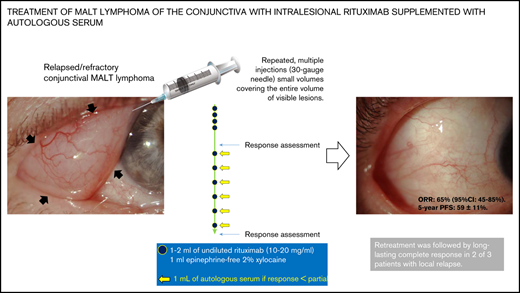
Key Points
Intralesional rituximab supplemented with autologous serum is a safe and active therapy for patients with relapsed conjunctival lymphoma.
Greater availability of effectors resulting from the addition of autologous serum may improve antitumor activity of intralesional rituximab.
Abstract
Patients with indolent conjunctival lymphomas exhibit good prognosis, with exceptional cases of dissemination, and are suitable candidates for intralesional therapies. We report the first prospective phase 2 trial using intralesional rituximab supplemented with autologous serum in adults with relapsed/refractory indolent CD20+ lymphoma of the conjunctiva (NCT01514344). Patients received 4 weekly intralesional injections of rituximab, followed by 6 monthly injections; 500 μL of autologous serum was added to rituximab in patients with lymphoma unresponsive to weekly doses. Safety, activity, and antitumor effect of autologous serum were investigated. Twenty patients with mucosa-associated lymphoid tissue (MALT)–type lymphoma were enrolled. Tolerability was excellent, with only 3 mild local reactions. After weekly injections, 11 patients achieved tumor regression, 8 had stable disease, and 1 experienced progressive disease; 9 patients received autologous serum, with response improvement in 4 cases (3 complete responses, 1 partial response). At the end of treatment, 12 patients achieved a complete remission, and 1 achieved a partial response, with an overall response rate of 65% (95% confidence interval, 45-85). At a median follow-up of 42 months (range, 10-78), 12 patients remain relapse free, with 5-year progression-free survival and time-to-next-treatment rates of 59% ± 11% and 69% ± 11%, respectively. Three patients with local relapse were retreated with intralesional rituximab and serum; 2 achieved a complete response that lasted 25+ and 38+ months. Thus, intralesional rituximab is a safe and active therapy in patients with relapsed conjunctival MALT lymphoma. The addition of autologous serum improves response in some cases. Retreatment of local relapses can result in a second durable remission.
Introduction
Ocular adnexal lymphoma (OAL) represents 5% to 15% of all extranodal non-Hodgkin lymphomas and 55% of all orbital malignancies.1 In the last decades, the incidence of OAL has increased, with an annual rate of 6%.2 Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT) is the most common tumor diagnosed in these organs, whereas a few cases are classified as follicular, diffuse large B-cell, or mantle cell lymphoma.3-6 One third of OALs primarily arise in the conjunctiva, a feature usually associated with a better prognosis in comparison with intraorbital lymphomas.5 Conjunctival lymphoma usually presents a “salmon red patch” appearance with swollen conjunctiva and patients complaining of watery eyes and discomfort. Bilateral involvement is reported in 10% to 15% of patients. Tumor growth is usually slow and is often characterized by high sensitivity to conventional therapies. However, local and, more frequently, contralateral relapses are recorded, with systemic dissemination occurring in <5% of cases.5 Conventional treatments for conjunctival lymphomas include radiotherapy, surgery, and mono-chemotherapy or poly-chemotherapy.7 In the last years, high efficacy rates were reported with different strategies, such as antibiotic therapy,8,9 topical chemotherapy,10 intralesional interferon,11,12 and IV rituximab.13 The last is a recombinant chimeric monoclonal anti-CD20 antibody, with antitumor activity related to antibody-dependent and complement-mediated cellular cytotoxicity,14,15 that is used as first-line and salvage therapies in a variety of non-Hodgkin B-cell lymphomas. Despite the high rates of expression of CD20 in marginal zone lymphomas, IV rituximab at conventional doses is associated with modest and transient benefits in patients with OAL,13,16 probably as a result of the poor bioavailability of the antibody and its effectors in the tumor microenvironment. The indolent course, exceptional cases of systemic dissemination, and good prognosis for conjunctival lymphoma point toward a less invasive and less toxic treatment. To this aim, local therapy with topical drugs and intralesional injections of interferon or rituximab have been reported, but data are limited to small and heterogeneous case series, and prospective trials are not available.
