Key Points
More than one-half of patients (55%) with AL amyloidosis in hematologic complete response were MRD positive.
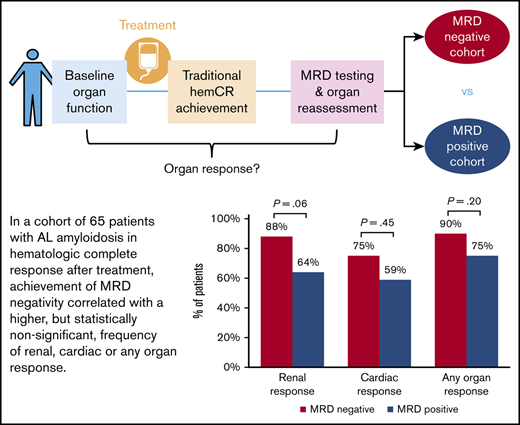
Achievement of MRD negativity correlated with a higher but statistically nonsignificant frequency of renal, cardiac, or any organ response.
Abstract
Despite achieving a hematologic complete response after treatment, many patients with AL amyloidosis do not attain recovery of organ function and/or experience hematologic relapse. A persistent plasma cell clone producing amyloidogenic light chains at levels below the detection threshold of traditional serologic methods is hypothesized to impede organ response in some patients. Assessment of minimal residual disease (MRD) may therefore have clinical importance as a more stringent treatment response tool for patients in a hematologic complete response. We used 2-tube, 10-color combination multiparametric flow cytometry to assess for MRD at a minimum sensitivity of 1 in 105 nucleated cells. Of 65 patients in hematologic complete response, 36 (55%) were found to have a residual clonal plasma cell population in the bone marrow. Comparing the MRD-negative and MRD-positive groups, renal response was observed in 88% vs 64% (P = .06), cardiac response in 75% vs 59% (P = .45), and any organ response in 90% vs 75% (P = .20) of patients. Depth of organ response as measured by the percent decrease in 24-hour proteinuria and brain natriuretic peptide was 96% vs 91% (P = .16) and 55% vs 46% (P = .66), respectively. These data suggest a possible correlation between MRD negativity and higher probability of organ response after treatment in AL amyloidosis. Future prospective studies with a larger cohort are needed to determine the clinical relevance of these improvements. This trial was registered at www.clinicaltrials.gov as #NCT00898235.
Introduction
Depth of hematologic response after treatment of AL amyloidosis predicts organ response and survival.1,2 Traditionally, a hematologic complete response (hemCR) is defined as monoclonal protein disappearance by serum/urine immunofixation electrophoresis (IFE) and serum free immunoglobulin light chain ratio normalization. Despite hemCR achievement, ∼20% of patients do not attain organ response, and 25% of those with cardiac progression are in hemCR.2,3 In addition, nearly one-third experience relapse of the underlying plasma cell (PC) dyscrasia.2,4 Minimal residual disease (MRD) assessment to define a deeper treatment response category than conventional hemCR is therefore of interest in AL amyloidosis.
In multiple myeloma, MRD detection using multiparametric flow cytometry (MFC) has prognostic value, with MRD negativity at a minimum sensitivity of 1 × 10−5 conferring superior progression-free and overall survival.5 Whether achievement of MRD negativity offers similar advantages in other PC dyscrasias remains uncertain. In AL amyloidosis, an MFC method using surface markers adopted from multiple myeloma was shown to detect aberrant PCs at the time of diagnosis with high sensitivity and the presence of <0.1% monotypic PCs by MFC after initial treatment to predict longer survival.6,7 Subsequent studies indicated longer progression-free survival and possibly improved organ response with MRD negativity.8-10 With small sample sizes in these previous investigations, we sought to elucidate the clinical relevance of MFC-based MRD assessment in patients with hemCR. Because organ dysfunction affects symptom burden, morbidity, and overall survival in AL amyloidosis, the association of MRD status with organ response was evaluated.
Methods
Fresh bone marrow aspirate samples from consented patients with AL amyloidosis were analyzed between February and November 2019 at the Amyloidosis Center at Boston University School of Medicine and Boston Medical Center. Patients were selected according to hemCR achievement on previous evaluation. Those with multiple myeloma–associated AL amyloidosis were excluded. A cross-validated, 2-tube, 10-color antibody panel was used to identify aberrant PCs by MFC (surface tube: anti-CD45/CD38/CD138/CD229/CD20/CD19/CD56/CD81/CD27/CD117; cytoplasmic tube: anti-CD45/CD38/CD138/CD229/CD19/CD56/CD20/κ/λ). Specimen processing was performed at PhenoPath Laboratories with a target of 2 million live cellular events acquired on a Beckman Coulter Gallios flow cytometer. Raw data were analyzed by using Kaluza software at Boston Medical Center by a board-certified hematopathologist (E.J.B.and J.C.L.).
