to 40%,31-33 not including patients with mild symptoms who do not require hospitalization. Further, variability in international regulatory guidance as to when patients should self-isolate or seek medical attention will influence hospital admission as an outcome variable to determine SARS-CoV-2 susceptibility. Our data in ICU-admitted patients did not reveal a difference in ABO blood group distribution compared with the normative national or provincial blood group distribution (Table 2). For the reasons explained above, we are unable to conclude that there is no relationship between ABO blood group and susceptibility to SARS-CoV-2 infection; rather our findings are an incentive for further research to assess this potential relationship using study designs (eg, population screening32 ) that are more inclusive of the full range of disease severity.
Our study demonstrates a link between ABO blood groups and mechanical ventilation, as well as the requirement for CRRT and ICU length of stay (Figure 1), reproducible outcome indices of the severity of COVID-19 across countries and jurisdictions. These factors are also directly relevant for risk stratification. Further, we demonstrated higher levels of AST, ALT, and peak serum creatinine in patients with blood group A or AB, which may indicate a multiorgan protective effect conferred by the anti-A antibody or other factors related to blood type (eg, von Willebrand factor); these data align with the multiorgan involvement of SARS-CoV-2 infection.10 Interestingly, we found a significant, albeit modest, elevation in fibrin D-dimers in patients with blood group A or AB compared with O or B. Because pulmonary vasculopathy and coagulopathy are increasingly recognized clinical pathophysiologic sequelae of COVID-19, this may hold relevance to manifestations of respiratory failure and morbidity,34 independent of the influence of the anti-A antibody on viral cell entry. Central to this observation, patients with blood group O have reduced levels of factor VIII and von Willebrand factor,35 which may account for an underlying protective effect against the development of vasculopathy within the pulmonary vasculature36 and other vital organ vascular beds.30 Indeed, ABO is a “histo-blood group,” and the associated antigens are present on many cell types, including endothelial cells and platelets, which relates to the overall function of these cell types.37 Although speculative, this mechanism requires further detailed prospective evaluation in COVID-19 patients.
Our study’s findings are congruent with a recent study of 1980 patients that demonstrated a link between ABO blood type and disease severity,9 although retrospective data have emerged to suggest otherwise.8 However, when comparing our study to that of Latz and colleagues,8 it is important to consider differences in mortality that may confound comparison between our cohorts. Indeed, our jurisdictional mortality rate for ICU-admitted patients was ∼15% (18% in the 95 patients assessed herein),25 whereas Latz et al had 123 patients admitted to the ICU and reported 89 deaths in the overall study cohort (if all deaths are attributable to ICU patients, this is a mortality rate of 72%).8 In light of these vastly different mortality rates, there may be valid concerns regarding the comparability of previous cohorts and the similarity of the underlying populations from which they came. It is unknown whether these differences are due to disease-specific factors, system-level public health factors, or a combination of both.
Although the host immune response to SARS-CoV-2 infection appears to be related to COVID-19 severity,11,14-21 we did not demonstrate group differences in serum inflammatory cytokines within our subcohort. Biologically, this may reflect a lack of blood group effect, because the juncture at which blood group and disease severity intersect is putatively at the level of cell entry.5 Whether immunological factors subsequent to SARS-CoV-2 infection differ by blood group remains unknown. Conversely, our negative finding may be a consequence of inadequate statistical power. This lack of statistical difference notwithstanding, the sixfold difference in IL-6 group medians between the A or AB and the O or B blood groups suggests that it is too early to disregard the host immune response as a factor that may differentially impact COVID-19 severity between ABO blood groups.
Our study has several limitations that warrant consideration. First, it is a retrospective analysis of observational data, precluding the ability to infer causality. Second, ∼25% of ICU-admitted patients did not have ABO blood group data from an admission group and screen. An important and unaddressed confounder in our study is the relationship between ethnic ancestry and outcomes in patients with COVID-19.38 Our analysis included 85% of all COVID-19 patients admitted to the ICU in our province during the study period, lending strength to our comparisons of blood type distributions with the larger provincial population (sample population). However, we acknowledge that our disease-severity comparisons made between A or AB and O or B patients in the ICU may be confounded by ethnic ancestry. Our subcohort consisted of a small sample; therefore, the resultant lack of statistical power precludes strong inferences related to serum inflammatory cytokines. These data should not be taken as conclusive; rather, they should be considered in future hypothesis generation. It is also important to consider that the titer of anti-A antibodies may affect COVID-19 severity. Therefore, another limitation of our study is that anti-A levels were not analyzed, but inferred, from patient blood group data. Future research will aim to address these limitations and unresolved questions.
In conclusion, we demonstrate that critically ill COVID-19 patients with blood group A or AB are associated with an increased risk for requiring mechanical ventilation, CRRT, and prolonged ICU length of stay compared with patients with blood groups O or B. Further research is required to delineate the biological mechanisms underpinning these findings.
A deidentified data set is held on a secure server at Vancouver Coastal Health. The data-sharing agreement prohibits the data from being publicly available. Data requests may be granted provided there is an appropriate data-sharing agreement that would encompass Vancouver Coastal Health and Fraser Health authorities.
Acknowledgments
This work was supported by the Vancouver General Hospital Foundation. M.S.S. is supported by the Vancouver Coastal Health Research Institute Clinician Scientist Award. D.E.G.G. is supported by a Michael Smith Foundation for Health Research Health Professional Investigator Award. D.V.D. is supported by a Burroughs Wellcome Fund Innovation in Regulatory Science Award. E.M.C. is supported by a Canada Research Chair and grants from the Canadian Institutes for Health Research and the Heart and Stroke Foundation of Canada. C.L.W. is supported by grants from the Canadian Institutes for Health Research, National Institutes of Health, Weston Brain Institute, and Cure Alzheimer Fund.
Authorship
Contribution: R.L.H., N.A.F., D.E.G.G., C.L.W., and M.S.S. designed the research; R.L.H., N.A.F., and M.S.S. wrote the manuscript; A.R.M., D.E.G.G., D.V.D., S.S., J.C., S.T., D.F., L.Y.C.C., A.Y.Y.L., E.M.C., and C.L.W. revised the manuscript for critical intellectual content; and all authors performed research and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mypinder S. Sekhon, Division of Critical Care Medicine, Department of Medicine, Faculty of Medicine, University of British Columbia, Room 2438, Jim Pattison Pavilion, 2nd Floor, 855 West 12th Ave, Vancouver V5Z 1M9, Canada; e-mail: mypindersekhon@gmail.com.
References
Author notes
R.L.H. and N.A.F. are joint first authors.
C.L.W. and M.S.S. are joint senior authors.