Intralesional injections of rituximab have been used in patients with indolent B-cell lymphoma of the skin, achieving lasting remissions with good tolerability.17,18 A pilot experience of 3 patients with relapsed conjunctival B-cell lymphoma treated with intralesional rituximab suggests that this approach is well tolerated and associated with long-lasting responses, even in patients who exhibit a prior failure after therapy with IV rituximab.19 Interestingly, 1 of the patients had concomitant infections with hepatitis B virus (HBV) and hepatitis C virus (HCV) and received intralesional rituximab without complications,19 which is an important issue considering that these infections are present in one third of OAL patients.20 Another of the 3 treated patients did not respond to intraconjunctival rituximab but achieved a durable complete response after injection of rituximab supplemented with autologous serum.19 The direct injection of rituximab and autologous serum within the tumor tissue could improve the above-mentioned poor bioavailability of the antibody and its effectors (ie, C3 and C4 proteins) in the tumor microenvironment and is in line with in vitro studies showing that rituximab lyses CD20+ lymphoma cell lines more effectively when it is supplemented with 10% serum.21
On this background, we designed a phase 2 study to assess the safety and activity of intralesional rituximab in patients with relapsed/refractory indolent CD20+ conjunctival lymphoma, with the addition of autologous serum in cases not responsive to the monoclonal antibody alone. Our results demonstrate that this approach is a simple, inexpensive, safe, and active treatment for indolent conjunctival lymphomas.
Patients and methods
Study design and population
The IRIS study is an open-label monocentric phase 2 trial focused on the tolerability and activity of intralesional rituximab supplemented with autologous serum in patients with relapsed or refractory CD20+ indolent lymphomas primarily arising in the conjunctiva (ClinicalTrials.gov, number NCT01514344). Inclusion criteria included age ≥18 years; HIV negativity; centrally reviewed histopathological diagnosis of CD20+ indolent lymphoma (marginal zone lymphoma, follicular lymphoma, small lymphocytic lymphoma, lymphoplasmacytic lymphoma); stage IEA disease limited to the conjunctiva (monolateral or bilateral disease); ≥1 measurable/parametrable lesion; and ≥1 previous line of treatment (patient refractory or relapsed after doxycycline was allowed; previous IV therapy was considered a prior line if ≥3 doses were administered). Patients with systemic symptoms (B symptoms) in the last 6 months or with postsurgical abnormalities that could hamper drug delivery and/or response assessment were excluded.
Staging work-up included physical examination, ophthalmological examination, assessment of seropositivity for HIV, HBV and HCV, assessment of Helicobacter pylori infection by breath test and antigen research in the feces, assessment of Chlamydia psittaci infection by polymerase chain reaction in peripheral blood mononuclear cells and conjunctival swabs,22 gadolinium-enhanced magnetic resonance imaging of the orbits, neck ultrasonography, total-body contrast-enhanced computed tomography, and bone marrow biopsy.
Written informed consent was obtained from each patient once eligibility was confirmed and after the patient received a complete explanation of protocol contents; in particular, treatment modalities, acute and late side effects, efficacy perspectives, and patient’s and physicians’ roles and responsibilities were discussed in detail before the patient signed any forms. This trial conformed to the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board of the San Raffaele Scientific Institute.
Experimental treatment
After trial registration, patients were treated with 4 weekly injections, followed by 6 monthly injections, of intralesional (ie, intraconjunctival) rituximab. Experienced ophthalmologists delivered treatment, performed physical examinations, visual acuity assessment, and slit-lamp and fundus examinations, and recorded any changes in disease using photographs. Each dose included 1 to 2 mL of undiluted rituximab (10-20 mg/mL) plus 1 mL of 2% epinephrine-free xylocaine. Intralesional drug delivery was preceded by eyelid border disinfection with iodopovidone 5% gauze and local anesthetic (2 drops of 0.4% Novesina) and consisted of repeated multiple injections (30-gauge needle) of small volumes of solution administered within and covering the entire volume of the visible lesions. Care was taken not to inject the solution into blood vessels. The entire procedure lasted ∼10 minutes. Patients were next treated with tobramycin ophthalmic eye drops, which were applied 3 times a day for 4 days, and bandages were applied.