MRD positivity was defined as a phenotypically abnormal PC population comprising ≥20 events with ≥2 aberrancies (minimum sensitivity, 1 in 105 nucleated cells). Hematologic and organ responses were classified according to consensus guidelines,1,11 along with brain natriuretic peptide–based cardiac staging and response criteria.12,13 Statistical differences between groups were estimated by using the Fisher's exact test and the Mann-Whitney U test (P < .05 considered significant).
Results
A total of 86 patients with AL amyloidosis with hemCR achievement on previous evaluation were tested for MRD; 19 of these patients were excluded from analysis due to detectable monoclonal protein by IFE at the time of MRD assessment. Another 2 patients were excluded because MFC showed aberrant PCs with cytoplasmic κ/λ incongruence from the original clone (uncertain whether from clonal evolution or a new clonal population). Of the remaining 65 patients in hemCR, 29 (45%) were MRD negative, and 36 (55%) were MRD positive.
Baseline characteristics of both groups are compared in Table 1. Notably, the median difference between involved and uninvolved serum free immunoglobulin light chain (dFLC) at diagnosis was significantly higher in the MRD-negative cohort (129 vs 70 mg/L; P = .02). Final treatments resulting in hemCR were similar in both groups. Two or more lines of therapy were required for 24 (37%) patients to achieve hemCR. Three patients were still receiving daratumumab at the time of hemCR achievement and MRD assessment.
Among patients with hemCR and MRD positivity, a median of 370 (range, 23-10 386) monotypic PCs were detected, corresponding to 0.03% (range, 0.002%-0.56%) PCs. The detection level of MRD positivity was 10−3 in 7 (19%) patients, 10−4 in 20 (56%) patients, and 10−5 in 9 (25%) patients. The most common phenotypic aberrancies were CD19 loss and CD56 expression, followed by CD20 expression, CD27 loss, and CD117 expression.
Organ response
Organ responses were evaluated at the time of MRD assessment (Figure 1). Comparing the MRD-negative and MRD-positive cohorts, renal response was attained in 21 (86%) of 24 patients vs 18 (64%) of 28 patients with kidney involvement (P = .06), respectively. Median percent change in 24-hour proteinuria level from baseline was −96% (range, −34% to −100%) vs −91% (range, −13% to −100%) (P = .16). Cardiac response was attained in 9 (75%) of 12 patients vs 10 (59%) of 17 patients with heart involvement (P = .45). Median percent change in brain natriuretic peptide was −55% (range, −92% to 391%) vs −46% (range, −98% to 289%) (P = .66). All patients with liver involvement exhibited hepatic response. Any organ response (kidney, heart, or liver) was seen in 26 (90%) patients with MRD negativity and 27 (75%) with MRD positivity (P = .20). These data correspond to MRD positivity detected in 10 (77%) of 13 patients without renal response, 7 (70%) of 10 patients without cardiac response, and 9 (75%) of 12 patients without any organ response.
Frequency of organ response at time of MRD assessment among patients in hemCR.
Timing of MRD assessment
MRD testing was performed at different points in time after hemCR achievement. The median duration from hemCR achievement to MRD assessment was longer in the MRD-negative cohort at 71 vs 32 months, although not statistically different (P = .27). Subgroup analysis according to more uniform time points of MRD testing (Table 2) indicated no organ response advantage of MRD negativity, except for early (≤12 months) renal response attained by 5 (100%) patients vs 1 (25%) patient (P = .04). Of 27 patients who underwent MRD testing ≥5 years after treatment completion, 11 (41%) were MRD positive. Despite MRD positivity, all patients with renal involvement (n = 8) exhibited durable renal response, and one-half with cardiac involvement (n = 6) exhibited durable cardiac response. However, 2 had progression of cardiac disease. Interestingly, 1 patient with MRD positivity demonstrated durable hemCR and renal response 21 years after last treatment.
Translocation t(11;14)
In AL amyloidosis, t(11;14) is implicated as an adverse prognostic factor with inferior benefit from bortezomib-based treatments.14,15 Cytogenetic data were readily available for 36 patients, of whom 14 (39%) had presence of t(11;14). The incidence of t(11;14) positivity was similar for both groups: 40% in the MRD-negative cohort vs 38% in the MRD-positive cohort (P = 1.0).
Treatments
Final treatment achieving hemCR was high-dose melphalan and stem cell transplantation (HDM/SCT) for 32 patients, bortezomib-based for 16 patients, and an anti-CD38 monoclonal antibody for 14 patients. The incidence of MRD negativity in our selected hemCR group was 43% for HDM/SCT, 38% for bortezomib-based regimens, and 57% for anti-CD38 monoclonal antibody therapy. Comparisons between treatments cannot be made, however, due to considerable differences in time intervals of MRD testing from last treatment: 78 months (range, 5-260 months) for HDM/SCT, 40 months (range, 3-91 months) for bortezomib-based therapy, and <1 month (range, 0-2 months) for anti-CD38 monoclonal antibody therapy.