Patients who did not achieve lymphoma regression after 4 weekly doses continued the therapeutic program and received intralesional injections of rituximab supplemented with autologous serum to increase the bioavailability of immunologic effectors in the tumor tissue. Autologous serum was collected on each treatment day, immediately before intralesional injection, as follows. Five milliliters of blood was collected under sterile conditions and centrifuged at 1000 rpm for 10 minutes; serum was collected and stored at 4°C to 8°C. Autologous serum (0.5 mL) was combined with 1.5 mL of undiluted rituximab and 0.5 mL of 2% epinephrine-free xylocaine, and this solution was administered as described above.
Toxicity and response assessment
Treatment side effects were assessed before each treatment with rituximab and graded according to the common toxicity criteria of the National Cancer Institute’s Common Terminology for Adverse Effects Version 3.0.23 Patients were considered assessable for toxicity whenever they received ≥1 dose of intralesional rituximab. The worst toxic effects per organ, per patient were considered for analyses.
Response to treatment was assessed after weekly and monthly doses of rituximab and at the end of the therapeutic program by ophthalmologic examination and photographs of the lesions. Response definition relied on changes in the size of conjunctival lesions and followed the National Cancer Institute standardized response criteria.24 In brief, complete remission was defined as the complete disappearance of all evidence of lymphoma; partial response was defined as ≥50% decrease in tumor size; progressive disease was defined as ≥25% increase in tumor size or the appearance of any new tumor lesion; and stable disease was defined as situations that did not meet any of the previous criteria. The maximum response recorded from treatment onset was considered for activity analyses. We measured the duration of response from the date of maximum response (complete remission or partial response) to the date of objective progression, or the last date of follow-up in the absence of progression.
At the end of treatment, disease was assessed every 6 months for the first 5 years and every year thereafter. After progression, patients were followed up every 6 months for survival; they returned to the previous follow-up schedule in the case of further remission.
Statistical considerations
Safety was the primary end point, and the response rate and the antitumor effect of the added autologous serum were secondary end points. All patients who received ≥1 dose of intralesional rituximab were considered for primary and secondary analyses. Estimated sample size was 20 patients, considering that every side effect of grade ≥3 should occur in ≥10% of patients to be considered clinically relevant. Survival curves were generated with the Kaplan-Meier method. Progression-free survival was calculated from the trial registration date to relapse, progression, or death or to the last date of follow-up. Death from any cause without relapse or progression was considered an event in the analysis of progression-free survival. Time-to-next-treatment rate was calculated like progression-free survival, but only the start of an additional line of treatment was considered an event. Survival rates were reported as 5-year progression-free survival and time-to-next-treatment with standard errors. We analyzed differences between patient subgroups using the Fisher’s exact test. All probability values were 2-sided. All analyses were performed with the Statistica 10.0 statistical package for Windows.
Results
Patients’ characteristics
Between October 2012 and August 2018, 20 patients were enrolled in the study (Table 1). Median age was 63 years (range, 30-81), with a male/female ratio of 0.66. All patients had an extranodal marginal zone B-cell lymphoma; 4 patients had concomitant HBV or HCV infection; and no patient had increased lactate dehydrogenase serum levels. Lymphoma involved the right eye in 6 patients, the left eye in 7 patients, and both eyes in 7 patients. All but 3 patients were registered in this trial at first relapse. Seven patients had lymphoma refractory to the prior line of treatment; 19 patients were previously treated with doxycycline.8 Two patients were refractory to IV rituximab. Complete assessment of C psittaci infection was available for 10 patients, and, in 2 of the patients, infection was still detected at the time of enrollment in the study.
Safety (primary end point)
Treatment tolerability was excellent: there were no interruptions or delays related to toxicity. Only 3 side effects were recorded, which were mild, local, and resolved spontaneously within 2 days (grade 1 edema, grade 1 hyperemia, and grade 2 bleeding). Grade 1 edema and grade 1 hyperemia were related to antitumor activity; in fact, both of the patients achieved a durable complete remission (38 and 29+ months) and did not require serum supplementation. The single episode of bleeding occurred after addition of the patient’s serum.
Activity and efficacy
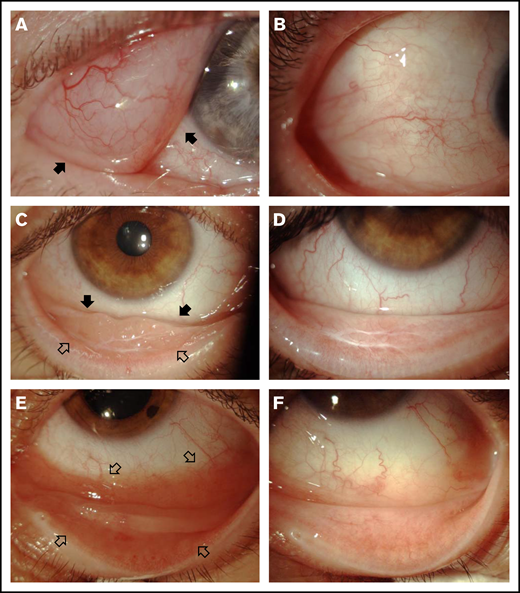
After the 4 weekly doses of rituximab, 11 patients achieved tumor regression, 8 patients had stable disease, and 1 patient experienced progressive disease. Autologous serum was added to subsequent rituximab injections in 7 of the 8 patients with stable disease and, as protocol violations, in 2 patients with partial response to weekly rituximab (Table 2). The best response after treatment included complete responses in 12 patients and a partial response in 1 patient (examples of responses are shown in Figure 1), with an overall response rate of 65% (95% confidence interval, 45-85); 5 patients had stable disease, and 2 patients experienced progressive disease.
Three examples of responses after intralesional rituximab. (A) Right eye: extensive infiltration of bulbar temporal conjunctiva of a lymphomatous mass with well-defined margins (arrows); the therapeutic target was well delimited. (C) Right eye: tumor infiltration of the inferior conjunctiva; the lesion of the bulbar conjunctiva was better defined (solid arrows), whereas infiltration of the palpebral conjunctiva was poorly delimited (open arrows). (E) Left eye: extensive infiltration of bulbar and palpebral inferior conjunctiva with intense hyperemia that hampered the definition of the borders of the lesion (open arrows) and required numerous subconjunctival injections of rituximab to avoid undertreatment. (B,D,F) Tumor regression after intralesional injections of rituximab.
Three examples of responses after intralesional rituximab. (A) Right eye: extensive infiltration of bulbar temporal conjunctiva of a lymphomatous mass with well-defined margins (arrows); the therapeutic target was well delimited. (C) Right eye: tumor infiltration of the inferior conjunctiva; the lesion of the bulbar conjunctiva was better defined (solid arrows), whereas infiltration of the palpebral conjunctiva was poorly delimited (open arrows). (E) Left eye: extensive infiltration of bulbar and palpebral inferior conjunctiva with intense hyperemia that hampered the definition of the borders of the lesion (open arrows) and required numerous subconjunctival injections of rituximab to avoid undertreatment. (B,D,F) Tumor regression after intralesional injections of rituximab.
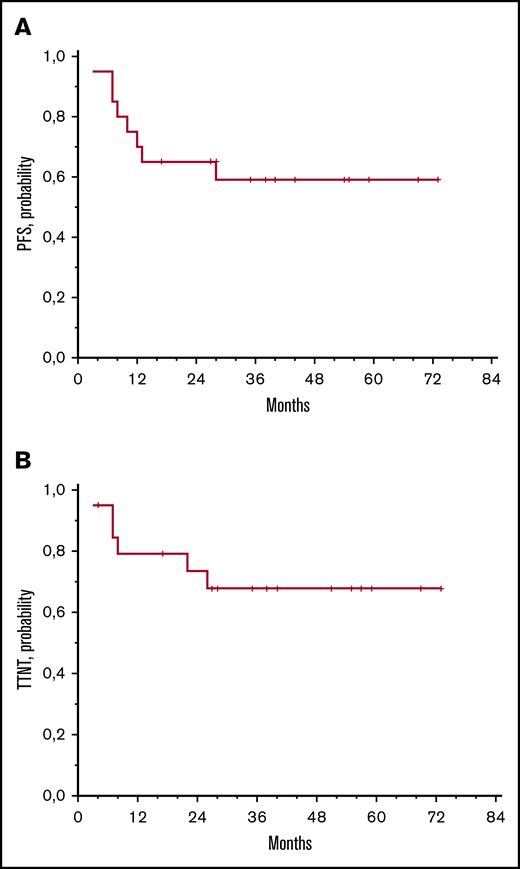
At a median follow-up of 42 months (range, 10-78), 12 patients remain progression-free; 2 of these patients did not experience progressive disease after intralesional rituximab, but they required palliative irradiation of the treated eye because of local symptoms after 4 or 22 months. Eight patients experienced progressive disease, which invariably affected the treated eye. Interestingly, 3 patients who experienced local relapse after injections of rituximab alone were treated with intralesional rituximab supplemented with autologous serum; 2 of them achieved a further complete response that lasted 25+ or 38+ months. The 5-year progression-free survival and time-to-next-treatment rates were 59% ± 11% and 69% ± 11%, respectively (Figure 2). All patients are alive, and visual acuity is preserved in all cases.