Role of MFC testing in hematologic very good partial response
Although MRD negativity by definition requires hemCR achievement according to the consensus criteria for multiple myeloma,5 MFC testing may also be relevant for patients who achieve a hematologic very good partial response (hemVGPR) after treatment. Of the 19 patients in our AL amyloidosis cohort who underwent MFC testing but were excluded from MRD analysis due to emergence of a detectable monoclonal protein by IFE, 100% were MFC positive, including 2 patients at a low level of detection of 10−5 (others detected at 10−4 or higher). Similarly, 2 other reports described MFC positivity in all patients with detectable monoclonal protein according to standard hematologic measures.9,10 Only one study using a minimum sensitivity of 1 × 10−4 reported MFC negativity among 16 of 38 patients meeting criteria for hemVGPR.8 The significance of MFC testing for patients with AL amyloidosis in hemVGPR remains to be established.
Discussion
The clinical relevance of flow cytometry–based MRD assessment and its predictive value on organ response in AL amyloidosis is of growing interest.6-10 Organ response is a crucial outcome for patients with this disease, yet the precise mechanisms of organ dysfunction and how hematologic response following treatment affects organ recovery are not well understood. Generally, a deep hematologic response (hemVGPR or better) is required for organ response, but even those who achieve hemCR can endure persistent organ dysfunction. It is believed that incomplete elimination of clonal PCs after treatment may hinder organ recovery.1 In this study, our goal was to determine whether achievement of MRD negativity by MFC provides an added organ response advantage for patients with AL amyloidosis. We adopted the minimum sensitivity threshold of 1 × 10−5 established for multiple myeloma.5
Among patients with AL amyloidosis in a conventional hemCR, 55% were found to be MRD positive according to MFC. Intriguingly, a substantial percentage (41%) of patients tested between 5 and 21 years since last treatment were MRD positive despite having a durable hemCR according to standard serologic measures and durable organ responses. The immunologic mechanisms in the bone marrow microenvironment keeping these residual aberrant PCs under control are not yet understood. Overall, the majority of patients in hemCR exhibited an organ response, regardless of MRD status. Although MRD negativity correlated towards a higher frequency of renal (24% more patients), cardiac (16% more patients), and any organ (15% more patients) response, statistical significance was not met. Furthermore, MRD negativity did not confer significantly deeper organ response according to percent improvement in biomarkers.
Although this study is one of the largest to date of MRD assessment in patients with AL amyloidosis in hemCR, small cohort size, referral center bias, and heterogeneity of patients are significant limitations. Results may be confounded by MRD testing occurring at a later time point after hemCR achievement in the MRD-negative cohort by ∼39 months (median, 72 vs 32 months), thereby allowing further time for organ recovery. This time difference between groups may especially affect data on renal response because previous studies of organ response kinetics indicate that kidney function can improve over long periods of time.3 Our subgroup analysis accounting for timing of MRD testing, however, showed an added advantage of MRD negativity for early renal response (within 12 months of hemCR achievement). In addition, the short time interval between hemCR achievement and MRD testing may not allow enough time to capture organ response for some patients in this study.
Notably, 25% of patients with MRD positivity in our cohort were detected at a level of 10−5 (between our sensitivity threshold of 1 × 10−5 and 1 × 10−4) and thus could have readily been misclassified as MRD negative had a less sensitive MFC technique been used. We cannot be certain what percentage of our MRD-negative cohort are false-negative findings due to the technical variability inherent in flow-based MRD assessment. To account for this, perhaps a higher sensitivity level for MRD detection (eg, 1 × 10−6) is important in AL amyloidosis, especially considering that the burden of the underlying PC dyscrasia is often low to begin with. Another potential confounder in our study may be the higher dFLC at diagnosis in the MRD-negative cohort, provided that higher dFLC was previously shown to predict increased likelihood of organ response.3
Due to the nonprospective nature of this study, duration of organ response after MRD assessment, as well as progression-free and overall survival, were not evaluated. Future directions include assessing the durability of clonal control and sustainability of MRD negativity. Given that the majority of patients (70%-77%) in our study who did not attain organ response were MRD positive, another question of interest is whether further treatment to achieve MRD negativity could enhance organ recovery in these patients.
In conclusion, MRD negativity as a deeper treatment response classification was correlated with higher organ response in patients with AL amyloidosis in hemCR, although differences were not statistically significant. A larger cohort and prospective study would be required to formally test whether the 15% to 24% improvements in organ responses found in this study are statistically and clinically relevant as characterized by duration of organ response and rate of organ progression. Obtaining MRD negativity may perhaps matter for a subset of individuals who have highly toxic amyloidogenic light chains even at very low concentrations, but these patients are not easily discernible in the current era.
Acknowledgment
The Amyloidosis Center database and repository is supported by the Amyloid Research Fund of Boston University School of Medicine.
Authorship
Contribution: A.S. collected and analyzed data, and wrote the manuscript in consultation with S.S. and J.M.S.; E.J.B. and J.C.L. interpreted the flow cytometry data; and V.S. conducted research and revised the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Vaishali Sanchorawala, Amyloidosis Center, 72 East Concord St, K-503, Boston, MA 02118; e-mail: vaishali.sanchorawala@bmc.org.