Survival data. Progression-free survival (PFS) (A) and time to next treatment (TTNT) (B).
Survival data. Progression-free survival (PFS) (A) and time to next treatment (TTNT) (B).
Effect of serum supplementation
The results of serum supplementation in the 9 patients who received this compound are summarized in Table 2. Autologous serum was added to rituximab in 2 patients with a partial response after weekly injections of rituximab (patients 7 and 15; Table 2); these patients achieved a complete remission that lasted 28 and 35+ months, respectively. In the 7 patients with stable disease after weekly injections of rituximab, the addition of autologous serum was followed by a complete response lasting 40+ months in patient 14 and by a partial response lasting 59+ months in patient 6. The other 5 patients did not have a benefit from serum supplementation.
Discussion
To the best of our knowledge, this is the first prospective trial using intralesional rituximab in patients with extranodal marginal zone lymphoma. This simple and inexpensive treatment was safe and active in patients with relapsed conjunctival MALT lymphoma, and the addition of autologous serum resulted in an improved response in 4 of 9 patients. Importantly, half of the responses lasted ≥3 years, and retreatment of local relapses resulted in a second durable tumor regression in 2 of the 3 retreated patients.
This study has a few limitations. First, the sample size was estimated to assess feasibility and not to demonstrate activity; thus, the number of registered patients may be considered small to fulfill this secondary end point. Indeed, the sample size was adequate to demonstrate that this treatment is safe, considering that no grade ≥3 toxicity was recorded, with only 3 mild local side effects; importantly, the number of responses was sufficient to provide the rationale for further investigations using this approach. Second, this trial does not demonstrate activity for intralesional rituximab in relapses within irradiated volume, because none of the patients received radiation therapy as first-line treatment. However, these data reflect routine practice, where in-field local relapses are uncommon (6%-8% of irradiated patients),25,26 a figure that impairs to carry ad hoc trials addressing the role of intralesional rituximab as postradiation salvage therapy. Accordingly, the routine use of intralesional rituximab in patients with in-field relapses should be performed with accurate monitoring of tolerability and activity.
Intralesional injections of rituximab seem to improve the activity of this antibody in conjunctival MALT lymphoma. Literature on the activity of systemic rituximab in this lymphoma consists of case reports and small case series including intraorbital and conjunctival lymphomas. In the largest series (10 patients), IV rituximab, at conventional doses, was associated with transient responses, with tumor recurrence requiring radiotherapy in 64% of patients at a median follow-up of 31 months.27 The comparison of the results of the IRIS prospective trial with the safety and activity profiles reported for other local therapies used in patients with conjunctival lymphoma is impaired by the availability of only small retrospective case series. Local therapies are better tolerated than IV and oral chemotherapies used in OAL patients, with the latter often associated with anemia (19% of cases), neutropenia (33%), paresthesia (33%), hepatotoxicity (21%), constipation (12%), and hyperglycemia (9%).28 Radiotherapy, the most widely used local treatment in OAL, is associated with grade 2-4 cataract in 38% of cases, retinopathy in 17% of cases, dry eyes in 17% of cases, and glaucoma in 2% of cases25,26 ; these were reduced to 6%, 5%, 27%, and 1%, respectively, when a smaller radiation dose was used.29 Mitomycin C drops have been used in a few cases of conjunctival lymphoma, with conjunctival injection and superficial punctate keratopathy that required a topical steroid and lubricant.10 A more extensive experience with ocular surface carcinomas has shown that topical mitomycin C is associated with an overall rate of side effects of 41%; specific treatment was required in 17% of cases.30 Intralesional interferon has been associated with a high rate of local disease control in a single retrospective series of 16 patients with conjunctival MALT lymphoma.31 Disappointingly, all treated patients had a combination of local and systemic side effects, such as conjunctival chemosis and flu-like syndrome, fever, and myalgias. Other local complications were pain (12% of cases), instant burning (12%), bleeding (6%), and photophobia (6%). The IRIS trial demonstrated that, when administered by an experienced ophthalmologist, intralesional rituximab was well tolerated; there were no delays or interruptions related to toxicity, and only 3 episodic side effects were observed that were local, mild, transient, and interpreted as related to antitumor effect. These favorable safety data, coupled with long-lasting responses in two thirds of treated patients, suggest that intralesional rituximab is a valid alternative to other local treatments for conjunctival lymphomas.
The clinical benefit achieved with the addition of autologous serum represents an innovative contribution of the IRIS trial. This antitumor effect is suggested by the complete or partial responses observed after the addition of autologous serum in 4 of 9 patients who did not achieve tumor remission after weekly injections of rituximab alone. Likewise, 2 long-lasting complete responses were observed among the 3 patients who were treated with intralesional rituximab plus autologous serum for local relapses that occurred after injections of rituximab alone. However, these findings should interpreted with caution, because rituximab was added concurrently, and delayed responses after rituximab are known, which might be the case after the 4 weekly injections. This biological effect needs to be assessed in a larger series, and it may be explained by a greater bioavailability of rituximab and its effectors in the tumor microenvironment, which is in line with the synergistic cytotoxic effect of rituximab supplemented with 10% serum reported in in vitro studies.21
Three major questions about the use of intralesional rituximab need to be addressed in forthcoming OAL trials. First, the use of this therapy in intraorbital lesions is debatable. In the IRIS trial, only patients with conjunctival MALT lymphoma and lesions fully appreciable at ophthalmologic examination were included. In a prior study,32 intralesional rituximab was associated with good disease control in 7 patients with concomitant involvement of lachrymal gland, eyelid, and medium/posterior orbital spaces, which suggests that this strategy may be also be applied to intraorbital MALT lymphomas. However, this issue should be specifically investigated to avoid the risk of inadequate treatment of deeper lesions, especially in lymphomas located in the lateral and superior quadrants of the orbit.19 Second, the use of intralesional rituximab in patients with CD20+ lymphomas other than MALT lymphoma needs to be investigated. Patients with such lymphomas were eligible for the IRIS trial, but the scarcity of these entities did not allow us to enroll any such patients; this experience suggests that it will be very difficult to recruit a sufficient amount of patients to draw reliable conclusions about this issue. Nevertheless, ≥2 cases of relapsed primary follicular lymphoma of the conjunctiva were successfully treated with intralesional injections of rituximab.19,33 This experience, albeit anecdotal, provides the rationale for the use of this therapy in uncommon forms of CD20+ conjunctival lymphomas. Third, the role of intralesional rituximab, or other anti-CD20 antibodies with improved activity in indolent lymphomas (ie, obinutuzumab), as first-line treatment in conjunctiva MALT lymphomas needs to be investigated. A single study of 7 patients with OAL who were treated with upfront intralesional rituximab seems to confirm the safety and activity of this strategy34 ; however, confirmatory studies are required before we can recommend the routine use of upfront intralesional rituximab.
In conclusion, the IRIS trial demonstrates that intralesional rituximab is an easy, safe, and active strategy for patients with relapsed conjunctival MALT lymphomas. The addition of autologous serum seems to improve the activity of intralesional rituximab, and it should be tested in other local immunotherapies. Finally, the IRIS trial represents a suitable background for the development of future clinical trials addressing local anti-CD20 therapies in localized indolent lymphomas, at diagnosis and at relapse.
Data sharing requests should be sent to Andrés J. M. Ferreri (ferreri.andres@hsr.it).
Acknowledgments
The authors thank the patients and their families for their commitment. They also acknowledge the excellent technical assistance and sustained scientific collaboration of oncologists, hematologists, pathologists, neuroradiologists, ophthalmologists, radiation oncologists, pharmacologists, research nurses, and data managers at the San Raffaele Scientific Institute.
The IRIS study was an academic trial; thus, it was performed without commercial funding and with no intent for profit.
Authorship
Contribution: A.J.M.F. conceived, designed, and supervised the study; A.J.M.F., M.S., E.M., S.G., and G.M. developed the methodology; M.E.C. provided pharmacologic support; L.A., F.Z., G.T., D.M., T.C., and S.P. evaluated and followed patients; E.M. and G.M. treated patients; M.P. performed histopathology and immunohistochemistry; M.S., S.M., C.C., and S.G. acquired clinical data; A.J.M.F., M.S., G.M., and M.P. analyzed and interpreted data and wrote the manuscript; and all authors revised and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andrés J. M. Ferreri, Lymphoma Unit, Department of Onco-Hematology, IRCCS San Raffaele Scientific Institute, Via Olgettina 60, 20132 Milan, Italy; e-mail: ferreri.andres@hsr.it.